Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand
2015-12-08DamrongpanThongwatNophawanBunchu
Damrongpan Thongwat, Nophawan Bunchu
1Department of Microbiology and Parasitology, Faculty of Medical Science, Naresuan University, Muang, Phitsanulok 65000, Thailand
2Centre of Excellence in Medical Biotechnology, Faculty of Medical Science, Naresuan University, Muang, Phitsanulok 65000, Thailand
Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand
Damrongpan Thongwat1,2*, Nophawan Bunchu1,2
1Department of Microbiology and Parasitology, Faculty of Medical Science, Naresuan University, Muang, Phitsanulok 65000, Thailand
2Centre of Excellence in Medical Biotechnology, Faculty of Medical Science, Naresuan University, Muang, Phitsanulok 65000, Thailand
ARTICLE INFO
Article history:
Received 10 August 2014
Received in revised form 15 October 2014
Accepted 15 December 2014
Available online 20 January 2015
Aedes aegypti
Insecticide susceptibility Temephos
Permethrin
Deltamethrin
Objective: To investigate the susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Ae. aegypti), collected from areas with high incidence of dengue hemorrhagic fever cases in Phitsanulok Province, Thailand. Methods: The F1 progenies of Ae. aegypti colony, originated from five sub-districts including Aranyik, Hua Ro, Nai Muang, Ban Krang and Tha Pho, were used in the bioassays following the procedures of World Health Organization. For larval bioassay, the late third or early fourth-instar larvae were tested with different concentrations of temephos. For adult bioassay, the females were exposed to 0.75 % permethrin or 0.05% deltamethrin. LC50value and mortality rate were analyzed to compare the insecticide susceptibility of the larvae and the adults in each area, respectively. Results: The LC50value of temephos for the larvae from Aranyik, Hua Ro, Nai Muang, Ban Krang and Tha Pho sub-districts was 0.017, 0.017, 0.026, 0.061, and 0.113 ppm, respectively. For permethrin, the highest mortality rate (86.84%) was found in the mosquitoes from Aranyik but the others were more resistant with the lower mortality rates (16.00-42.67%). The adult mortality rates after exposing to deltamethrin were higher (82.34-98.67%) in all areas. Conclusions: Ae. aegypti larvae were still susceptible to temephos. Conversely, most tested adults tended to resist the permethrin and deltamethrin.
1. Introduction
Dengue (DF) and dengue hemorrhagic fevers (DHF) are the vector-borne diseases of public health importance in the temperate, subtropical, and tropical region of the world[1-3]. In Thailand, the first case and the outbreak were observed in 1949 and 1958, respectively[4]. Subsequently, these diseases have spread gradually throughout Thailand and the trend of incidence has continued to increase. In this study we focused on the DF/DHF in Phitsanulok Province not only because DF/DHF cases have been reported annually in many areas, but also because this province is currently known as a strategic location where the Indo-China intersection and it will be one of the centers of economic and social communications of ASEAN Economic Community of Thailand in 2015. Therefore, the prevention of disease outbreaks is primary public health missions in this province.
The main problem of vector-borne disease outbreaks is the insecticide resistance of the vector. Aedes aegypti (Linnaeus, 1762) (Ae. aegypti) is recognized as the main vector of DF/DHF in tropical and subtropical region. For control program, many synthetic insecticides have been applied to control the Aedes populations. Organochlorines (DDT), organophosphates (temephos, fenitrothion, malathion, and chlorpyrifos), carbametes (propoxur, pirimiphosmethyl, and bendiocarb) and pyrethroids (permethrin, deltamethrin, lambda-cyhalothrin, and etofenprox) are the insecticides that have been used for controlling Aedes mosquitoes in Thailand[5]. DDT has been used to control Aedes mosquito since the first DHF outbreak in Thailand found. After that, the first report of DDT resistance in Ae. aegypti
in this country was published[6]. During 1986-1993, the resistance of Ae. aegypti to temephos, malathion, and fenitrothion was continuously reported from many regions of Thailand[5]. Up to date, the resistance of Ae. aegypti to insecticides, including temephos, DDT, permethrin, deltamethrin, have been reported from several areas of Thailand[7-11]; however, no information for Phitsanulok Province was found.
