可视化黄色荧光石油焦基碳量子点高效检测Cu2+
2015-10-24吴文婷吴明铂孙洪迪
王 月,吴文婷,吴明铂,孙洪迪,
谢 辉1,胡 超2,吴雪岩1,邱介山2
(1.中国石油大学重质油国家重点实验室,山东青岛266580;2.大连理工大学精细化工国家重点实验室,化工与环境生命学部炭素材料研究室,辽宁大连116024)
可视化黄色荧光石油焦基碳量子点高效检测Cu2+
王 月1,吴文婷1,吴明铂1,孙洪迪1,
谢 辉1,胡 超2,吴雪岩1,邱介山2
(1.中国石油大学重质油国家重点实验室,山东青岛266580;2.大连理工大学精细化工国家重点实验室,化工与环境生命学部炭素材料研究室,辽宁大连116024)
以石油焦为碳源,采用超声辅助的化学氧化法直接制备可视化黄色荧光碳量子点(CQDs)。作为非标记的探针,该CQDs无需任何修饰即可成功用于实际水样中Cu2+的检测。该荧光探针制备方法简单、经济,可快速响应(3 s),具有良好的选择性、灵敏性和可重复利用性,并且可实现“混合即检测”的快速检测目的。其线性检出范围较宽,为0.25~10 μmol/L,检出限为0.029 5 μmol/L。光诱导电子转移机理可很好地解释Cu2+猝灭CQDs的过程,本文提出的CQDs-Cu2+-EDTA“off-toon”检测机理为金属离子荧光探针的开发奠定了理论基础。石油焦基CQDs在实际水样Cu2+的检测中具有快速响应性,在实际传感器应用领域具有很好的实际应用价值。
石油焦;碳量子点;黄色荧光;快速检测;Cu2+检测
1 Introduction
Water pollution caused by heavy metal ions has become a critical issue worldwide.As a kind of heavy metals,a trace amount of copper is essential for many living organisms.However,electroplating wastewater with a plenty of copper is currently becoming a serious threat to human's health and the environment[1,2].Conventional analysis methods,such as atomic absorption spectrometry,electrochemical method,inductively coupled plasma-mass spectrometry and inductively plasma-mass emission spectrometry,have been widely used for the detection of Cu2+[3-5].However,the above-mentioned methods are usually time consuming,complicated to prepare sample,even with toxic reagents,and generally unsuitable for online application or in-field detection[6].Therefore,it is highly desired to develop a practicable method with a high accuracy and sensitivity for simple,rapid,green,and low-cost tracking of Cu2+in environmental,biological and tap water.
Previously,the organic dye/organic complex fluorophore-based and quantum dots(QDs)-based probes,as fluorescent probes,have been proved to selectively respond to Cu2+due to their relatively high sensitivity[7-11].However,the employed organic dyes and QDs are easy to be oxidized,toxic and often lack photostability,which restrict their performance in practical applications[12-14].
The emergency of fluorescent carbon quantum dots(CQDs)provides a new way to detect Cu2+,which could meet most requirements in Cu2+detection.As a new star in QDs family,CQDs show a considerably low toxicity,excellent aqueous solubility,high stability,resistance to photobleaching,and stable fluorescence,which make them promising in fields like fluorscent probe,optoelectrical devices,sensor cell imaging,etc[15-17].Based on the merits mentioned above,CQDs are more suitable as probe for thedetectionofCu2+thanotherflurescent materials.
Some excellent works on CQDs as a probe for Cu2+detection have been reported.CQD-based Cu2+probe was firstly developed by Liu et al.in 2012[18].They explored the feasibility of photoluminescent polymer nanodots for Cu2+detection,which exhibit a blue fluorescent emission.The detection range is from 5×10-11to 5×10-5mol/L with a limit of detection(LOD)of 1 nmol/L,and their response time to Cu2+is 10 min.Recently,Wang et al.[19]developed a facile approach to prepare the blue-fluorescent graphene quantum dots via a hydrothermal re-oxidation of graphene oxide to detect Cu2+,the calibration curve of which displays a linear region of 0-15 μm with a detection limit of 0.226 μm.
Here we prepared a new kind of yellow fluorescent CQDs from petroleum coke,which show a high selectivity,sensitivity and fast response for Cu2+detection.It should be noted that the high value-added utilization of heavy oil is still urgently needed,although lots of efforts have been reported[20-22].As a by-product in oil refining process,petroleum coke used to prepare CQDs can achieve a high value-added utilization.More importantly,the yellow fluorescent CQDs are easier to be distinguished by naked eyes compared with blue fluorescent CQDs.CQDs fluorescent intensity could be recovered by ethylene diamine tetraacetic acid(EDTA)and reused,whereas it could hardly be quenched by other metal ions,such as Al3+,Zn2+,Ca2+,Mg2+,Cd2+,Cr6+,Ba2+,Na+,As3+,K+,Ce3+and Sn4+.Moreover,the proposed CQDs probing system has been successfully applied for the determination of Cu2+both in natural water and tap water.
