氰根桥连的FeⅢMnⅡ链中磁性作用的调控
2015-11-30郑慧徐杨段春迎
郑慧 徐杨 段春迎
氰根桥连的FeⅢMnⅡ链中磁性作用的调控
郑慧 徐杨 段春迎*
(大连理工大学精细化工重点实验室,大连116024)
基于三氰构筑单元合成了两个氰基桥联的FeⅢ-MnⅡ一维链,{[Fe(Tp)(CN)3]2[Mn(bib)]}·CH3OH·2H2O(1)和{[Fe(pzTp)(CN)3]2[Mn(bib)]}·3H2O(2)。1和2中的双之字链通过刚性的双齿配体固定排列方式并连接成二维结构,链内FeⅢ和MnⅡ之间的磁相互作用可以通过改变链中配体的位阻调控。
氰基;之字链;双齿配体;磁相互作用
0 Introduction
One-dimensional magnetic systems,especially single chain magnets(SCMs),are attracting considerable interest in the field of chemistry,physics and material science[1].Such materials present both spontaneous magnetization and quantum behavior, providing an ideal model for studying the coexistence of classic and quantum behavior[2-5].Moreover,SCMs can retain spin information over long periods of time, providing potential applications in high-density information storage,quantum computing,and spintronics[6-7].Thereinto,cyanide-bridged complexes are of particular interest,because their structures can be rationally designed utilizing the linear linking mode of cyano-bridge of cyanometallate[8-10].Moreover,it is possible to reasonably predict the type of magnetic interaction and in some cases the magnitude of the magnetic anisotropy with knowledge of the exchange interaction nature through the cyanide bridge[11-12].In these systems,metal ions with strong anisotropy are usually adopted.However,the apical directions of the metal ions are not uniform and are tilted with an angle in general case,which will decrease the blocking temperature of the system or destroy the SCMs behavior[13].Even if the apical directions ofmetal ions within one chain are parallel,the apical directions of metal ions in neighboring chains may be different.In this case,the anisotropy of the system is hard to increase because the neighboring chains rotate an angle relatively[14-15].To solve this problem,a rational way is to use structurally ditopic,magnetically inert rigid organic linkers,which will link the chain into two-dimensional networks without significant changing the nature of the chains.This rigid linker can control the arrangement of chains,and force the apical directions of metal ions to parallel with each other, maximizing the anisotropy of the whole system.In the same time,the interchain interactions could also be controlled via choosing ditopic linkers with different lengths and electric natures.With such strategy,we have synthesized light-responsive SCMs,whose magnetic properties can be tuned via light-induced charge transfer or spin-crossover[16].Both MnⅡions and FeⅢions possess differentredox valence states,and charge transfer properties between them have been reported[17]. We aimed to incorporating MnⅡions and paramagnetic FeⅢions into a cyanide-bridged{FeⅢ2MnⅡ}chain utilizing tricyanometallate building block.Moreover,a rigid ditopic linker was adopted to fix the orientation of the chains and link the chains to form a layer. With such strategy,two heterometallic Fe-Mn compounds,{[Fe(Tp)(CN)3]2[Mn(bib)]}·CH3OH·2H2O(1) (Tp=hydrotris(pyrazolyl)borate;bib=1,4-bis(1-imidazolyl)benzene)and{[Fe(pzTp)(CN)3]2[Mn(bib)]}·3H2O (2)(pzTp=tetrakis(pyrazolyl)borate),were synthesized. Unfortunately,no charge transfer was observed. However,the interchain linkage and apical directions of MnⅡions were fixed with the rigid linker,providing a chance to tune the intrachain magnetic interactions via changing the capping ligand of FeⅢions of cyanometallate building blocks.
