植物多糖和吡格列酮对小鼠肺腺癌干预效果的研究
2015-11-24邓增华谢辉陈振岗王广舜张成岗
邓增华,谢辉,陈振岗,王广舜△,张成岗
植物多糖和吡格列酮对小鼠肺腺癌干预效果的研究
邓增华1,谢辉1,陈振岗1,王广舜1△,张成岗2△
目的观察植物多糖和吡格列酮对小鼠肺腺癌的干预效果,探讨炎症和肺腺癌之间的关系,为临床肺腺癌的治疗提供理论基础。方法100只小鼠分为对照组、模型组、植物多糖组、吡格列酮组、植物多糖和吡格列酮联合干预组(联合组),每组20只;植物多糖组给予植物多糖溶液500 mg/kg,吡格列酮组给予吡格列酮溶液15 mg/kg,联合组给予500 mg/kg植物多糖+15 mg/kg吡格列酮;对照组和模型组给予等量生理盐水(10 mL/kg),均1次/d,5 d/周;共20周。观察各组不同时间小鼠肺腺癌成瘤情况,分别于第12周和20周,处死小鼠后检测各组核因子(NF)-κB、肿瘤坏死因子(TNF)-α、白细胞介素(IL)-1β和IL-6含量。结果对照组小鼠体质量平稳上升,其余组小鼠在乌拉坦注射期体质量持续下降,然后持续上升;第20周,对照组小鼠肺表面未见结节,其余组均见明显的肺结节,且小鼠肺的脏器指数明显高于对照组;在第12周和20周时,模型、吡格列酮、植物多糖和联合组小鼠体内的NF-κB、TNF-α、IL-1β和IL-6含量均高于对照组,吡格列酮、植物多糖和联合组小鼠体内的NF-κB、TNF-α、IL-1β和IL-6含量均低于模型组。结论持续的炎症反应是肺腺癌发生发展的危险因素之一,植物多糖和吡格列酮均能降低肺腺癌小鼠体内的炎症水平,提示可将其用于临床肺腺癌的药物辅助治疗。
植物多糖;吡格列酮;腺癌;肺肿瘤;NF-κB;肿瘤坏死因子α;白细胞介素1β;白细胞介素6
肺癌是对人体健康和生命威胁最大的肿瘤之一[1]。其在病理上分为小细胞肺癌(small cell lung cancer,SCLC)和非小细胞肺癌(non-small cell lung cancer,NSCLC),NSCLC又主要分为大细胞肺癌、肺鳞癌和肺腺癌,其中肺腺癌已成为肺癌中最主要的类型[2]。核因子(NF)-κB家族在调节炎症、免疫反应以及细胞增殖中均起重要的作用[3-4],其与肺腺癌之间的关系一直受到人们的关注[5-6]。相关研究表明NF-κB促进肺腺癌的发生与其增强肿瘤相关的炎症反应,促进血管生成以及肿瘤转移有关[7]。
植物多糖是从植物中提取的天然化合物,具有广泛的生理生化作用,已有研究显示多种植物多糖均有抗肿瘤的作用[8-10]。吡格列酮属于噻唑烷二酮类降糖药,是过氧化物酶体增殖物激活型受体(PPAR)-γ激动剂,研究证明PPAR-γ激动剂对肺腺癌有抑制作用[11-12]。且植物多糖[10]和PPAR-γ激动剂[11]均能抑制NF-κB信号通路,从而控制炎症。本研究使用植物多糖和吡格列酮干预小鼠肺腺癌模型,通过检测小鼠肺部成瘤情况和体内炎症水平,探讨炎症与肺腺癌的关系。
1 材料与方法
1.1 材料乌拉坦(国药集团化学试剂有限公司,化学纯);植物多糖(苏州科景生物医药科技有限公司,配料为膳食纤维、虫草多糖、人参多糖、灵芝多糖、海藻多糖、猴头菇多糖、枸杞多糖);盐酸吡格列酮(北京太平洋药业有限公司);小鼠ELISA试剂盒(美国Rapidbio公司,CK-E02226、CKE02538M、CK-E20012M、CK-E20073M);10%多聚甲醛(实验室自制)。Bio-Rad公司680型酶标仪和光学显微镜(OLYMPUS BX41)。SPF级雄性Balb/c小鼠100只(北京维通利华实验动物技术有限公司),体质量18~22 g。
