Reduced grain chalkiness and its possible physiological mechanism in transgenic rice overexpressingL-GalLDH
2015-11-12LeYuYonghiLiuJinhuTongJunhuiDingRuozhongWngChnglinPengLngtoXio
Le Yu,Yonghi Liu,*,Jinhu Tong,Junhui Ding,Ruozhong Wng,Chnglin Peng,Lngto Xio
aCollege of Life Sciences,Zhaoqing University,Zhaoqing 526061,China
bHunan Provincial Key Laboratory of Phytohormones and Growth Development,Hunan Agricultural University,Changsha 410128,ChinacCollege of Life Sciences,South China Normal University,Guangzhou 510631,China
Reduced grain chalkiness and its possible physiological mechanism in transgenic rice overexpressingL-GalLDH
Le Yua,Yonghai Liua,*,Jianhua Tongb,Junhui Dingb,Ruozhong Wangb,Changlian Pengc,Langtao Xiaob
aCollege of Life Sciences,Zhaoqing University,Zhaoqing 526061,China
bHunan Provincial Key Laboratory of Phytohormones and Growth Development,Hunan Agricultural University,Changsha 410128,ChinacCollege of Life Sciences,South China Normal University,Guangzhou 510631,China
A R T I C L E I N F O
Article history:
24 December 2014
Accepted 16 February 2015
Available online 23 February 2015
Grain chalkiness
Ascorbic acid
Abscisic acid
Jasmonic acid
Rice
Chalkiness is one of the key factors determining rice quality and price.Ascorbic acid(Asc)is a
major plant antioxidant that performs many functions in plants.L-Galactono-1,4-lactone dehydrogenase(L-GalLDH,EC1.3.2.3)is an enzyme that catalyzes the final step of Asc biosynthesis in plants.Here we show that theL-GalLDH-overexpressing transgenic rice,GO-2,
which has constitutively higher leaf Asc content than wild-type(WT)plants,exhibits significantly reduced grain chalkiness.Higher foliar ascorbate/dehydroascorbate(Asc/DHA)ratios at 40,60,80,and 100 days of plant age were observed in GO-2.Further investigation showed that the enhanced level of Asc resulted in a significantly higher ribulose-1,5-bisphosphate(RuBP)carboxylase/oxygenase(Rubisco)protein level in GO-2 at 80 days.In addition,levels of abscisic acid(ABA)and jasmonic acid(JA)were lower in GO-2 at 60,80,and 100 days.The results we present here indicate that the enhanced level of Asc is likely responsibleforchangingredoxhomeostasisinkeydevelopmentalstagesassociatedwithgrain filling and alters grain chalkiness in theL-GalLDH-overexpressing transgenic by maintaining photosynthetic function and affecting phytohormones associated with grain filling.
©2015 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Ascorbic acid(Asc)is considered an excellent water-soluble antioxidant in plants and animals.It is widely associated with photosynthetic function and stress tolerance in plants and must be obtained regularly from dietary sources by animals,which cannot synthesize it[1].The importance of endogenous Asc in plants has been proposed to be its function as a cofactor for many enzymes and as a regulator of cell division and growth,as well as in signal transduction[2].Asc has also been proposed to regulate the development of senescence[3,4],defend against pathogens,and control flowering time in plants[5-8].Recently,it has been reported that Asc appears to be involved in a complex phytohormone-mediated signaling network that ties together ozone and pathogen responses and influencestheonset of senescence[9].Given the importance of Asc to plant and animal health,much research has focused on developing strategies to increase Asc contentin plants to improve their nutritional value and stress responses[10,11].
Although four Asc biosynthetic pathways in higher plants have been proposed[12-15],the Smirnoff-Wheeler pathway has been shown to be the primary pathway in most plant organs[1].In the Smirnoff-Wheeler pathway,the enzyme L-GalLDH (EC1.3.2.3)catalyzestheultimate stepof Asc biosynthesis[16].L-GalLDH is attached to complex I of the mitochondrial electron transport chain,which represents a major fragment of the“membrane arm”of complex I[17]and usesL-galactono-1,4-lactone(L-GalL)as an electron donor to reduce cytochrome c between complexes III and IV,while L-GalL is converted into Asc[16].
The role ofL-GalLDH in the control of cell,organ and plant growth has been studied using mainly antisense or RNA interference(RNAi)approaches,and a deficiency of Asc and L-GalLDH has been observed to affect not only the division and growth of tobacco BY-2(Nicotiana tabacum cv.Bright Yellow 2)cell culture[18]but also the growth and development of tomato(Solanum lycopersicum)[19]and Arabidopsis[20].Recently,we reported the development by RNAi of homozygous L-GalLDH-suppressed transgenic rice plants with approximately 20%to 50%of the Asc content of wild-type(WT)plants[21].Further study showed thatL-GalLDH-suppressed rice plants displayed reduced growth rate,seed set,and tiller number[22,23].Several studies have been performed to investigate whether the overexpression ofL-GalLDH would affect the accumulation of Asc.For example,it has been reported that overexpression ofL-GalLDH leads to an increase of Asc content in tobacco suspension cells[24].In another study,a cDNA encodingL-GalLDH from sweet potato was introduced into tobacco plants and an increase ofL-GalLDH activity was observed in the transgenic plants[25].Our recent results showed that overexpression of authenticL-GalLDH resulted in a substantial increase of foliar Asc in rice;moreover,L-GalLDH-overexpressing rice plants displayed increased seed set compared with the WT[22].