1 Experimental
1.1 Materials and physicalmeasurements
Manganese(Ⅱ)perchloratehexahydrate(Mn(ClO4)2· 6H2O,99%)and methanol(CH3OH)were ofanalytical grade from Tianjin Kemiou Chemical Reagent Co., Ltd.China.All chemical reagents were obtained from commercialsources and were used as received without further purication unless otherwise noted.The compounds Bu4N[Fe(Tp)(CN)3][18],Bu4N[Fe(pzTp)(CN)3][19]and ligand bib were synthesized by a modified literature method[20].Elemental analyses(carbon,hydrogen,and nitrogen)were carried out on an Elementar Vario ELⅢanalyzer.Infrared spectra were recorded as fine powder samples with a JASCO FT/IR-600 Plus spectrometer in the 400~4 000 cm-1region and a spectral resolution of 1 cm-1.Magnetic measurements of the samples were performed on a Quantum Design SQUID (MPMS XL-7)magnetometer.Data were corrected for the diamagnetic contribution calculated from Pascal constants.
1.2 Preparation of complexes 1 and 2
1.2.1 Synthesis of{[Fe(Tp)(CN)3]2[Mn(bib)]}·CH3OH· 2H2O(1)
4.0 mL water solution of 0.05 mmol(18.1 mg) Mn(ClO4)2·6H2O was placed at the bottom in one side of an H-shaped tube,while 4.0 mL methanol solution of 0.1 mmol(58.9 mg)[Bu4N][Fe(Tp)(CN)3]and 0.4 mmol(84.0 mg)bib was placed in the other side. Then 4.0 mL methanol solution was layered upon solutions of both sides to provide diffusion pathway. Crystallization took several weeks and gave crystals in a yield of 55%based on Mn(ClO4)2·6H2O.Element analysis:Calcd.for C37H38B2Fe2MnN22O3(%):C 43.26, H 3.73,N 30.01;Found(%):C 43.21,H 3.89,N 29.13.IR(solid KBr pellet,ν/cm-1):3 443 vs,3 123 m, 2 508 w,2 134 s,2 123 s,1 632 s,1 530 s,1 501 m,1 408 s,1 313 s,1 262 w,1 214 m,1 116 s,1 064 s, 1048 s,988 w,961 m,929 w,836 m,769 m,766 s, 711 m,658 m,614 m,536 m,492 m.
1.2.2 Synthesis of{[Fe(pzTp)(CN)3]2[Mn(bib)]}·3H2O (2)
The preparation of complex 2 was carried out using a procedure similar to thatemployed for complex 1.[Bu4N][Fe(pzTp)(CN)3](0.1 mmol,65.5 mg)was used as the building block,and the red prisms crystals were obtained in a yield of 70%based on Mn(ClO4)2· 6H2O.Elementanalysis:Calcd.for C42H41B2Fe2MnN26O3(%):C 44.01,H 3.60,N 31.77;Found(%):C 44.09,H 3.65,N 31.70.IR(solid KBr pellet,ν/cm-1):3 439 vs,3 137 m,2 141 m,1 633 m,1 530 s,1 501 m,1 441 w, 1 408 s,1 388 m,1 307 s,1 243 w,1 213 s,1 107 s, 1 092 w,1 065 s,962 w,932 w,858 m,809 m,794 m, 768 s,655 m,614 m,536 m.
1.3 Crystallographic refinement details
The data was collected on a Bruker Smart APEXⅡX-diffractometer equipped with graphite monochromatic Mo Kαradiation(λ=0.071 073 nm)using the SMART and SAINT programs.Indexing and unit cell refinement were based on all observed reflections.The structures were solved in the space group by direct method and refined by the full-matrix least-squares fitting on F2using SHELXTL-97.All non-hydrogen atoms were treated anisotropically.Hydrogen atoms bound to carbon were placed in calculated positions using a riding model while those attached to some water lattice solvents were located in the difference Fourier map and refined using fixed isotropic thermal parameters based on their respective parent atoms. The details of crystallographic data for compounds 1 and 2 are listed in Table 1.Selected bond distances and angles are listed in Table 2,respectively.
CCDC:1001946,1;1001947,2.