2 Experimental
2.1Chemicals and materials
All chemical agents used were analytical-grade pure.98.0 wt.%sulfuric acid,65.0 wt.%nitric acid,28.0 wt.%ammonia,EDTA and all metal salts were provided by Sinopharm Chemical Reagent Company,China.Aqueous solutions of Ba2+,Ce3+and Na+were prepared from their nitrate salts,aqueous solutions of Mg2+,Zn2+,Ca2+,Sn4+and K+were prepared from their chloride salts,aqueous solution of Cu2+,Cd2+and Al3+were prepared from their sulfuric acid salts.Aqueous solutions of As3+and Cr6+were prepared from K2Cr2O7and Na3AsO4,respectively.Nitrate and chloride salts of Cu2+as well as chloride salts and sulfuric acid salts of Na+were also used.Quinine(97.0 wt.%)was from Aladdin Industrial Corporation at Shanghai of China.Inorganic filter membrane(0.22 μm)and 3500 Da molecular weight cut off(MWCO)membranes(Amicon Ultra-4,Millipore)were bought from Shanghai Green Bird Science&Technology Development Co.of China.The water used throughout the experiments was deionized(DI)water.
2.2Synthesis and purification of CQDs
CQDs were obtained from petroleum coke via the ultrasonic-assisted chemical oxidation approach[23].In brief,2 g petroleum coke was added into a mixture of concentrated H2SO4(45 mL)and HNO3(15 mL).The solution was sonicated at 700 W in a flask for 2 h and then stirred under reflux in an oil bath at 120℃for 24 h.After the reaction,the mixture was cooled to room temperature(RT),then diluted ten times and adjusted to neutral with ammonia.The neutralized mixture was filtered with a 0.22 μm membrane and dialyzed in a dialysis bag(MWCO 3500 Da)for 72 h to remove the remaining salts and tiny fragments to obtain a CQD solution.The CQD solution exhibited a yellow emission under UV light at 365 nm.
2.3Detection of Cu2+
In a typical run,a standard 0.1 mol/L Cu2+solution was prepared by dissolving 0.125 g CuSO4· 5H2O in DI water and adjusting the volume to 5.0 mL in a volumetric flask.
A fixed concentration of CQDs(0.02 mg/mL)was transferred to a fluorescent cuvette.The fluorescence intensity of the solution was recorded from 435 to 800 nm with an excitation wavelength of 420 nm.The fluorescence intensity of the solution was then recorded according to the amount of added Cu2+.Similar procedure was performed for various predetermined concentrations of Cu2+,EDTA and other metal ions.For the sake of comparison,the volume of CQD solution was fixed at 2 mL before the addition of Cu2+.All measurements were made at RT.
In this experiment,two kinds of water,i.e.natural water obtained from Tangdao Bay of Qingdao,Shandong province,China and tap water from our lab were used to evaluate the CQD-based probe for Cu2+detection.Before analysis,natural water was firstly filtered through a 0.22 μm filtered membrane,then spiked Cu2+at different concentrations.The tap water was spiked with different concentrations of Cu2+solutions directly without any pretreatment.
2.4Characterization
All fluorescence measurements were carried out withaF-97Profluorescencespectrophotometer(Shanghai Lengguang Technology Co.,Ltd.,China).UV-Vis absorption spectra were measured by a GoldSpectrumlab54UV/Visspectrophotometer(Shanghai Lengguang Technology Co.,Ltd.,China).The transmission electron microscopic(TEM)image was obtained by the JEOL JEM-2100UHR microscope with an accelerating voltage of 200 kV.Fourier transform infrared(FT-IR)spectra were recorded on a Nicolet 6700 spectrometer.The time decay was measured on a Fluoro Max-4 fluorometer(Horiba Jobin Yvon Inc,France).An excitation wavelength of 405 nm was used and emission was collected at 510 nm.
3 Results and discussion
3.1Preparation and characterization of the CQDs
With the help from mixed strong acids and ultrasonic treatment for 24 h,yellow fluorescent CQDs could be directly obtained from petroleum coke without any further passivation or modification.The yellow fluorescence intensity of the CQDs was strong enough for Cu2+detection,and the quantum yield of as-prepared CQDs was as high as 9.8%.