Seed set is an important agronomic trait that determines rice yield,while chalkiness is a major concern in rice breeding because it is one of the key factors determining quality and price[26].Chalky kernels have a lower density of starch granules than do vitreous ones,and are accordingly more prone to breakage during milling[27].Many studies have shown that multiple factors contribute to the formation of kernel chalkiness,including starch synthesis and starch granule structure and arrangement[28-30].The rice endosperm in the storage phase undergoes a series of coordinated cellular and metabolic events,including starchy endosperm cell death,starch synthesis,and starch granule packaging followed by an endogenous H2O2burst 15 days after flowering(DAF)[31].Liu et al.observed high grain chalkiness characteristics in a near-isogenic line,CSSL50-1,and suggested that reactive oxygen species(ROS)play a critical role in regulating rice endosperm chalkiness[32].However,grain chalkiness is a complex quantitative genetic trait and the molecular mechanisms underlying its formation are still poorly understood[32].In this study,we investigated how a change in Asc content with overexpression ofL-GalLDH in rice plants leads to reduced grain chalkiness.The consequences of an increase in Asc(by use of homozygousL-GalLDH-overexpressing transgenic rice,GO-2,which has constitutively higher leaf Asc content)to grain chalkiness,lipid peroxidation,photosynthetic function,phytohormone changes,and their interactions were compared between GO-2 and the WT.The results suggested that Asc plays a role in grain chalkiness through maintaining photosynthetic function and affecting phytohormones associated with grain filling.
2.Materials and methods
2.1.Plant materials
Oryza sativa L.cv.Zhonghua 11(WT)and the previously described homozygousL-GalLDH-overexpressing transgenic rice plant(GO-2)[22]were used as experimental plants.GO-2 has constitutively higher(about 1.4-fold)leaf Asc content than the WT plant.
2.2.Growth conditions and treatments
Germinated seeds of WT and GO-2 were pre-grown with complete Kimura B nutrient solution in a greenhouse for 30 days.The seedlings were grown until the emergence of the fourth leaf blade.They were then taken out gently and transferred to earthen pots 30 cm in diameter and 32 cm in depth filled with 6.0 kg of sieved,sterilized dry paddy soil(the contents of soil organic matter,alkaline hydrolytic nitrogen,effective phosphorus,and available potassium were 14.2%,66.2 mg kg-1,8.5 mg kg-1,and 8.0 mg kg-1,respectively,and soil pH was 5.4)amended with 1.0 g(NH4)2SO4,0.8 g P2O5,and 0.6 g K2O per kg soil,where they grew until the seeds were harvested.The plants were grown under natural conditions with average temperature of 32°C/24°C(day/night),relativehumidity65-85%,photosynthetically active radiation 600 to 1200 μmol m-2s-1,and photoperiod of 14/10 h(day/night).Young fully expanded leaves of WT and GO-2 from the tops of plants were sampled at 40,60,80,100,and 120 days after germination,representing the initial tillering,peak tillering,heading,milk ripe,and dough ripe stages,respectively.The sampled leaves were stored at-70°C for analysis.
2.3.Measurement of chalkiness and grain size
Mature panicles were harvested and dried at 37°C for at least 3 days to 15%moisture content.The seeds were then manually threshed and machine dehulled.The degree of chalkiness,grain length(GL)and grain width(GW)were evaluated following the method of Xiao et al.[33].
2.4.Scanning electron microscopy(SEM)
Morphological properties of rice kernels were examined.Rice kernels were broken along natural fracture planes and the pieces mounted on stubs.The specimens were coated with gold using a SC7610 sputter coater(Fisons Ins.,England). ThesesampleswereobservedbyJSM-6380LVscanning electron microscope(Jeol,Akishima-Shi,Japan)at a magnification of 2000×.
2.5.Measurement of Asc and DHA
Asc(reduced form)and total Asc(Asc plus DHA)contents were measured in 0.1 g of fresh leaves,according to the method of Kampfenkel et al.[34]with slight modification. DHA was determined as the difference between total and reduced Asc.
2.6.Determination of chlorophyll content
Fresh leaf material(0.1 g)was extracted with 80%(v/v)acetone,and the absorption of the extracts was measured at 663 and 645 nm.Chlorophyll(Chl)a and b were determined using the redetermined extinction coefficients and equations established by Lichtenthaler[35]:Chl a(mg L-1)=12.21 A663-2.81 A645,Chl b(mg L-1)=20.13 A645-5.03 A663.
2.7.Lipid peroxidation assay
Lipid peroxidation was evaluated by measuring malondialdehyde(MDA)content in 0.1 g of fresh leaves,according to the method of Heath and Packer[36]with slight modification.
2.8.Determination of Rubisco content
Rubisco protein determination was performed according to Liu et al.[22].Briefly,0.5 g of leaves was homogenized in 5 mL of grinding medium and the homogenate was centrifuged at 15,000×g for 20 min.Then 100 μL of supernatant was added to anequalvolumeofsamplebufferandincubatedinboilingwater for 3 min before electrophoresis.The samples(10 μL)were loaded onto 12.5%(w/v)resolving polyacrylamide gels(PAG)with a 5%(w/v)PAG stacker.Electrophoresis was performed at a constant current of 15 mA(Beijing Liuyi Instrument Factory,China).After the gel was stained with Coomassie Brilliant Blue R-250(Sigma,USA),it was destained overnight with gentle shaking to ensure that its background had turned colorless. Then the large subunit and smallsubunit bands ofRubiscowere cut out of the gel and eluted with 1.0 mL of formamide at 50°C for 5 h with shaking.The light absorption of the resulting solution was measured at 595 nm.A calibration curve was constructed with authentic Rubisco(Bio-Rad,USA).
2.9.Net photosynthesis rate measurements
The net photosynthetic rate was measured with a portable photosynthesis system(Li-6400,Li-Cor Inc.,Lincoln,NE,USA). The measurement was conducted between 11:00 and 13:00 on the measuring day,and the youngest fully expanded leaf on each plant at 40,60,80,100,and 120 days of plant age was used for the determination.The measurement conditions were as follows:leaftemperature32±2°C,photonfluxdensity 1000 μmol m-2s-1,relative humidity 79-83%,and CO2concentration 340 μmol mol-1.