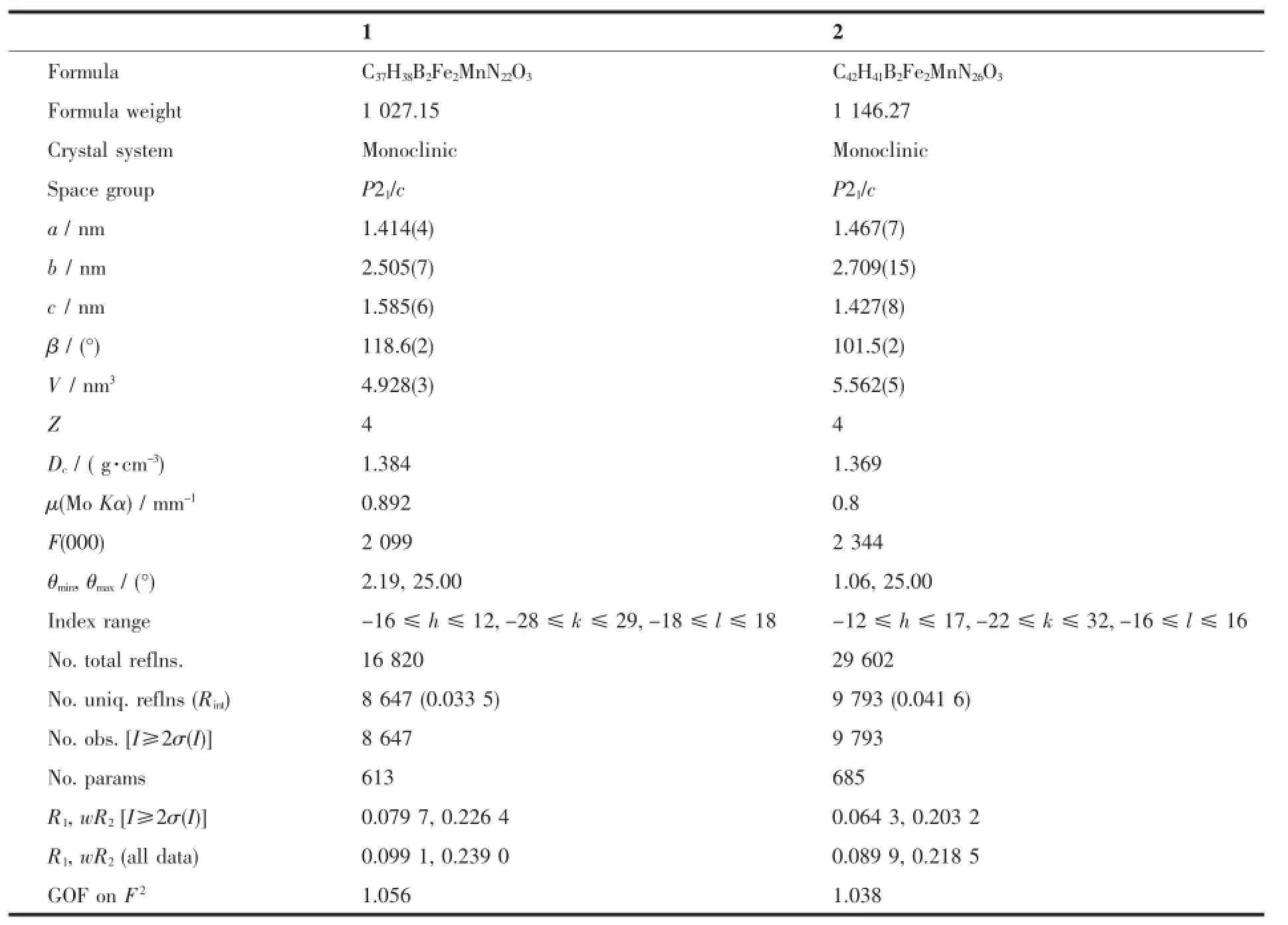
Table 1 Selected crystal data and structure refinement parameters of 1 and 2

Table 2 Selected bond distances(nm)and angles(°)in 1 and 2

Continued Table 1
2 Results and discussion
2.1 Crystalstructure of compound 1 and 2
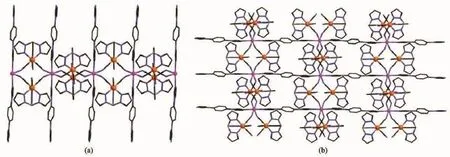
Fig.1(a)Side view of 1D double zigzag chain of complex 1,(b)Side view of the 2D layer for complex 1; Hydrogen atoms and crystallization water molecules are omitted for clarity;Atoms not involved in bridging are omitted for clarity;Fe orange;Mn pink;C dark;N blue;B dark yellow
Single-crystal X-ray diffraction analysis revealed that compound 1 crystallizes in a monoclinic P21/c space group.The crystal structure comprises neutral bimetallic[FeⅢ(Tp)(CN)3]2MnⅡ(bib)layers,with uncoordinated water and methanolmolecules located between the layers.In the neutral chain,the[Fe(Tp)(CN)3]-unit bridges two MnⅡions through two of its three cyanide groups in the cis position,whereas each MnⅡcoordinates to four nitrogen atoms from the CN-bridges,forming double zigzag chains(Fig.1a),which is similar to the reported examples[21-22].The neutral chains are further linked by bib ligands along the apical directions of the manganese centers to a layer framework(Fig.1b).The crystalunitconsists oftwo FeⅢcenters and one unique MnⅡcenter.Each FeⅢcenter is located in an octahedral environment with three nitrogen atoms from the tridentate Tp unit and threecyanide carbon atoms,whereas the coordination sites of the MnⅡcenter are occupied by two nitrogen atoms from the bidentate bib and four cyanide nitrogen atoms in an compressed N6coordination sphere.At 296 K,The Mn-NCNbond lengths are 0.220 2~0.224 1 nm and the Mn-Nbibbond distances are 0.221 7~0.221 8 nm,which are in good agreement with those observed previously in related compounds containing a high spin(HS)MnⅡion[13].The FeⅢ-C bond distances are 0.189 9~0.192 8 nm and 0.185 1~0.190 9 nm.The