Therefore, the objective of this study was to determine the current susceptibility of the Aedes larvae and adults collected from the areas, found the high incidence of DF/ DHF cases of Phitsanulok Province, to three currently used insecticides including temephos, permethrin and deltamethrin. The information on the insecticide susceptibility rate of the mosquito will be useful for selecting the appropriate insecticide and their concentrations that provide the most effective for Ae. aegypti controlling in these areas.
2. Materials and methods
2.1. Collection sites
During November - December 2010, Aedes mosquito larvae were collected from five sub-districts in Muang district of Phitsanulok Province, Thailand. The collection sites included Aranyik (N 16o48.064’ E 100o16.525’), Hua Ro (N 16o51.386’ E 100o16.410’), Nai Muang (N 16o44.447’E 100o14.712’), Ban Krang (N 16o52.601’ E 100o11.744’) and Tha Pho (N 16o46.478’ E 100o10.071’) sub-districts. According to vector-borne disease annual report 2009-2010 of Phitsanulok Provincial Health Office, Muang district was found to have the highest number of DF/ DHF cases in Phitsanulok. The five selected sub-districts were the top five in the list of the areas where DF/ DHF cases were detected.
2.2. Insecticide source
The temephos solution and the permethrin and deltamethrin impregnated papers were purchased from Vector Control Research Unit, Penang, Malaysia.
2.3. Mosquito collection and colonization
Aedes larvae were gently collected from their breeding habitats using plastic pipettes and transferred into a plastic cup. The collected larvae were maintained in the laboratory at Department of Microbiology and Parasitology, Faculty of Medical Science, Naresuan University. The emerging adults were identified morphologically following the illustrated keys to the mosquitoes of Thailand [12]. Ae. aegypti mosquito from each site were pooled and transferred into a mosquito cage (30 cm × 30 cm × 30 cm). They were fed with 5% sugar mixed with 5% multivitamin syrup solution. After emerging for 2-3 days, they were fed on a blood meal by using an artificial membrane feeding technique [13]. After the bloodfed female became gravid, they were allowed to lay eggs on a filter paper in the rearing cage. Filter paper found eggs on was transferred to a plastic tray filled with the tab water for larval hatching to produce F1 progeny. The larvae were daily fed with the crushed dog biscuits. The third to fourth instar larvae from each sub-district were used for larval susceptibility test. The remaining larvae were reared until they became adults, then only the mosquito females were subjected to test for permethrin and deltamethrin susceptibility.
2.4. Bioassays
2.4.1. Larval bioassay
This assay was designed to evaluate susceptibility of Ae. aegypti larvae to temephos. Late third or early fourth instar larvae were exposed to several concentrations of temephos following the procedure of World Health Organization [14]. One mL of temephos with the concentration 1.25, 6.25, 31.25 or 156.25 mg/L was dispensed in 249 mL of tap water in a plastic cup (7 cm in diameter and 5 cm in depth), resulting in temephos final concentrations of 0.005, 0.025, 0.125 or 0.625 μg/mL, respectively. After that, 25 larvae were gently transferred into each cup. Absolute ethanol was used as a control reagent. Four replicates per concentration and two control replicates were done. All the containers were held in a laboratory at room temperature. After 24 hour-exposing time, the mortality rate was recorded. Mortality of larvae was detected by lightly stirring them with a needle. Unmoved larvae were recorded as dead. Probit analysis was used to calculate the lethal concentration 50 (LC50) and resistance ratio[15] by using the software Ldp Line (http://embakr.tripod. com/ldpline). The lowest LC50was selected to be a baseline for comparing resistance level among studied areas.