2.10.Detection of phytohormones
Young,fully expanded leaves from GO-2 and WT(0.5 g)were harvested for abscisic acid(ABA)and jasmonic acid(JA)extraction.The harvested tissues were immediately ground to afinepowderinliquidN2,andthenexposedtoextractionbuffer(1.0 mL of 80%methanol)at 4°C overnight.The samples were centrifuged at 10,000×g for 5 min,and the residues were re-extracted with 0.6 mL of 80%methanol(HPLC grade,Merck,Germany).The dried extracts were obtained according to Liu et al.[23]andthendissolvedin40 μLof50%methanolandusedfor LC/MS assay in a Waters Acquity SQD(LC/MS)system(Waters,USA)according to Liu et al.[37].
2.11.Statistical analysis
All experiments were conducted in at least triplicate.Values were expressed as means±SE.All results were subjected to a one-way analysis of variance with a least significant difference(LSD)test between means.Data were processed with the SigmaPlot software(version 10.0,SYSTAT Software Inc.,Richmond,CA,USA).Principal component(PC)analysis was performed with mean-centered data using statistical software SPSS(version 20.0,IBM Corp,Armonk,NY,USA).Loading plots were used to detect the parameters responsible for the separation between different plant stages or genotypes in the data.
3.Results
3.1.L-GalLDH-overexpressing rice plants display reduced grain chalkiness
A significant difference in grain chalkiness degree was observed between GO-2 and WT.GO-2 kernels displayed a reduced(6.21%)grain chalkiness degree,but WT kernels displayed 9.32%(P=0.0008,n=50,Fig.1A).Furthermore,WT kernels displayed higher chalkiness with less translucence compared to GO-2(Fig.1B).Scanning electron microscopy showed that the GO-2 endosperm consisted of densely packed andlargestarchgranules,whileWTendospermalso consisted of densely packed and large starch granules,but with some round and loosely packed starch granules with air spaces(Fig.1C).As chalkiness may be affected by grain size,GL,GW,and grain length-to-width ratio(GLWR)of GO-2 and WT were measured.No significant difference between GO-2 and WT in GL,GW,or GLWR was observed(Table 1).
3.2.Contents of Asc,chlorophyll,and lipid peroxidation inL-GalLDH-overexpressing rice plants at different plant growth stages
To investigate the causes of the different grain chalkiness shown between GO-2 and WT,we evaluated plant growth rate(including plant height and tiller number),Asc content,Asc/DHA ratio,Chl pool,and MDA content in GO-2 and WT plants at 40,60,80,100,and 120 days of age.No significant differences between GO-2 and WT in plant height and tiller number were observed at different plant growth stages(Table 2),whereas the level of foliar Asc of GO-2 increased significantly compared with that of the WT(Fig.2A).The level of Asc in young fully expanded leaves of GO-2 was increased to 137.0%,142.1%,152.4%,140.1%,and 116.8%at 40,60,80,100,and 120 days,respectively,of the level in the correspondingleaves of the WT(Fig.2A).Significant increases were also observed in the Asc/DHA ratio of GO-2 plants at 40,60,80,and 100 days,of 2.04,1.43,2.11,and 2.41,respectively,compared to the WT(1.32,1.00,1.15,and 1.29),whereas no significant difference was observed in GO-2 leaves relative to the WT at 120 days(Fig.2B).The level of lipid peroxidation,as measured byMDAassay,wassignificantlylowerinyoungfully expanded leaves of GO-2 at 40 and 120 days(0.05±0 and 0.05±0 mg g-1DW)than in the corresponding leaves of the WT(0.07±0 and 0.06±0 mg g-1DW),whereas no significant difference was observed in GO-2 leaves relative to the WT at 60,80,and 100 days(Fig.2C).
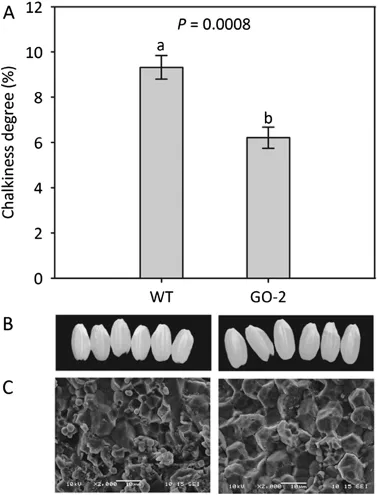
Fig.1-Grain chalkiness and starch granule morphology comparison between GO-2 and WT.(A)Chalkiness degree of GO-2 and WT grains.(B)Polished grain of WT(left)and GO-2(right).(C)Scanning electron microscopy images of transverse sections of WT(left)and GO-2(right)grains.Bars,10 μm.Data in A are presented as means±SE of at least 50 replicates,and those that were significantly different from one another according to Fisher's protected LSD test are indicated by different letters(P<0.05).

Table 1-Grain appearance quality traits of GO-2 and WT.
To determine whether Asc content affects the pool size of Chl a and b,Chl levels were measured in leaves of GO-2 and WT at different plant age.No significant difference in the level of Chl a was observed in GO-2 leaves relative to the WT at 60,80,100,and 120 days,but the difference was significant in GO-2 compared with the WT at 40 days,and no significant difference in the level of Chl b and the Chl a to Chl b ratio was observed in GO-2 leaves relative to the WT(Table 3).
3.3.Changes in Rubisco level and net photosynthesis rate in L-GalLDH-overexpressing rice plants
To determine whether the amount of Rubisco protein varied in GO-2 plants,we evaluated by SDS-PAGE the content of Rubisco protein in young,fully expanded leaves of GO-2 and WT plants at different plant ages.The results showed that the level of Rubisco protein was significantly higher in young,fully expanded leaves of GO-2 at 40 and 80 days of plant age(42.66±1.73 and 66.40±1.06 mg g-1DW)than in the correspondingleavesoftheWT(33.89±2.24and58.56± 1.33 mg g-1DW),whereas no significant difference was observed between GO-2 and WT at 60,100,and 120 days(Fig.3A).The net photosynthesis rate was also measured in young,fully expanded leaves in GO-2 and WT leaves atdifferent plant ages,and no significant difference was observed between GO-2 and WT plants(Fig.3B).