1.2 方法
1.2.1 模型建立及实验分组参照文献中常用的方法建立小鼠肺腺癌模型,即腹腔注射乌拉坦(1 000 mg/kg,1次/周,连续8周)[13-15]。小鼠实验室适应性喂养2周后,按体质量随机分为5组:对照组、模型组、植物多糖组、吡格列酮组、植物多糖和吡格列酮联合干预组(联合组),每组20只。前8周,后4组均腹腔注射乌拉坦1 g/kg,对照组注射等量生理盐水。从第4周开始,植物多糖组给予植物多糖溶液500 mg/kg,吡格列酮组给予吡格列酮溶液15 mg/kg,植物多糖和吡格列酮所用剂量均参照相关文献[8,16],联合组给予植物多糖和吡格列酮混合液(500 mg/kg植物多糖+15 mg/kg吡格列酮);对照组和模型组给予等量生理盐水(10 mL/kg),均1次/d,5 d/周。整个干预方案一直到实验结束(共20周,从第1次注射乌拉坦始算)。
1.2.2 各组小鼠肺部成瘤情况及炎症因子测定每周一记录小鼠体质量,并观看其生长状态;第12周时,每组处死6只小鼠,心尖取血分离血清(5 000 r/min,10 min),检测各组NF-κB、肿瘤坏死因子(TNF)-α、白细胞介素(IL)-1β和IL-6的含量,具体步骤参照试剂盒说明书。取肺组织并计算肺的脏器指数=肺的质量/小鼠体质量,然后将其固定于10%多聚甲醛溶液中,以备做石蜡包块、病理切片(5 μm)和HE染色;第20周时处死剩余的小鼠,同第12周进行HE染色和病理分析。
1.3 统计学方法应用SPSS 17.0统计软件进行数据处理,应用GraphPad Prism 5软件绘制图表,采用方差分析进行统计学检验,组间多重比较用LSD-t检验。以P<0.05为差异有统计学意义。
2 结果
2.1 小鼠生长情况对照组小鼠体质量在整个实验周期基本保持平稳上升,生长状况良好。其余组小鼠在乌拉坦注射期(前8周)体质量持续下降,到第9周时降到最低,在第9周以后体质量开始持续上升,到20周时各组间小鼠体质量基本一致,见图1。

Fig.1The changes of body weights in different mouse groups图1 各组小鼠体质量变化
2.2 各组小鼠肺部成瘤情况至第20周,对照组小鼠肺表面未见结节,其余组均见明显的肺结节,模型组、吡格列酮组、植物多糖组和联合组间的肺表面结节数差异无统计学意义(F=2.816,P=0.055),见图2。对照组小鼠肺的脏器指数明显小于其他4组(F= 12.680,P<0.05),而模型组、植物多糖组、吡格列酮组和联合组间差异无统计学意义,见图3。各组间小鼠肺部病理改变见图4,其中对照组小鼠全部正常,其余组的每只小鼠均出现肺腺癌。

Fig.2The number of lung nodules at the 20thweek in different groups图2 第20周时各组间小鼠肺表面结节数
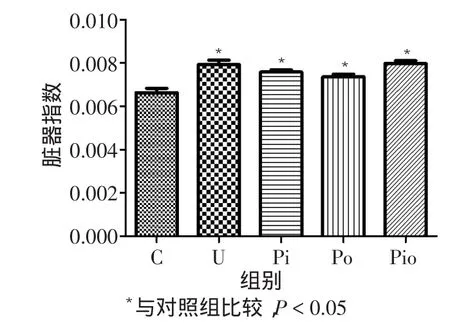
Fig.3The index of lung in different groups图3 各组小鼠肺的脏器指数

Fig.4The pathological changes of lung tissues in different groups(HE,×100)图4 各组小鼠肺组织病理改变(HE,×100)