Fig.1a shows the TEM image of the CQDs,which exhibits a spherical shape with a narrow size distribution.The corresponding particle size distribution of CQDs in Fig.1b indicates that their diameters are in the range of 2.0-4.5 nm(averaged 2.8 nm).
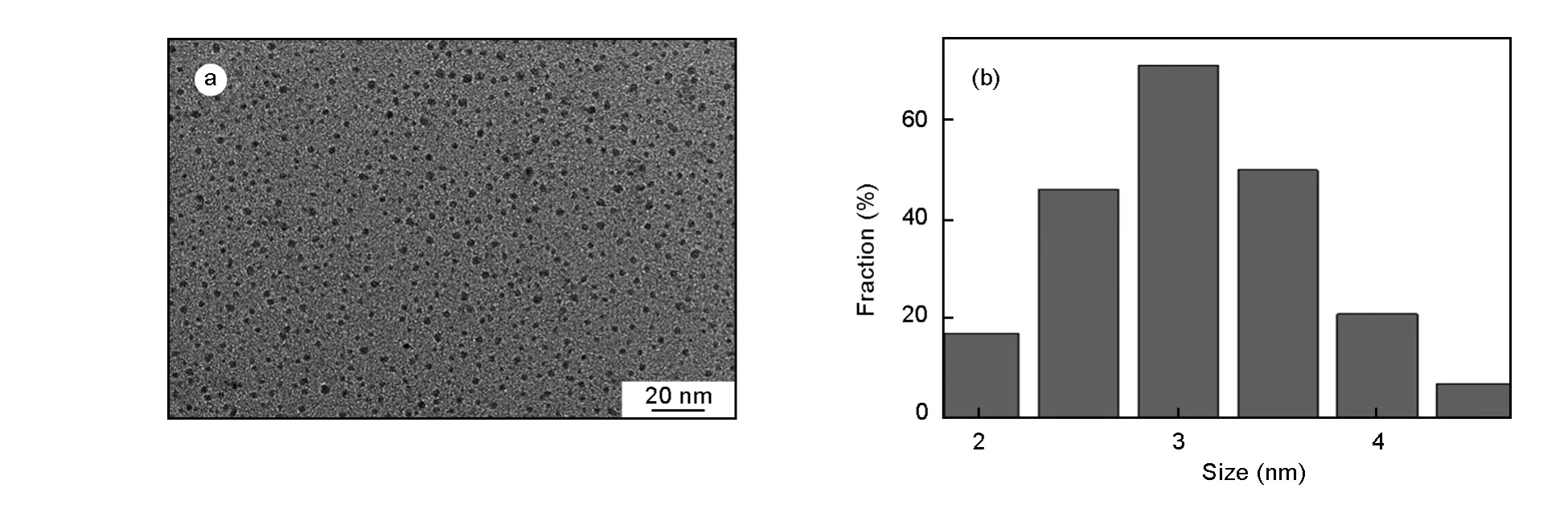
Fig.1(a)TEM image and(b)the corresponding particle size distribution of CQDs.
The FT-IR spectra of petroleum coke and the CQDs are shown in Fig.2.For petroleum coke,no obvious peaks are found except the small peaks at 2 918 and 2 857 cm-1,which are originated from —CH2vibration.However,rich hydrophilic groups including carboxyl and hydroxyl groups are found in CQDs.The typical peak at 3 441 cm-1is assigned to —OH group and the small peaks at 2 918 and 2 857 cm-1belongs to—CH2.The peaks at 1 608 and 1 763 cm-1arise from the stretching vibration of C==Oband in carboxylic moiety.Peak around 1 439 cm-1belongs to C—OH or C—H stretchingvibration,and peak at 1 128 cm-1can be identified as C—Ostretchingvibration[24,25].Obviously,the abundant hydrophilic groups help to greatly improve aqueous solubility of the CQDs,which are beneficial for their applications in water system.
The optical properties of as-prepared CQDs were further explored.The UV—Vis absorption spectrum and fluorescence spectra of the CQDs are shown in Fig.3.The UV—Vis absorption spectrum of the aqueous CQDs(Fig.3a)shows an absorption band at ca.228 nm,attributing to π-π*electron transition of graphitic sp2domains[26].The normalized fluorescence intensities of the CQDs are plotted in Fig.3b.With the excitation wavelength increases from 300 to 580 nm,the emission peak red-shifts from 480 to 600 nm,indicating the excitation-dependent luminescent behavior of the CQDs.This may be associated with the aromaticC==Cbonds and surface defects resulted from C—OH andC==Ogroups in the CQDs[27,28].