Table 2-Plant height and tiller number of GO-2 and WT plants at 40,60,80,100,and 120 days of age.
3.4.Changes in phytohormones associated with senescence in L-GalLDH-overexpressing rice plants
In this study,two phytohormones(ABA and JA)associated with senescence and grain filling were measured at different plant growth stages.The level of ABA was not significantly different in GO-2 leaves relative to the WT at 40 and 120 days of plant age,whereas it was significantly lower in leaves of GO-2 plants at 60,80,and 100 days than in WT plants,being reduced to respectively 81.86%,43.87%,and 62.98%of the WT(Fig.4A).Similar differences between GO-2 and WT were observed in the level of JA,with JA content in GO-2 reduced to 76.22%,59.78%,and 57.15%of the WT at 60,80,and 100 days plant age,respectively,whereas no significant difference was observed between GO-2 and WT at 40 and 120 days of plant age(Fig.4B).
3.5.Correlation analysis of physiochemical indexes at 60,80,and 100 days of plant age
To assess the effect on chalkiness of growth status after heading,especially during the grain-filling stage,we focused on analyzing the association of chalkiness with physiological indices inGO-2 and WT atkey developmentalstagesassociated with grain filling(60,80,and 100 days of plant age).The relationships between foliar Asc and MDA content,Chl pool size,Rubiscocontent,CO2assimilation,andABAand JAcontent in GO-2 and WT plants at 60,80,and 100 days of plant age are described in Fig.5.Pearson correlation analysis was performed on the least-squares means for each parameter pair.Values are given for the correlation coefficient(r).Parameter pairs that are positively or negatively correlated with significance greater than P<0.05 and P<0.01 are highlighted by shading(see legend for details).Asc showed a strongly positive trend with respect to Asc/DHA,Rubisco,and net photosynthesis rate(Photo)(Asc versus Asc/DHA,r=0.940;Asc versus Rubisco,r= 0.603;Asc versus Photo,r=0.654),but a strongly negative trend with respect to ABA and JA (Asc versus ABA,R=-0.837;Asc versus JA,r=-0.763).Asc/DHA showed a positive trend with respect to Rubisco and Photo(Asc/DHA versus Rubisco,r=0.554;Asc/DHA versus Photo,r=0.530)and a strongly negative trend with respect to ABA and JA(Asc/DHA versus ABA,r=-0.721;Asc/DHA versus JA,r=-0.700).There was also a strongly positive correlation between Rubisco and Photo(R=0.849),but a strongly negative correlation between ABA content and Rubisco(r=-0.893)and between ABA and Photo(r=-0.870).
3.6.PC analysis of Asc and other correlated parameters in L-GalLDH-overexpressing rice plants at 60,80,and 100 days of plant age
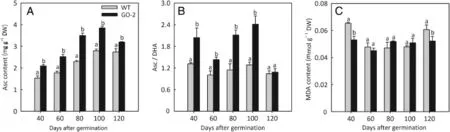
Fig.2-Asc content(A),Asc/DHA ratio(B),and MDA content(C)were measured in young,fully expanded fresh leaves of plants 40,60,80,100,and 120 days old.Data in A,B,and C are presented as means±SE of at least four replicates,and those that were significantly different from one another at the same growth stage according to Fisher's protected LSD test are indicated by different letters(P<0.05).
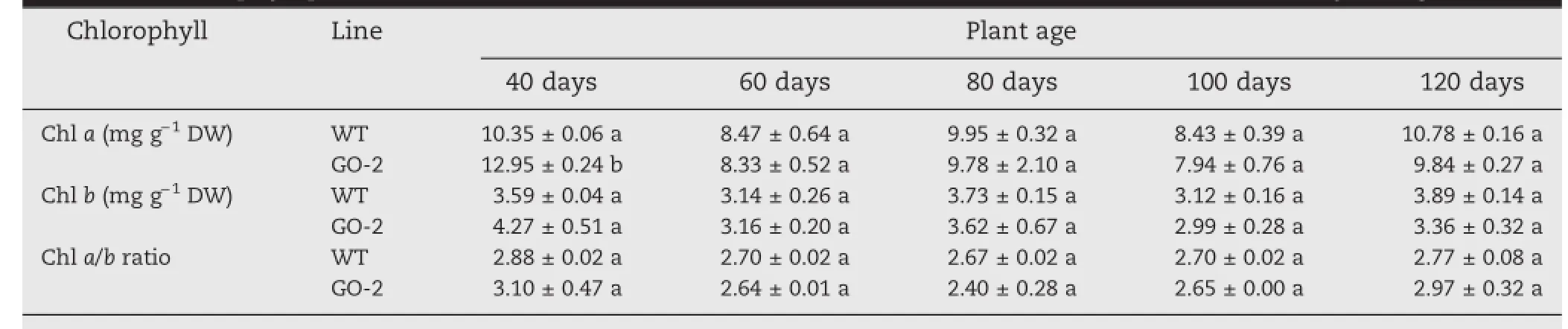
Table 3-Chlorophyll pool size and Chl a/b ratio in GO-2 and WT rice leaves at 40,60,80,100,and 120 days of age.
Principal component(PC)analysis was applied to the concentrationsofall metabolitesofleafsamplestogivean overallview of the differences between genotypes and between key stages(60,80,and 100 days of plant age).The first two PC analysis scores explained 77.64%of total variation(Fig.6A).The first PC(PC1),which explained 55.55%of total variation,was positively correlated with Asc content,redox state(Asc/DHA),Rubisco content and net photosynthesis rate,and negative correlations with ABA and JA content,thus confirming the relevance of the changes in these parameters at different plant growth stages(Fig.6B).The second PC(PC2),which explained 22.09%of the totalvariation(Fig.6A),separated60and100 daysofplantstage from 80 days of plant stage in the WT and GO-2(Fig.6A). Examination of PC2 loadings suggested that this difference between 60,80,and 100 days of plant stage involved Chl(a+b)and JA content on the positive side(Fig.6B).This analysis confirmed and summarized the general trends identified in Fig.5.