2.3 各组小鼠炎症因子表达情况在第12周和20周时,模型组、吡格列酮、植物多糖和联合组小鼠体内的NF-κB、TNF-α、IL-1β和IL-6含量均高于对照组,吡格列酮、植物多糖和联合组小鼠体内的NF-κB、TNF-α、IL-1β和IL-6含量均低于模型组,见表1、2。
Tab.1The expression levels of inflammation factors in different groups at the 12thweek表1 各组小鼠12周炎症因子表达水平比较(n=6,ng/L,)
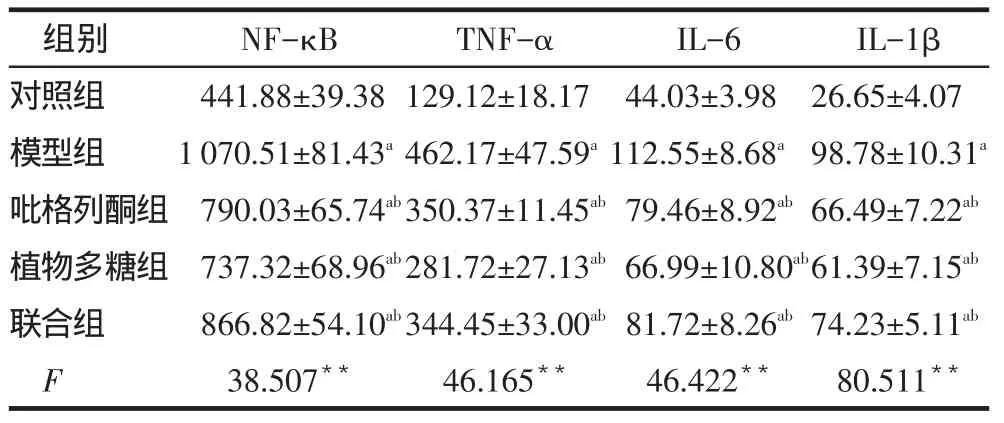
Tab.1The expression levels of inflammation factors in different groups at the 12thweek表1 各组小鼠12周炎症因子表达水平比较(n=6,ng/L,)
**P<0.01;a与对照组比较,b与模型组比较,P<0.05;表2同
I L -1 β 2 6 . 6 5 ± 4 . 0 7 9 8 . 7 8 ± 1 0 . 3 1 a 6 6 . 4 9 ± 7 . 2 2 a b 6 1 . 3 9 ± 7 . 1 5 a b 7 4 . 2 3 ± 5 . 1 1 a b 8 0 . 5 1 1**组别对照组模型组吡格列酮组植物多糖组联合组F N F -κ B 4 4 1 . 8 8 ± 3 9 . 3 8 1 0 7 0 . 5 1 ± 8 1 . 4 3 a 7 9 0 . 0 3 ± 6 5 . 7 4 a b 7 3 7 . 3 2 ± 6 8 . 9 6 a b 8 6 6 . 8 2 ± 5 4 . 1 0 a b 3 8 . 5 0 7**T N F -α 1 2 9 . 1 2 ± 1 8 . 1 7 4 6 2 . 1 7 ± 4 7 . 5 9 a 3 5 0 . 3 7 ± 1 1 . 4 5 a b 2 8 1 . 7 2 ± 2 7 . 1 3 a b 3 4 4 . 4 5 ± 3 3 . 0 0 a b 4 6 . 1 6 5**I L -6 4 4 . 0 3 ± 3 . 9 8 1 1 2 . 5 5 ± 8 . 6 8 a 7 9 . 4 6 ± 8 . 9 2 a b 6 6 . 9 9 ± 1 0 . 8 0 a b 8 1 . 7 2 ± 8 . 2 6 a b 4 6 . 4 2 2**
Tab.2The expression levels of inflammation factors in different groups at the 20thweek表2 各组小鼠20周炎症因子表达水平比较(n=14,ng/L,)

Tab.2The expression levels of inflammation factors in different groups at the 20thweek表2 各组小鼠20周炎症因子表达水平比较(n=14,ng/L,)