2.4.2. Adult bioassay
The objective of this assay was to determine the susceptibility of Ae. aegypti adults to the permethrin and deltamethrin. The experiment was carried out according to the recommended protocol of World Health Organization[16]. A group (25 adults) of F1 unfed with blood females (2-3 days old) from each area was exposed to 0.75 % permethrin or 0.05% deltamethrin impregnated papers for 1 hour in an exposure tube and this tube was held vertically in the laboratory at room temperature. Paper without any insecticide was used as a control. Twelve replicates of each
insecticide and four control replicates were used in this study. The knock down rate of each replicate was recorded after 1 hour-exposing time. After that, the mosquitoes were transferred into a resting tube and provided with 5% sugar solution and kept at room temperature with~70% relative humidity. The mortality rate was recorded after 24 hour-exposing time. The mortality rates of the adult mosquitoes were interpreted to susceptibility rate following the interpretation guideline of World Health Organization recommendation: 98%-100% mortality indicates susceptibility; 80%-97% mortality suggests the possibility of resistance; and < 80% mortality suggests resistance[16].
For both larval and adult bioassays, when the mortality rate in the control groups was over 5%, but less than 20%, correction of the mortality rate was made by applying the Abbott’s formula[17]. When the mortality rate in the controls was over 20%, the tests were discarded.
3. Results
The summary result of the larval bioassay is demonstrated in Table 1. LC50of temephos for Aranyik, Hua Ro, Nai Muang, Ban Krang, and Tha Pho larvae were 0.017, 0.017, 0.026, 0.061, and 0.113 μg/mL, respectively. Moreover, the resistance ratio revealed that Tha Pho larvae were more resistant to temephos with 6.65 times than Aranyik larvae while the resistance ratio of Ban Krang and Nai Muang larvae compared to the Aranyik ones were only 3.59 and 1.53 times, respectively (Table 1).
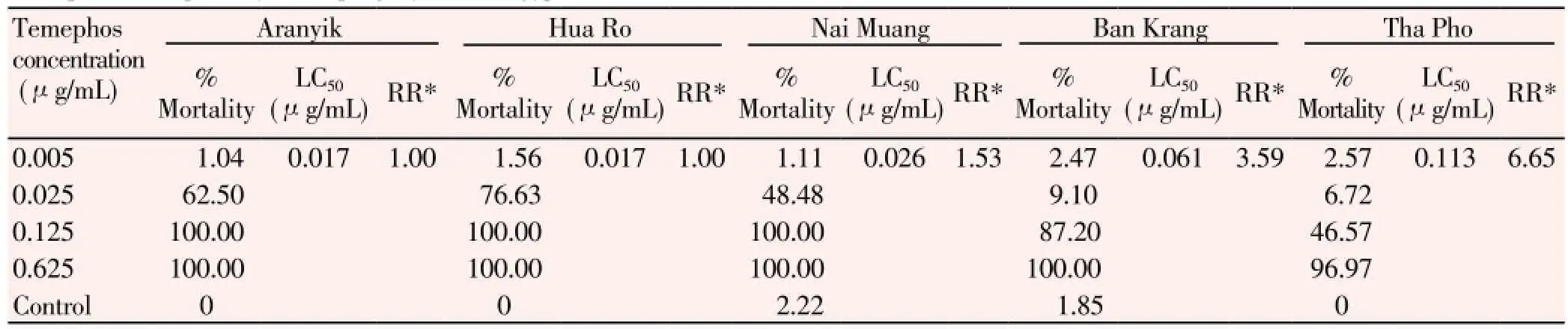
Table 1 Temephos susceptibility of F1 progeny of Ae. aegypti larvae from five collection areas.
The results of the adult bioassay are summarized in Table 2. For permethrin susceptibility test, the knock down rates and the mortality rates were ranging 14.34%-62.17% and 16.00%-86.84%, respectively. The mosquito from Aranyik provided the highest of knock down (62.17%) and mortality (86.84%) rates while these parameters for the mosquitoes from other areas were lower (Table 2). All the results indicated that the mosquitoes from all areas were resistant to permethrin. According to the classification of WHO, only the mosquitoes from Nai Muang and Aranyik sub-districts was susceptible to deltamethrin with knock down and mortality rates 96.33%-98.67% and 98.00%-98.67%, respectively. In contrast, the others showed the possibility of resistance with knock down and mortality rates 82.84%-96.67% and 82.34%-96.67%, respectively. Among those, the mosquitoes from Tha Pho were more tolerant to deltamethrin than the ones from Ban Krang and Hua Ro with the lower of knock down and mortality rates (Table 2).