FeⅢ-N bond distances are 0.197 5~0.198 1 nm and 0.196 0~0.197 5 nm,for Fe1 and Fe2,respectively, which are typical values for LS FeⅢions.In neutral double zigzag chains,the square units exhibit two orientations of their mean planes(FeⅢ2MnⅡ2)with a 51.2°dihedralangle(here after denoted asφ).For the bond angles related to cyano bridge and metal ions, the N-C-Fe angles are 173.5°~174.8°,deviating slightly from the strict linearity.The C-N-Mn angles are 144.0°~156.0°,departing significantly from 180°.The shortest intrachain Fe-Mn,Mn-Mn,and Fe-Fe distancesare 0.498 5,0.689 9,and 0.691 6 nm,respectively, and the shortest interchain Fe-Mn,Mn-Mn,and Fe-Fe distances are 1.349,1.390,and 1.083 nm,respectively, along the b axis.The shortest interchain parallel pyrazolyl-pyrazolyl separation(0.362 nm)indicates that there exist weak interchainπ-πstacking interactions along the b axis.
The structure of complex 2 is isostructuralto that of 1.The distortion of the square units in complex 2 is slightly smaller than that in complex 1 because the steric hindrance of[Fe(pzTp)(CN)3]-in 2 is larger than the[Fe(Tp)(CN)3]-unitin 1.Theφvalue is 47°,smaller than that in 1.At 296 K,the lengths of the Mn-NCNcoordination bonds are 0.222 6~0.224 5 nm and the Mn-Nbibbond distances are 0.223 9 nm and 0.224 8 nm,providing an elongated N6coordination environment.Within the cyano bridged squares,the N≡C-Fe bond angels are 174.4°and 175.6°,being nearly linear;whereas the C≡N-Mn angles are 147.6°~153.1°,departing slightly smaller from linearity than that of 1.The shortest intrachains of Fe-Mn,Fe-Fe, Mn-Mn are 0.507 5,0.712 0,and 0.706 8 nm.The shortest interchain Fe-Mn,Mn-Mn,and Fe-Fe distances are 1.122,1.350,and 1.066 nm along the b axis,separating by bib,respectively.There are weak interchain interactions from parallel quasi-eclipsed stacking pyrazolyl rings from adjacent chains along the b axis,and the shortest distance between them is 0.362 13 nm.