组别对照组模型组吡格列酮组植物多糖组联合组F N F -κ B 4 8 2 . 7 5 ± 4 6 . 3 8 1 1 2 9 . 0 7 ± 9 0 . 3 0a8 2 6 . 5 7 ± 7 2 . 4 9ab8 3 7 . 7 1 ± 7 0 . 4 9ab9 7 3 . 0 2 ± 8 8 . 8 3ab1 1 7 . 7 1 4**T N F -α 1 7 3 . 7 3 ± 1 6 . 8 2 4 8 3 . 9 1 ± 4 3 . 6 8a3 4 4 . 2 2 ± 3 1 . 7 2ab3 4 9 . 7 6 ± 3 6 . 2 7ab4 0 6 . 8 1 ± 3 4 . 5 2ab1 2 1 . 7 2 2**I L -6 4 0 . 4 9 ± 4 . 8 9 1 0 6 . 4 2 ± 9 . 3 3a7 9 . 1 3 ± 6 . 3 3ab7 9 . 1 9 ± 5 . 8 4ab9 4 . 2 3 ± 9 . 0 4ab1 3 5 . 6 3 5**I L -1 β 2 7 . 7 5 ± 6 . 3 5 9 4 . 7 0 ± 9 . 9 9a7 0 . 4 4 ± 7 . 2 1ab6 6 . 2 5 ± 7 . 2 4ab8 1 . 7 4 ± 8 . 5 9ab1 2 7 . 4 9 6**
3 讨论
乌拉坦的化学名为氨基甲酸乙酯,是烟草和吸烟烟雾中致癌成分之一,并且广泛存在于发酵食品中[17],并在2007年被国际癌症研究机构认定为2A类致癌物(可能对人体致癌)。乌拉坦作为一种胆碱酯酶抑制剂,由于其稳定的麻醉效果,现仍广泛应用于动物麻醉。本次实验结果证实乌拉坦能稳定地诱导出小鼠肺腺癌,提醒人们应该重视其对人体的潜在巨大危害。
NF-κB是一种核转录因子,主要存在于细胞核中,是炎症通路中关键因子之一,能调控多种炎症介质的表达。已有研究显示其与肺腺癌的发生发展密切相关[3,15,18],本次实验再次证实乌拉坦诱导的小鼠肺腺癌中NF-κB和其他炎症因子一直处于高表达状态,说明慢性炎症反应是肺腺癌形成的机制之一。相关研究表明,慢性阻塞性肺疾病小鼠模型患肺癌的概率增大与其体内持续高水平的炎症状态有关[19],与本研究结果相符。
植物多糖是从植物中提取的天然化合物,具有广泛的生理生化作用,且对人体基本没有毒副作用,研究表明许多种植物多糖(如纤维素、枸杞多糖、人
参多糖)均具有抗肿瘤的作用[9,20]。但是以往关于植物多糖和肺腺癌的研究均停留在细胞实验或者种植瘤实验的水平,尚鲜见用植物多糖干预乌拉坦诱导小鼠肺腺癌模型的报道。吡格列酮属于噻唑烷二酮类降糖药,是PPAR-γ激动剂,相关研究显示PPAR-γ信号通路和NF-κB信号通路存在交叉部分,PPAR-γ激动剂能抑制NF-κB的表达[21-22]。但是PPAR-γ激动剂能否通过抑制NF-κB控制炎症从而达到抑制肺腺癌,尚鲜见文献报道。本实验结果显示,植物多糖和吡格列酮均能降低肺腺癌小鼠体内的炎症水平,但是药物干预组(植物多糖、吡格列酮和联合组)小鼠体内的炎症水平仍然高于对照组,这很可能是两种药物未能进一步抑制肺腺癌的主要原因。能否将这两种药作为临床治疗肺腺癌的辅助用药,尚需进一步实验研究。
[1]DeSantis CE,Lin CC,Mariotto AB,et al.Cancer treatment and survivorship statistics,2014[J].CA Cancer J Clin,2014,64(4):252-271. doi:10.3322/caac.21235.
[2]Jemal A,Bray F,Center MM,et al.Global cancer statistics,2011[J]. CA Cancer J Chin,2011,61(2):69-90.doi:10.3322/caac.20107.