4.Discussion
L-GalLDH catalyzes the terminal step of the major pathway of Asc biosynthesis in higher plants by convertingL-GalL into Asc[38].Many studies have been performed to investigate the role ofL-GalLDH in the control of cell,organ and plant growth using mainly antisense or RNAi approaches[19],whereas only a few studies have been performed to investigate whether the overexpression ofL-GalLDH affects the accumulation of Asc. For example,tobacco suspensioncells overexpressingL-GalLDH showed an increase in Asc content[24].Tobacco plants overexpressing sweetpotatoL-GalLDHshoweda3-fold increase inL-GalLDH activity,but no effect on Asc level[25].Recently,we introduced authenticL-GalLDH into rice to investigate whether theoverexpression ofL-GalLDHledtochanged Asc content,and found that the overexpression ofL-GalLDH resulted in a substantial increase in foliar Asc content at 30 days or the grain-filling stage;moreover,thatL-GalLDH-overexpressingrice plants displayed increased seed set compared with the WT[22].
In this study,we investigated how a change in Asc content leadstoachangedgrainchalkinessusingL-GalLDH-overexpressing rice(GO-2)in which the foliar Asc content has increased to 1.4-fold of the WT.We observed a significant lower grain chalkiness degree in GO-2 than in the WT(Fig.1A).GO-2 kernels have higher density starch granules than WT kernels(Fig.1C).Grain chalkiness is an important factor in determining rice quality and price,and is influenced by multiple factors including starch synthesis and starch granule structure and arrangement[39].Programmed starchy endosperm cell death occurred after starch granule packaging,and an endogenous H2O2burst was detectable at 15 DAF in rice endosperm[31].As the most abundant water-soluble antioxidant in plants,Asc plays a pivotal role in the network of characterized events involved in wheat kernel maturation,such as protein synthesis andstorage,programmedcelldeath(PCD)ofstarchyendosperm,and tissue dehydration[40].We observed substantially increased levels of foliar Asc content at different plant ages and increased foliar Asc/DHA ratios at 40,60,80,and 100 days of age in GO-2 compared to the WT(Fig.2A,B).On the other hand,an insufficient supply of photosynthate from source to sink organs is considered to be one of the causes of chalkiness[41].In our study,the observation that increasing L-GalLDH expression correlated with increased Rubisco content and net photosynthesis rate was consistent with the foliar levels of Asc at key developmental stages associated with grain filling(Figs.2A,3A,5,6).Moreover,the correlation between the level of Rubisco,net photosynthesis rate,and Asc/DHA suggested that redox state is important in maintaining photosynthetic function,resulting in the reduction of chalky grain formation(Figs.5 and 6).
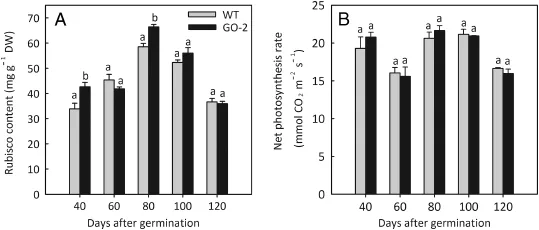
Fig.3-Levels of Rubisco protein and net photosynthesis rate of GO-2 and WT plants at 40,60,80,100,and 120 days of plant age.Rubisco protein content(A)and net photosynthesis rate(B)were measured in young,fully expanded leaves of WT and GO-2 rice at 40,60,80,100,and 120 days of plant age.Data in A and B are presented as means±SE of at least four replicates,and those that were significantly different from one another at the same growth stage according to Fisher's protected LSD test are indicated by different letters(P<0.05).
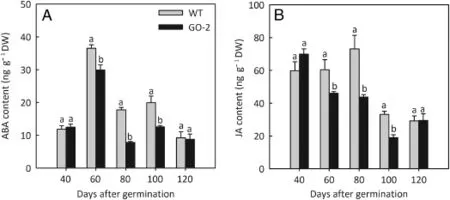
Fig.4-ABA and JA content of GO-2 rice at 40,60,80,100,and 120 days of plant age.ABA(A)and JA(B)were measured in young,fully expanded leaves of WT and GO-2 rice at 40,60,80,100,and 120 days of plant age.Data in A and B are presented as means±SE of at least three replicates,and those that were significantly different from one another at the same growth stage according to Fisher's protected LSD test are indicated by different letters(P<0.05).
Phytohormonesare keyregulatorsofseeddevelopment[42]. Genes encoding late embryogenesis abundant(LEA)proteins are activated by ABA through OsVP1 to protect the embryo from dehydration[43].Our previous study showed thatL-GalLDH-suppressed rice plants,which contain very low foliar Asc levels(<30%of WT levels),exhibit significantly higher levels of foliar ABA at 40,60,and 80 days of plant age than with the WT and show reduced tiller number[23].In the present study,the L-GalLDH-overexpressing rice plant(GO-2),which showed higher foliar Asc levels(about 1.4-fold of the WT),exhibited significantly lower levels of foliar ABA at 60,80,and 100 days of plant age compared with the WT,while no significant difference was observed at 40 and 120 days.We also noticed that the increase in percent of Asc in GO-2 plants compared with WT at 60,80,and 100 days of plant age was greater than 40%,whereas it was less than 40%at 40 and 120 days(Fig.2A).A strongly negative correlation was also observed between ABA and Asc at 60,80,and 100 days of plant age(Figs.5,6).Previous studies have shown that the Asc-deficient Arabidopsis mutant vtc1 has higher levels of ABA than the WT[44].A recent study also showed that the vtc1 and vtc2 mutants,which have low abundance of Asc,show enhanced ABA levels compared with the WT[45].Asc is required as a co-substrate for the activity of 2-oxoacid-dependent dioxygenases,a class of enzymesthat are involved in the biosynthesis of ABA[4,46].These results seem somewhat contradictory,as the ABA biosynthetic pathway requires Asc.However,the authors hypothesized that this ABA increase was due to differential gene expression of vtc1 with respect to WT plants[40,47].Pastori et al.speculated that 9-cis-epoxycarotenoid dioxygenase(NCED)transcripts are upregulated in response to Asc deficiency in vtc1,perhaps ascompensation for decreased cofactor availability and to increasemaximal catalytic capacity[44].Zhu et al.reported that a group of starch metabolism-related genes showed enhanced expression profiles and had higher transcript levels in superior spikelets than in inferior ones at the early and middle rice kernel filling stages,and the expression of the ABA synthesis genes NCED1 and NCED5 declined with development of the caryopses[48].Here we presumed that there was an Asc increased threshold value(>40%of the WT levels)that affects ABAlevel,andthelowerlevelsoffoliarABAinGO-2plantinkey stages associated with grain filling are due to the expression changes of ABA biosynthetic genes,possibly as a consequence of the enhanced Asc level,and possibly accounting for the significantly reduced grain chalkiness observed in GO-2 plants.