2.2 Magnetic properties of complexes 1 and 2
The temperature dependent magnetic susceptibilities ofcomplexes 1 and 2 in the form ofχT vs T plots were measured on polycrystalline samples in the range of2~300 K under the applied magnetic field of 1 000 Oe(Fig.2).TheχT values per FeⅢ2MnⅡunit at room temperature are 5.24 cm3·mol-1·K and 5.38 cm3·mol-1·K for 1 and 2,respectively.These values are a little larger than the spin-only value of 5.125 cm3·mol-1·K expected for the magnetically dilute system including one high-spin MnⅡion(S=5/2)and two low-spin FeⅢions(S=1/2),where the spin-only value is calculated by assuming gMn=gFe=2.0.However,this is not surprising and simply indicates that the persistence of a residual unquenched orbital contribution due to the2T2gstate of low-spin FeⅢatoms.The effective g value of FeⅢis often found to be different from 2.0 because of an important orbital contribution.As the temperature is lowered,theχT value gradually decreases until 25 K,where a value of 3.70 cm3·mol-1·K is attained.Upon further cooling,theχT value sharply decreases to 1.26 cm3·mol-1·K at 2 K for 1.For 2, theχT values decrease smoothly down to 4.33 cm3·mol-1·K at 50 K and then abruptly reaching 1.33 cm3·mol-1·K at 2 K.The magnetic susceptibilities data conform well to the Curie-Weiss law of 2~300 K, which gives the Curie constant of 5.35 cm3·mol-1·K for 1 and 5.29 cm3·mol-1·K for 2,respectively.The negative Weiss temperatures(θ=-15.610 K for 1 and θ=-10.726 K for 2)suggest the presence of a dominant antiferromagnetic coupling between FeⅢand MnⅡions via the cyanide bridge within the 2D layer.
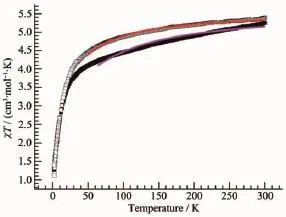
Fig.2 Temperature dependence ofχT for the complexes 1((Ⅱ))and 2(□)measured at 1 000 Oe;The solid line represents the best fit obtained with the model in Fig.3
For compound 1 and 2,to simplify the simulation model,the double zigzag chain structure could be treated as a 1D chain model(see Fig.3)and the Hamiltonian can be described as the following:H=-2J (S1S2+S1S3+S2S4+S3S4+S4S5+S4S6+S5S7+S6S7+S7S8+S7S9+ S8S10+S9S10+S10S11+S10S12+S11S1+S12S1),where S1=S4=S7= S10=5/2,S2=S3=S5=S6=S8=S9=S11=S12=1/2,and J is the coupling parameter between neighboring FeⅢand MnⅡ. An excellent agreement with the experimental data is obtained by calculating the susceptibility data with the MAGPACK package[21],with the following set of parameters:J=-7.64 cm-1,g=gFe=gMn=2.09 with R= 1.99×10-4{R=∑[(χMT)obs-(χMT)cal]2/∑(χMT)obs2}for 1, and J=-3.78 cm-1,g=gFe=gMn=2.04 with R=2.16×10-5for 2,respectively.As expected on the basis of their structural similarity,the magnetic behaviors of the two complexesare essentially identical.The above behaviors were consistent with an intramolecular FeⅢ-MnⅡantiferromagnetic interaction,which can be rationalized in terms of the magnetic orbitals ofthe low-spin FeⅢand high-spin MnⅡcenters.An arrangement that normally gives rise to antiferromagnetic coupling.However, because of the enhancement of the steric effect of the tridentate ligand in[LM(CN)3]-building blocks,the coupling constant J in compound 1 is over twice as large as in 2.Therefore,the tridentate ligand in [LM(CN)3]-building blocks plays a role in constructing molecule magnets,which not only greatly affects the structure of the resulting magnetic assemblies,but also induces a diverse hindrance effecting the different magnetic interactions.Because the steric hindrance of pzTp in 2 is larger than Tp in 1,the bond lengths of Mn-NCNin 2 are larger than that in 1.Such difference results in that the antiferromagnetic interaction between FeⅢ-MnⅡions in 1 is larger than that in 2.