[3]Hopewell EL,Zhao W,Fulp WJ,et al.Lung tumor NF-kappaB signaling promotes T cell-mediated immune surveillance,2013[J].J Chin Invest,2013,123(6):2509-2522.doi:10.1172/JCI67250.
[4]Hayden MS,Ghosh S.Signaling to NF-kappaB,2004[J].Genes Dev,2004,18(18):2195-2224.
[5]Basseres DS,Baldwin AS.Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression,2006[J].Oncogene,2006,25(51):6817-6830.
[6]Meylan E,Dooley AL,Feldser DM,et al.Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma,2009[J]. Nature,2009,462(7269):104-107.doi:10.1038/nature08462.
[7]Hanahan D,Coussens LM.Accessories to the crime:functions of cells recruited to the tumor microenvironment,2012[J].Cancer cell,2012,21(3):309-322.doi:10.1016/j.ccr.
[8]Lee H,Kim JS,Kim E.Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3KAkt-mTOR pathways,2012[J].PLoS One,2012,7(11):e50624.doi: 10.1371/journal.pone.0050624.
[9]Li C,Cai J,Geng J,et al.Purification,characterization and anticancer activity of a polysaccharide from Panax ginseng,2012[J].Int J Bio Macromol,2012,51(5):968-973.doi:10.1016/j.ijbiomac.
[10]Peng Q,Liu H,Shi S,et al.Lycium ruthenicum polysaccharide attenuates inflammation through inhibiting TLR4/NF-kappaB signaling pathway,2014[J].Int J Bio Macromol,2014,67:330-335.doi: 10.1016/j.ijbiomac.2014.03.023.
[11]Lyon CM,Klinge DM,Do KC,et al.Rosiglitazone prevents the progression of preinvasive lung cancer in a murine model,2009[J].Carcinogenesis,2009,30(12):2095-2099.doi:10.1093/carcin/bgp260.
[12]Keshamouni VG,Arenberg DA,Reddy RC,et al.PPAR-gamma activation inhibits angiogenesis by blocking ELR+CXC chemokine production in non-small cell lung cancer,2005[J].Neoplasia,2005,7(3):294-301.
[13]Busch SE,Moser RD,Gurley KE,et al.ARF inhibits the growth and malignant progression of non-small-cell lung carcinoma,2014[J].Oncogene,2014,33(20):2665-2673.doi:10.1038/onc.2013.2 08.
[14]Balli D,Zhang Y,Snyder J,et al.Endothelial cell-specific deletion of transcription factor FoxM1 increases urethane-induced lung carcinogenesis,2011[J].Cancer Res,2011,71(1):40-50.doi:10.1158/ 0008-5472.
[15]Narayan C,Kumar A.Constitutive over expression of IL-1beta,IL-6,NF-kappaB,and Stat3 is a potential cause of lung tumorgenesis in urethane(ethyl carbamate)induced Balb/c mice,2012[J].J Carcinog,2012,11:9.doi:10.4103/1477-3163.98965.
[16]Wang Y,James M,Wen W,et al.Chemopreventive effects of pioglitazone on chemically induced lung carcinogenesis in mice,2010[J]. Mol Cancer Ther,2010,9(11):3074-3082.doi:10.1158/1535-7163. MCT-10-0510.
[17]Ough CS.Ethylcarbamate in fermented beverages and foods.I.Naturally occurring ethylcarbamate,1976[J].J Aqir Food Chem,1976,24(2):323-328.
[18]Zaynagetdinov R,Stathopoulos GT,Sherrill TP,et al.Epithelial nuclear factor-kappa B signaling promotes lung carcinogenesis via recruitment of regulatory T lymphocytes,2012[J].Oncogene,2012,31(26):3164-3176.doi:10.1038/onc.2011.480.
[19]Moghaddam SJ,Li H,Cho SN,et al.Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model,2009[J].Am J Respir Cell Mol Biol,2009,40(4):443-453.doi:10.1165/rcmb.2008-0198OC.