Table 2 Permethrin and deltamethrin susceptibility of F1 progeny of Ae. aegypti adult from five collection areas.
4. Discussion
DH and DHF have been recognized as seriously vectorborne disease worldwide. Control programs for limiting the population of the vector, are necessary especially when the outbreak occurs. Applying insecticide is the primary method to control the outbreak. Based on the results of this study, F1 larvae from all the areas were susceptible to temephos because all of them died after the contact with 0.625 1 μg/mL temephos. This concentration is below the
recommendable concentration, 1μg/mL, for using to control the Aedes larvae in the household water container[18]. Therefore, temephos is still the effective insecticide for controlling Ae. aegypti larvae in these areas. The susceptible of Ae. aegypti larvae to temephos was previously reported from many areas in Thailand, including Ubon Ratchathani, Sri Sa Ket, Yasothon, Amnat Charoen, Kalasin, Nonthaburi, Ratchaburi, Nakhon Sawan, Kamphaeng Phet, Nakhon Ratchasima, Buri Ram, Surat Thani and Bangkok Provinces [8,11,19]. Conversely, the resistance to temephos was found in Ae. aegypti larvae from some areas of Thailand, such as Tak (Mae Sot), Mukdahan, Nakhon Sawan, Phatthalung, Sakon Nakhon and Surat Thani Provinces[8,20]. Although the mosquito larvae from all the areas of our study were susceptible to temephos, the larvae from Tha Pho sub-district showed the lowest mortality rate in all tested temephos concentrations when compared with other areas. Therefore, it is possible that Ae. aegypti from Tha Pho subdistrict may develop insecticide resistance rapider than the others or to develop cross-resistance to other insecticides as found in the previous studies[21-24]. In terms of the knock down rates and the mortality rates from the adult bioassays, F1 females from all studied areas were more resistant to permethrin than to deltamethrin. Moreover, we were able to find the susceptibility of mosquitoes to deltamethrin only in two areas (Aranyik and Nai Muang sub-districts). Therefore, an alternative choice of insecticides or other control measures to control Ae. aegypti adults should be studied and applied in these studied areas immediately.
The mosquitoes collected in Tha Pho sub-district had the lowest mortality rates for both insecticides. This result indicated that the mosquitoes from this area were stronger and more tolerance to the insecticides than the other four studied areas. It might be due to the difference of land use types. Only Tha Pho sub-district is an agricultural area with the rice cultivation fields, while the others are the residential areas. For the agricultural management, insecticides have been frequently applied for controlling pests resulting with pesticide residues contaminated in the environment [25-28]. Consequently, the native insects including the mosquitoes could probably be affected by this operation. The continuous receiving of various types and concentrations of the contaminating insecticides and their residues might be the cause of the development of multi-resistant to the insecticides in the mosquito vectors as was found in many countries, including Thailand[29-31]. Therefore, the intensive study in Tha Pho sub-district should be performed in the future. Moreover, the use of ineffective concentrations of insecticides in control program can also cause the development of insecticide resistance in mosquito vectors. Adaptation behavior when the mosquitoes contact with un-lethal dose of insecticide was reported as one of the important factors for developing insecticide resistance mechanisms. The previous studies found that after growth of several generations under the selective pressure of temephos, the resistance ratio for the insecticide of Ae. aegypti offspring increased[21,24,32-34]. To prevent insecticide resistance in mosquito from inadequate application with un-lethal dose applying, the evaluation of the insecticide susceptibility status of the vectors in a small area should be studied. Moreover, the preliminary susceptibility tests for the currently used insecticides should be conducted. It will allow selection of the most effective chemicals and their concentrations before applying any insecticides on a large scale.
The results of this study confirmed that one of the important factors in DH/DHF outbreaks in these areas were from the insecticide resistance in Ae. aegypti adults. However, other factors such as insecticide-applying methods, community education, social mobilization and communication are also important and should be studied further. Moreover, it is necessary to survey and evaluate regularly the data on the insecticide susceptibility of the vectors in each particular area in order to prevent the developing of the vectors insecticide resistance.
Conflict of interest statement
Authors declare no conflict of interest.