The field dependence of the magnetization(0~50 kOe)measured at 1.8 K is shown in Fig.4.The magnetization increases almost linearly with the applied magnetic field,reaching a value of 3.01Nβfor 1 and 3.38Nβfor 2 at 50 kOe.This behavior is consistent with the antiferromagnetic behavior of 1 and 2.

Fig.3 Representation of the magnetic exchange pathways between neighboring metal ions in 1 and 2
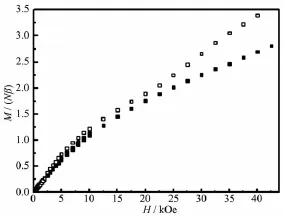
Fig.4 Field dependence of the magnetization at 1.8 K for complexes 1((Ⅱ))and 2(□)
3 Conclusions
In summary,two isostructural cyano-bridged {FeⅢ2MnⅡ}2D layers were synthesized.The interchain linkage and apical directions of MnⅡcould be fixed via the rigid ditopic ligand bib.The antiferromagnetic interaction between FeⅢand MnⅡions could be tuned by changing the capping ligand of FeⅢions with different steric hindrances.Such results provide a strategy to synthesize 1D magnetic systems with tunable intrachain magnetic interactions.
References:
[1](a)Caneschi A,Gatteschi D,Lalioti N,et al.Angew.Chem. Int.Ed.,2001,40:1760-1763
(b)Miyasaka H,Julve M,Yamashita M,et al.Inorg.Chem., 2009,48:3420-3437
[2](a)Zhang X M,Wang Y Q,Wang K,et al.Chem.Commun., 2011,47:1815-1817
(b)Miyasaka H,Madanbashi T,Sugimoto K,et al.Chem. Eur.J.,2006,12:7028
(c)Bai Y L,Tao J,Wernsdorfer W,et al.J.Am.Chem.Soc., 2006,128:16428-16429
[3](a)Coronado E,Mascarós J R G and Gastaldo C M.J.Am. Chem.Soc.,2008,130:14987-14989
(b)Zhang Y Z,Tong M L,Chen X M,et al.Angew.Chem. Int.Ed.,2006,45:6310-6314
(c)Venkatakrishnan T S,Sahoo S,Bréfuel N,et al.J.Am. Chem.Soc.,2010,132:6047-6057
(d)Coulon C,Clérac R,Wernsdorfer W,etal.Phys.Rev.Lett., 2009,102:167204-167207
[4](a)Lescouzec R,Vaissermann J,Ruiz-Prez C,et al.Angew. Chem.,2003,115:1521-1524;Angew.Chem.Int.Ed.,2003, 42:1483-1486
(b)Toma L M,Lescouzec R,Lloret F,et al.Chem.Commun., 2003,24:1850-1851
where r is the radial mode number, nr=n1/n2, n1 and n2 are the refractive indices of microcavity and ambient materials, respectively, η(r) is the Airy function solution and equals 2.338 if r=1, L is the polarization characteristic coefficient and L=1/nr for TM modes and L=nr for TE modes.