[20]Ji NF,Yao LS,Li Y,et al.Polysaccharide of Cordyceps sinensis enhances cisplatin cytotoxicity in non-small cell lung cancer H157 cell line,2011[J].Integr Cancer Ther,2011,10(4):359-367.doi: 10.1177/1534735410392573.
[21]Castrillo A,Diaz-Guerra MJ,Hortelano S,et al.Inhibition of kappa B kinase and kappa B phosphorylation by 15-deoxy-Delta(12,14)-prostaglandin J(2)in activated murine macrophages,2000[J].Mol Cell Biol,2000,20(5):1692-1698.
[22]Wu JP,Guo ZX.Effects of telmisartan on the expression of adiponectin receptor 1 in cardiomyocytes and its mechanism[J].Med J Chin PLA,2015,40(1):30-34.[吴杰萍,郭志新.替米沙坦对心肌细胞脂联素受体1表达的影响及其可能机制研究[J].解放军医学杂志,2015,40(1):30-34].doi:10.11855/j.issn.0577-7402.2015.01.07.
(2015-05-20收稿 2015-06-20修回)
(本文编辑 闫娟)
The effects of polysaccharides and pioglitazone on mouse model of pulmonary adenocarcinoma
DENG Zenghua1,XIE Hui1,CHEN Zhengang1,WANG Guangshun1△,ZHANG Chenggang2△
1 Baodi Clinical Hospital,Tianjin Medical University,Tianjin 301800,China;2 Beijing Institute of Radiation Medicine,State Key Laboratory of Proteomics,Cognitive and Mental Health Research Center of the PLA△
ObjectiveTo provide theoretical reference for clinical therapy of pulmonary adenocarcinoma by evaluating the effects of polysaccharides and pioglitazone on mouse model of pulmonary adenocarcinoma and to explore the relationship between inflammation and pulmonary adenocarcinoma.MethodsOne hundred mice were averagely divided into five groups,including control group,model group,polysaccharides group,pioglitazone group,polysaccharides and pioglitazone group(unite group).Polysaccharides solution(500 mg/kg)was given to polysaccharides group,pioglitazone solution(15 mg/kg)was given to pioglitazone group,polysaccharides solution(500 mg/kg)and pioglitazone solution(15 mg/kg)were given to unite group;and the equal volume of saline(10 mL/kg)was given to control and model group(1 t/d,5 d/w,continuously 20 w).The pulmonary adenocarcinoma induced by urethane was evaluated in each group at different time points.The levels of NF-κB,TNF-α,IL-1β and IL-6 were measured in each group at the 12thweek and the 20thweek respectively.Results The body weights were increased in the control group,which were decreased in other groups during urethane-injection,but increased continuously after the injection.At the 20thweek,nodules were found in lung surfaces in all mice except mice of control group.The lung index was higher in all mice except mice of control group.The levels of NF-κB,TNF-α,IL-1β and IL-6 were significantly higher at 12thweek and 20thin model group,polysaccharides group,pioglitazone group,polysaccharides and pioglitazone group than those of control group.The levels of NF-κB,TNF-α,IL-1β and IL-6 were significantly lower in polysaccharides group,pioglitazone group,polysaccharides and pioglitazone group than those of model group.ConclusionSustained inflammatory response is one of the risk factors for the development of lung adenocarcinoma.Polysaccharides and pioglitazone can reduce the level of inflammation in mouse lung adenocarcinoma,suggesting that both of them can be used as potential adjuvant in the clinical treatment of lung adenocarcinoma.
polysaccharides;pioglitazone;adenocarcinoma;lung neoplasms;NF-kappa B;tumor necrosis factor-alpha;interleukin-1β;interleukin-6
R734.2
A
10.11958/j.issn.0253-9896.2015.12.011
国家自然科学基金资助项目(81271206)
1天津医科大学宝坻临床学院(邮编301800);2军事医学科学院放射与辐射医学研究所,蛋白质组学国家重点实验室,全军军事认知与心理卫生研究中心,北京
邓增华(1990),男,硕士在读,主要从事肺腺癌发病机制和药物干预研究
△通讯作者E-mail:wgs@bdhospital.com;zhangcg@bmi.ac.cn