Acknowledgments
This study was financially supported from faculty of Medical Science and Centre of Excellence in Medical Biotechnology, Naresuan University, Thailand. We acknowledge Mr. Aussanee Jeenam, Ms. Panida Jamnongjit and Ms. Sirinya Puangsri (former students of Faculty of Medical Science, Naresuan University) for their assistances during the experiments. We are grateful to the Phitsanulok Provincial Health Office for providing data of DF/DHF annual report in 2009-2010. Dr. Svetlana Kocherginskaya is also acknowledged for her intensive proofreading of our manuscript.
[1] Halstead SB. Dengue. Lancet 2007; 370: 1644-1652.
[2] Guzman A, Isturiz RE. Update on the global spread of dengue. Int J Antimicrob Agents 2010; 36(Suppl 1): S40-S42.
[3] Wilder-Smith A. Dengue infections in travellers. Paediatr Int Child Health 2012; 32(S1): 28-32.
[4] Prasittisuk C, Andjaparidze AG, Kumar V. Current status of dengue/dengue haemorrhagic fever in WHO South-East Asia region. Dengue Bull 1998; 22: 1-15.
[5] Chareonviriyaphap T, Aum-aung B, Ratanatham S. Current insecticide resistance patterns in mosquito vectors in Thailand. Southeast Asian J Trop Med Public Health 1999; 30(1): 184-194.
[6] Neely M. Insecticide resistance studies on Aedes aegypti in Thailand. Bull WHO 1964; 35: 91-92.
[7] Thanispong K, Sathantriphop S, Chareonviriyaphap T. Insecticide resistance of Aedes aegypti and Culex quinquefasciatus in Thailand. J Pestic Sci 2008; 33(4): 351-356.
[8] Sornpeng W, Pimsamarn S, Akksilp S. Resistance to temephos of Aedes aegypti Linnaeus larvae (Diptera: Culicidae). J Health Sci 2009; 18(5): 650-654.
[9] Pimsamarn S, Sornpeng W, Akksilp S, Paeporn P, Limpawitthayakul M. Detection of insecticide resistance in Aedes aegypti to organophosphate and synthetic pyrethroid compounds in the north-east of Thailand. Dengue Bull 2009; 33: 194-202.
[10] Chuaycharoensuk T, Juntarajumnong W, Boonyuan W, Bangs MJ, Akratanakul P, Thammapalo S, et al. Frequency of pyrethroid resistance in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Thailand. J Vector Ecol 2011; 36(1): 204-212.
[11] Komalamisra N, Srisawat R, Phanbhuwong T, Oatwaree S. Insecticide susceptibility of the dengue vector, Aedes aegypti (L.) in metropolitan Bangkok. Southeast Asian J Trop Med Public Health 2011; 42(4): 814-823.
[12] Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE, Richardson JH. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J Trop Med Public Health 2010; 41(Suppl 1): 1-225.
[13] Rutledge LC, Ward RA, Gould DJ. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq News 1964; 24: 407-419.
[14] World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. Document WHO/CDS/WHOPES/ GCDPP/2005.13. Geneva: World Health Organization; 2005.
[15] Finney DJ. Probit analysis. 3 ed. London: Cambridge University Press; 1971, p. 68-78.
[16] World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector, bio-efficacy and persistence of insecticides on treated surfaces. Report of the WHO Informal Consultation, 28-30 September 1998, WHO/HQ, Document WHO/CDS/CPC/MAL/98.12. Geneva: World Health Organization; 1998.
[17] Abbott, WS. A method of computing the effectiveness of an insecticide. J Econ Entomol 1925; 18: 265-7.
[18] Rozendaal JA. Vector control. Methods for use by individuals and communities. Geneva: World Health Organization; 1997, p. 132-133.
[19] Paeporn P, Supaphathom K, Wattanachai P, Sathatriphop S, Yaumphan P, Deesin V. Insecticide susceptibility of Aedes aegypti in different parts of Thailand. J Trop Med Parasitol 2006; 29: 1-5.
[20] Ponlawat A, Scott JG, Harrington LC. Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J Med Entomol 2005; 42(5): 821-825.