(c)Wang S,Zuo J L,Gao S,et al.J.Am.Chem.Soc.,2004, 126:8900-8901
(d)Toma L M,Lescouzec R,Lloret F,et al.J.Am.Chem. Soc.,2006,128:4842-4853
[5](a)Ferbinteanu M,Miyasaka H,Wernsdorfer W,et al.J.Am. Chem.Soc.,2005,127:3090-3099
(b)Lescouzec R,Toma M L,Vaissermann J,etal.Coord.Chem. Rev.,2005,249:269-27291
(c)Kou H Z,Ni Z H,Liu C M,et al.New J.Chem.,2009,33: 2296-2299
[6](a)Rinehart J D,Long J R.Chem.Sci.,2011,2:2078-2085
(b)Sun H L,Wang Z M,Gao S.Coord.Chem.Rev.,2010, 254:1081-1101
(c)Aubin S M J,Sun Z,Pardi L,et al.Inorg.Chem.,1999, 38:5329-5340
(b)Zuo J L,Bartlett B M,You X Z,et al.J.Am.Chem.Soc., 2006,128:7162-7163
(c)Kostakisa G E,Perlepesb S P,Blatovc V A,et al.Acc. Chem.Res.,2005,38:325-334
(d)Berseth P A,Sokol J J,Shores M P,et al.J.Am.Chem. Soc.,2000,122:9655-9662
[8]Kostakisa G E,Perlepesb S P,Blatovc V A,et al.Acc.Chem. Res.,2005,38:325-334
[9]Berseth P A,Sokol J J,Shores M P,et al.J.Am.Chem.Soc., 2000,122:9655-9622
[10]Wen H R,Zuo J L,et al.Inorg.Chem.,2006,45:582-590
[11](a)Liu T,Dong D P,Kanegawa S,et al.Angew.Chem.Int. Ed.,2012,51:4367-4370
(b)Wang S,Zuo J L,Zhou H C,et al.Angew.Chem.Int. Ed.,2004,43:594-597
(c)Dong D P,Liu T,Zheng H,et al.Inorg.Chem.Comm., 2012,24:153-156
[12]Zhang Y Z,Wang B W,Sato O,et al.Chem.Commun., 2010,46:6959-6961
[13]Song X J,Muddassir M,Chen Y,et al.Dalton Trans.,2013, 42:1116-1121
[14]Bogani L,Vindigni A,Sessoli R,et al.J.Mater.Chem., 2008,18:4750-4758
[15](a)Harris T D,Bennett M V,Clérac R,et al.J.Am.Chem. Soc.,2010,132:3980-3988
(b)Bernot K,Bogani L,Caneschi A,et al.J.Am.Chem.Soc., 2006,128:7947-7956
(c)Clérac R,Miyasaka H,Yamashita M,et al.J.Am.Chem. Soc.,2002,124:12837-12844
[16](a)Liu T,Zheng H,Kang S,et al.Nat.Commun.,2013,4: 2826-2833
(b)Liu T,Zhang Y J,Kanegawa S,et al.Angew.Chem.Int. Ed.,2010,49:8645-8648
(c)Liu T,Zhang Y J,Kanegawa S,et al.J.Am.Chem.Soc., 2010,132:8250-8251
[17]Ohkoshi S,Tokoro H,Utsunomiya M,et al.J.Phys.Chem. B,2002,106:2423-2425
[18]Lescouëzec R,Vaissermann J,Lloret F,et al.Inorg.Chem., 2002,41:5943-5945
[19]LI Dong-Feng(李东峰),Clérac R,Parkin S,et al.Inorg. Chem.,2006,45:5251-5253
[20]Vlahakis J Z,Mitu S,Roman G,etal.Bioorganic&Medicinal Chemistry,2011,19:6525-6545
[21]DONG Da-Peng(董大鹏),LIU Tao(刘涛),ZHENG Hui(郑慧),et al.Scince China B:Chem.(中国科学:化学),2012,55 (6):1018-1021
[22]CHEN Man-Sheng(陈满生),LUO Li(罗莉),SUN Wei-Yin (孙为银),et al.Chinese J.Inorg.Chem.(无机化学学报), 2010,16(12):2227-2232
Tuning Intrachain Magnetic Interactions of Cyanide-bridged FeⅢMnⅡChain
ZHENG Hui XU Yang DUAN Chun-Ying*
(State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian,116024,China)
Two cyanide bridged FeⅢ-MnⅡdouble zigzag chains{[Fe(Tp)(CN)3]2[Mn(bib)]}·CH3OH·2H2O(1)and {[Fe(pzTp)(CN)3]2[Mn(bib)]}·3H2O(2)were synthesized utilizing tricyanometallate building blocks.The chains were further linked via long ditopic ligand to form two dimensional compounds.With the alignment of chains fixed by the rigid ditopic linker,the intrachain magnetic interactions between FeⅢand MnⅡcould be further tuned via changing the capping ligand of FeⅢions.CCDC:1001946,1;1001947,2.
cyanide;zigzag chain;ditopic linker;magnetic interactions
0614.7
A
1001-4861(2015)07-1460-07
10.11862/CJIC.2015.177
2015-04-07。收修改稿日期:2015-05-08。
国家自然科学基金(No.91122031)资助项目。
*通讯联系人。E-mail:cyduan@dlut.edu.cn;会员登记号:S06N6732S1406。