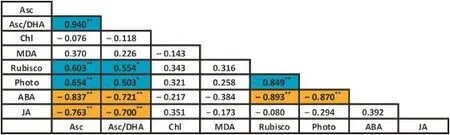
Fig.5-Genetic correlations(Pearson coefficients)between different metabolic parameters in GO-2 and WT plants at 60,80,and 100 days of plant age.For calculating correlations,the least-squares means of the metabolites of each accession averaged across three experiments were used.The results are based on three independent experiments.Relations that are significant at P<0.01 and P<0.05 are indicated by dark and light shading,with positive and negative correlations being distinguished by blue and orange.

Fig.6-PC analysis of Asc,ABA,JA and other correlated parameters in GO-2 and WT plants at 60,80,and 100 days of plant age. PC analysis of Asc content(Asc),redox state(Asc/DHA),Chl(a+b)contents(Chl),net photosynthesis rate(Photo),MDA content(MDA),ABA content(ABA)and JA content(JA)in fully expanded fresh leaves of GO-2 and WT plants at 60,80,and 100 days of plant age.(A)Scores obtained by PC1 and PC2 for GO-2 plants(black symbols)and the WT(gray symbols).Individual values are given for each biological triplicate.Each number corresponds to days of plant age.(B)PC1 and PC2 loadings for each analyzed parameter.
Given that exogenous application of methyl jasmonate(MeJA)stimulates de novo biosynthesis of Asc in N.tabacum andArabidopsissuspensioncells,Asccontentseemstobeunder hormonal control[49].Sasaki-Sekimoto et al.found that among the transcripts affected by JA are genes involved in Asc biosynthesis and recycling[50].For example,exogenous application of JA induced an increase in transcript levels ofL-GalLDH andstimulatedAscbiosynthesisinthemonocotyledon Agropyron cristatum[51].However,the effects of JA may show species specificity;for example,the expression ofL-GalLDH mRNA in broccoli florets was suppressed by treatment with MeJA and ABA and was accompanied by the acceleration of Asc degradation[49,52].In addition,in contrast to its effect in Arabidopsis,applicationofMeJAintomatotendstosuppressAsc accumulation[53].JA is involved in plant development and the defense response[54].Grain yield was greatly reduced in transgenic rice in which the MeJA level was 6-fold those of nontransgenic controls as a result of overexpression of the Arabidopsis JA carboxyl methyltransferase gene[55].In our study,the level of JA was significantly lower in GO-2 leaves than in the WT at 60,80,and 100 days of plant age,and no significant difference was observed at 40 and 120 days of plant age(Fig.4B).Moreover,a strongly negative correlation was observedbetweenJAandAscat60,80,and100 daysofplantage(Figs.5 and 6).Here we suggest that the reduced JA content in GO-2 plant leaves was a consequence of the high level of endogenous Asc and may also account for the significantly reduced grain chalkiness observed in GO-2 plants.
In summary,our results suggest that the level of Asc plays a role in the reduction of grain chalkiness inL-GalLDH-overexpressingriceplants.ThehigherleveloffoliarAscinGO-2leadsto achangedredoxhomeostasis,whichisimportantinmaintaining photosynthetic function in grain filling stages.The loss of ABA andJAinGO-2leavesmaybeaconsequenceofthehigherlevelof Asc present in grain filling stages,and may account for the reduced rice chalkiness observed in GO-2.Thus,these observations support the conclusion that an enhanced level of Asc alters grain chalkiness in theL-GalLDH-overexpressing transgenic through maintaining photosynthetic function and affecting contents of phytohormones associated with grain filling.
Acknowledgments
The authors are very grateful to Professor Xingxiang Peng(South China Agricultural University,China)for the generous gift of rice seeds.This work was supported by the National Natural Science Foundation of China(31270287,31301244,31471432)and the Natural Science Foundation of Guangdong Province,China(2014A030313663,S2012010010680).
R E F E R E N C E S
[1]D.R.Gallie,The role ofL-ascorbic acid recycling in responding to environmental stress and in promoting plant growth,J.Exp.Bot.64(2013)433-443.
[2]C.Pignocchi,C.H.Foyer,Apoplastic ascorbate metabolism and its role in the regulation of cell signaling,Curr.Opin. Plant Biol.6(2003)379-389.
[3]C.Barth,W.Moeder,D.F.Klessig,P.L.Conklin,The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1,Plant Physiol.134(2004)1784-1792.
[4]C.Barth,M.De Tullio,P.L.Conklin,The role of ascorbic acid in the control of flowering time and the onset of senescence,J.Exp.Bot.57(2006)1657-1665.
[5]V.Pavet,E.Olmos,G.Kiddle,S.Mowla,S.Kumar,J.Antoniw,M.E.Alvarez,C.H.Foyer,Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis,Plant Physiol.139(2005)1291-1303.