[21] Rodriguez MM, Bisset J, Ruiz M, Soca A. Cross-resistance to pyrethroid and organophosphorus insecticides induced by selection with temephos in Aedes aegypti (Diptera: Culicidae) from Cuba. J Med Entomol 2002; 39(6): 882-888.
[22] Thongwat D, Bunchu N. Cross-resistance to deltamethrin in Aedes aegypti (Diptera: Culicidae) adult induced by selection with temephos in larvae. Lanna Public Health J 2011; 7: 240-250.
[23] Poupardin R, Reynaud S, Strode C, Ranson H, Vontas J, David JP. Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti; impact on larval tolerance to chemical insecticide. Insect Biochem Mol Biol 2008; 38(5): 540-551.
[24] Tikar SN, Kumar A, Prasad GB, Prakash S. Temephos-induced resistance in Aedes aegypti and its cross-resistance studies to certain insecticides from India. Parasitol Res 2009; 105: 57-63.
[25] Thapina A, Hudak PF. Pesticide use and residual occurrence in Thailand. Environ Monitor Assess 2000; 60: 103-114.
[26] Musa S, Gichuki JW. Raburu PO, Aura CM. Organochlorine and organophosphorus pesticide residues in water and sediment from Yala/Nzoia river within lake Victoria basin, Kenya. J Ecol Nat Environ 2011; 3(12): 392-399.
[27] Wang Y, Zhang B, Zhang X. Residues and spatial distribution of organochlorine pesticides in arable land from Liaocheng. Adv Mater Res 2012; 588-589: 2060-2063.
[28] Velasco A, Rodriguez J, Castillo R, Ortiz I. Residues of organochlorine and organophosphorus pesticides in sugarcane crop soils and river water. J Environ Sci Health, Part B 2012; 47(9): 833-841.
[29] Brogdon WG and McAllister JC. Synopses: insecticide and vector control. Emerg Infect Dis 1998; 4: 605-613.
[30] Hemingway J, Ranson H. Insecticide in insect vectors of human diseases. Annu Rev Entomol 2000; 45: 371-391.
[31] Jirakanjanakit N, Rongnoparut P, Saengtharatip S, Chareonviriyaphap T, Duchon S, Bellec C, et al. Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003-2005. J Econ Entomol 2007; 100(2): 545-550.
[32] Wirth MC, Georghiou GP. Selection and characterization of temephos resistance in a population of Aedes aegypti from Tortola, British Virgin Islands. J Am Mosq Control Assoc 1999; 15(3): 315-320.
[33] Paeporn P, Komalamisra N, Deesin V, Rongsriyam Y, Eshita Y, Thongrungkiat S. Temephos resistance in two forms of Aedes aegypti and its significance for the resistance mechanism. Southeast Asian J Trop Med Public Health 2003; 34(4): 786-792.
[34] Paeporn P, Ya-umphan P, Supaphathom K, Savanpanyalert P, Wattanachai P, Patimaprakorn R. Insecticide susceptibility and selection for resistance in a population of Aedes aegypti from Ratchaburi Province, Thailand. Trop Biomed 2004; 21(2): 1-8.
ment heading
10.1016/S1995-7645(14)60180-2
*Corresponding author: Dr. Damrongpan Thongwat, Faculty of Medical Science, Naresuan University, Amphur Muang, Phitsanulok 65000, Thailand.
E-mail: damrongpanth@nu.ac.th
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium maackii
- Anti-hypercholesterolemic effect of kenaf (Hibiscus cannabinus L.) seed on high-fat diet Sprague dawley rats
- Cloning, expression, purification and bioinformatic analysis of 2-methylcitrate synthase from Mycobacterium tuberculosis
- Prevalence of shiga toxins (stx1, stx2), eaeA and hly genes of Escherichia coli O157:H7 strains among children with acute gastroenteritis in southern of Iran
- Larvicidal, ovicidal and repellent activities of marine sponge Cliona celata (Grant) extracts against Anopheles stephensi Liston (Diptera: Culicidae)
- Characterization of mycobacterium isolates from pulmomary tuberculosis suspected cases visiting Tuberculosis Reference Laboratory at Ethiopian Health and Nutrition Research Institute, Addis Ababa Ethiopia: a cross sectional study