[6]C.J.Botanga,G.Bethke,Z.Chen,D.R.Gallie,O.Fiehn,J.Glazebrook,Metabolite profiling of Arabidopsis inoculated with Alternaria brassicicola reveals that ascorbate reduces disease severity,Mol.Plant Microbe Interact.25(2012)1628-1638.
[7]A.Attolico,M.De Tullio,Increased ascorbate content delays flowering in long-day grown Arabidopsis thaliana(L.)Heynh,Plant Physiol.Biochem.44(2006)462-466.
[8]S.O.Kotchoni,K.E.Larrimore,M.Mukherjee,C.F.Kempinski,C.Barth,Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis,Plant Physiol.149(2009)803-815.
[9]M.Mukherjee,K.E.Larrimore,N.J.Ahmed,T.S.Bedick,N.T. Barghouthi,M.B.Traw,C.Barth,Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide,salicylic acid,and the NPR1 gene,Mol. Plant Microbe Interact.23(2010)340-351.
[10]R.D.Hancock,R.Viola,Improving the nutritional value of crops through enhancement ofL-ascorbic acid(vitamin C)content:rationale and biotechnological opportunities,J.Agric.Food Chem.53(2005)5248-5257.
[11]S.Naqvi,C.Zhu,G.Farre,K.Ramessar,L.Bassie,J. Breitenbach,D.P.Conesa,G.Ros,G.Sandmann,T.Capell,Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways,Proc.Natl.Acad.Sci.U.S.A.106(2009)7762-7767.
[12]N.Smirnoff,P.L.Conklin,F.A.Loewus,Biosynthesis of ascorbic acid in plants:a renaissance,Annu.Rev.Plant Biol. 52(2001)437-467.
[13]B.A.Wolucka,M.Van Montagu,GDP-mannose 3′,5′-epimerase forms GDP-L-gulose,a putative intermediate for the de novo biosynthesis of vitamin C in plants,J.Biol. Chem.278(2003)47483-47490.
[14]A.Lorence,B.I.Chevone,P.Mendes,C.L.Nessler,myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis,Plant Physiol.134(2004)1200-1205.
[15]V.Valpuesta,M.A.Botella,Biosynthesis ofL-ascorbic acid in plants:new pathways for an old antioxidant,Trends Plant Sci.9(2004)573-577.
[16]C.G.Bartoli,G.M.Pastori,C.H.Foyer,Ascorbate biosynthesis in mitochondria is linked tothe electron transport chain between complexes III and IV,Plant Physiol.123(2000)335-344.
[17]P.Schertl,S.Sunderhaus,J.Klodmann,G.E.Grozeff,C.G. Bartoli,H.P.Braun,L-Galactono-1,4-lactone dehydrogenase(GLDH)forms part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana,J.Biol.Chem.287(2012)14412-14419.
[18]K.Tabata,K.Ôba,K.Suzuki,M.Esaka,Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA forL-galactono-1,4-lactone dehydrogenase,Plant J.27(2001)139-148.
[19]M.Alhagdow,F.Mounet,L.Gilbert,A.Nunes-Nesi,V.Garcia,D. Just,J.Petit,B.Beauvoit,A.R.Fernie,C.Rothan,Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato,Plant Physiol.145(2007)1408-1422.
[20]B.Pineau,O.Layoune,A.Danon,R.De Paepe,L-Galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I,J.Biol.Chem. 283(2008)32500-32505.
[21]L.Yu,J.Jiang,C.Zhang,L.Jiang,N.Ye,Y.Lu,G.Yang,E.Liu,C. Peng,Z.He,Glyoxylate rather than ascorbate is an efficient precursor for oxalate biosynthesis in rice,J.Exp.Bot.61(2010)1625-1634.
[22]Y.Liu,L.Yu,R.Wang,Level of ascorbic acid in transgenic rice forL-galactono-1,4-lactone dehydrogenase overexpressing or suppressed is associated with plant growth and seed set,Acta Physiol.Plant.33(2011)1353-1363.
[23]Y.Liu,L.Yu,J.Tong,J.Ding,R.Wang,Y.Lu,L.Xiao,Tiller number is altered in the ascorbic acid-deficient rice suppressed forL-galactono-1,4-lactone dehydrogenase,J.Plant Physiol.170(2013)389-396.
[24]T.Tokunaga,K.Miyahara,K.Tabata,M.Esaka,Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA forL-galactono-1,4-lactone dehydrogenase,Planta 220(2005)854-863.
[25]T.Imai,M.Niwa,Y.Ban,M.Hirai,K.Ôba,T.Moriguchi,Importance of theL-galactonolactone pool for enhancing the ascorbate content revealed byL-galactonolactone dehydrogenase-overexpressing tobacco plants,Plant Cell Tissue Organ 96(2009)105-112.
[26]Y.Yoshioka,H.Iwata,M.Tabata,S.Ninomiya,R.Ohsawa,Chalkiness in rice:potential for evaluation with image analysis,Crop Sci.47(2007)2113-2120.
[27]A.R.Del Rosario,V.P.Briones,A.J.Vidal,B.O.Juliano,Composition and endosperm structure of developing and mature rice kernel,Cereal Chem.45(1968)225-235.
[28]A.M.Myers,M.K.Morell,M.G.James,S.G.Ball,Recent progress toward understanding biosynthesis of the amylopectin crystal,Plant Physiol.122(2000)989-998.
[29]H.G.Kang,S.Park,M.Matsuoka,An G White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene(OsPPDKB),Plant J.42(2005)901-911.
[30]H.Yamakawa,T.Hirose,M.Kuroda,T.Yamaguchi,Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray,Plant Physiol.144(2007)258-277.
[31]S.Xu,H.Yu,L.Yan,T.Wang,Integrated proteomic and cytological study of rice endosperms at the storage phase,J.Proteome Res.9(2010)4906-4918.
[32]X.Liu,T.Guo,X.Wan,H.Wang,M.Zhu,A.Li,N.Su,Y.Shen,B.Mao,H.Zhai,Transcriptome analysis of grain filling caryopses reveals involvement of multiple regulatory pathways in chalky grain formation in rice,BMC Genomics 11(2010)730.
[33]K.Kampfenkel,M.V.Motagu,D.Inzè,Extraction and determination of ascorbate and dehydroascorbate from plant tissue,Anal.Biochem.225(1995)165-167.
[34]L.Xiao,W.Lin,D.Li,B.Hong,A method to measure the rice kernel chalkiness objectively,Chin.Rice Res.Newsl.9(2001)12-13.
[35]H.K.Lichtenthaler,Chlorophylls and carotenoids:pigments of photosynthetic biomembranes,Methods Enzymol.148(1987)350-382.
[36]R.L.Heath,L.Packer,Photoperoxidation in isolated chloroplasts:I.Kinetics and stoichiometry of fatty acid peroxidation,Arch.Biochem.Biophys.125(1968)189-198.
[37]X.Liu,Y.L.Yang,W.H.Lin,J.H.Tong,Z.G.Huang,L.T.Xiao,Determination of both jasmonic acid and methyl jasmonate in plant samples by liquid chromatography tandem mass spectrometry,Chin.Sci.Bull.21(2010)2231-2235.
[38]G.L.Wheeler,M.A.Jones,N.Smirnoff,The biosynthetic pathway of vitamin C in higher plants,Nature 393(1998)365-369.
[39]M.Kusano,A.Fukushima,N.Fujita,Y.Okazaki,M.Kobayashi,N.F.Oitome,K.Ebana,K.Saito,Deciphering starch quality of rice kernels using metabolite profiling and pedigree network analysis,Mol.Plant 5(2012)442-451.
[40]A.Paradiso,M.C.De Pinto,V.Locato,L.De Gara,Galactone-γ-lactone-dependent ascorbate biosynthesis alters wheat kernel maturation,Plant Biol.14(2012)652-658.
[41]Q.Liu,X.Zhou,L.Yang,T.Li,Effects of chalkiness on cooking,eating and nutritional qualities of rice in two indica varieties,Rice Sci.16(2009)161-164.
[42]L.Xue,J.Zhang,H.Xue,Genome-wide analysis of the complex transcriptional networks of rice developing seeds,PLoS ONE 7(2012)1-15.
[43]T.Hattori,T.Terada,S.T.Hamasuna,Sequence and functional analyses of the rice gene homologous to the maize Vp1,Plant Mol.Biol.24(1994)805-810.
[44]G.M.Pastori,G.Kiddle,J.Antoniw,S.Bernard,S.Veljovic-Jovanovic,P.J.Verrier,G.Noctor,C.H.Foyer,Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling,Plant Cell 15(2003)939-951.
[45]P.I.Kerchev,T.K.Pellny,P.D.Vivancos,G.Kiddle,P.Hedden,S.Driscoll,H.Vanacker,P.Verrier,R.D.Hancock,C.H.Foyer,The transcription factor ABI4 is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis,Plant Cell 23(2011)3319-3334.
[46]O.Arrigoni,M.C.De Tullio,Ascorbic acid:much more than just an antioxidant,Biochim.Biophys.Acta 1569(2002)1-9.
[47]P.Conklin,C.Barth,Ascorbic acid,a familiar small molecule intertwined in the response of plants to ozone,pathogens, and the onset of senescence,Plant Cell Environ.27(2004)959-970.
[48]G.Zhu,N.Ye,J.Yang,X.Peng,J.Zhang,Regulation of expression of starch synthesis genes by ethylene and ABA in relation to the development of rice inferior and superior spikelets,J.Exp.Bot.62(2011)3907-3916.
[49]B.A.Wolucka,A.Goossens,D.Inzé,Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions,J.Exp.Bot.56(2005)2527-2538.
[50]Y.Sasaki-Sekimoto,N.Taki,T.Obayashi,M.Aono,F. Matsumoto,N.Sakurai,H.Suzuki,M.Y.Hirai,M.Noji,K.Saito,Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis,Plant J.44(2005)653-668.
[51]C.Shan,Z.Liang,Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress,Plant Sci.178(2010)130-139.
[52]F.Nishikawa,M.Kato,H.Hyodo,Y.Ikoma,M.Sugiura,M. Yano,Ascorbate metabolism in harvested broccoli,J.Exp.Bot. 54(2003)2439-2448.
[53]W.P.Suza,C.A.Avila,K.Carruthers,S.Kulkarni,F.L.Goggin,A.Lorence,Exploring the impact of wounding and jasmonates on ascorbate metabolism,Plant Physiol. Biochem.48(2010)337-350.
[54]J.J.Cheong,Y.D.Choi,Methyl jasmonate as a vital substance in plants,Trends Genet.19(2003)409-413.
[55]E.H.Kim,Y.S.Kim,S.H.Park,Y.J.Koo,Y.Do Choi,Y.Y.Chung,I.J.Lee,J.K.Kim,Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice,Plant Physiol.149(2009)1751-1760.
5 July 2014
in revised form
.Tel.:+86 7582716359
E-mail address:534537324@qq.com(Y.Liu).
Peer review is under the responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
http://dx.doi.org/10.1016/j.cj.2014.12.001
2214-5141/©2015 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
The Crop Journal的其它文章
- Effects of cultivation of OsrHSA transgenic rice on functional diversity of microbial communities in the soil rhizosphere
- An AFLP marker linked to the leaf rust resistance gene LrBi16 and test of allelism with Lr14a on chromosome arm 7BL
- Genetic variation for phytic acid content in mungbean(Vigna radiata L.Wilczek)
- Quantifying cardinal temperatures and thermal time required for germination of Silybum marianum seed
- Characterization and mapping of QTLs on chromosome 2D for grain size and yield traits using a mutant line induced by EMS in wheat
- Bed planting of wheat(Triticum aestivum L.)improves nitrogen use efficiency and grain yield compared to flat planting