Fig.4a depicts the comparison of fluorescent spectra before and after 6 months.As shown in Fig.4a,the fluorescence intensity of the CQDs remains unchangedfor6months,suggestingthestable fluorescent properties of the CQDs.As molecular oxygen is regarded as one of the best-known collisional quenchers for fluorescent materials[19],the effect of dissolved oxygen on Cu2+detection via the CQD probe was investigated.The dissolved oxygen was removed by filling N2into cuvette for 30 min,detection result without oxygen was then obtained.Fig.4b shows that the fluorescence intensities of the CQD solutions are almost the same before and after the removal of dissolved oxygen.Therefore,in our case,the effect of dissolved oxygen can be neglected in Cu2+detection via the CQD probe,which is an advantage for CQD probing,especially in oxygen existing circumstance.

Fig.2 FT-IR spectra of CQDs and petroleum coke.

Fig.3(a)UV-Vis absorption spectrum and(b)normalized fluorescence spectra of CQDs.

Fig.4(a)The stability of CQDs and(b)the effect of dissolved oxygen.
3.2Detection of Cu2+
To confirm the quenching is solely caused by Cu2+and not due to the associated anions,various salts with different types of anions were added and investigated(Fig.5a).All solutions containing Cu2+and different salts show similar quenching effect,proving that Cu2+is responsible for fluorescence quenching of the CQDs(Fig.5b).To prove that the quenching is not due to a synergistic effect of ions and their counter anions,the similar experiments were carried out by various Na+salts substituting above mentioned Cu2+salts(Fig.5c).Interestingly,none of the Na+salts show fluorescence quenching of the CQDs,further confirming that it is Cu2+that solely cause the fluorescence quenching of the CQDs.(Fig.5d).

Fig.5(a,b)Fluorescent intensity responses of CQDs towards different salt solutions of Cu2+(50 μmol/L)and(c,d)Na+(50 μmol/L).
The fluorescence intensity of the CQD solution versus scan time at 513 nm of emission wavelength are given in Fig.6.After a fast titration of Cu2+,the fluorescence intensity at 513 nm immediately decreases to the lowest value within 3 s,then reaches a constant value,which indicates a fast and completed reaction between CQDs and Cu2+.It is noted that the response time is 3 s,which is the fastest response probe compared to other CQD probes reported in literatures[18,19,29,30].Therefore,the on-line detection and “mix-and-detect”protocol can be realized.
The insets of Fig.7a show the photographs of the CQD aqueous solution(left)and the CQDs with 50 μmol/L Cu2+(right)under UV light.Compared with other CQD probes(blue emission)[18,19,31],petroleum coke-based CQDs prepared here show a long wavelength(yellow fluorescence),which could be easier to be distinguished by naked eyes.After the titration of Cu2+,it can be clearly seen that the yellowvisual fluorescent CQDs is effectively quenched by Cu2+.The fluorescence intensity(F/F0,F0and F are the fluorescence intensities before and after the addition of Cu2+)gradually decreases by about 93%of its initial value when the concentration of added Cu2+increases to 50 μmol/L(Fig.7b).Particularly,with the addition of Cu2+,an obvious blue shift is observed(Fig.7a).The fluorescence intensity of the CQDs versus Cu2+concentration in the range of 0.25-10 μmol/L exhibits a good linearity(inset of Fig.7b).Under the current experimental conditions,the LOD of Cu2+is estimated to be 0.029 5 μmol/L based on 3Sb/k(here Sbis the standard deviation of the corrected blank signals of CQDs and k is the slope of the calibration curve),which can meet the limit of Cu2+in drinking water(20 μmol/L)set by U.S.Environmental Protection Agency[6],and is comparable to or even better than most previous reported nanoparticle-based probes[19,32-34].Recently,modified carbon dots or doped CQDs have been reported with a high LOD[18,30,35,36],which make us believe that the LODof as-made CQDs from petroleum coke can be increased in near future in our lab.
The different F/F0ratios of the CQD solutions in the absence and presence of various metal ions were also calculated.The F/F0ratios of other metal ions are almost 4-5 times as high as that of Cu2+.In order to assess the selectivity of the proposed probing method on Cu2+,the possible interferences of coexisting cations in Cu2+aqueous solution were also tested(Fig.8).Those employed ions include Ba2+,Na+,Mg2+,Zn2+,Ca2+,Sn4+,K+,Mn2+,Cd2+,Al3+,As3+and Cr6+common in our living environment.Notably,Cu2+can substantially quench the fluorescence intensity of the CQDs,and the effect of other metal ions can be neglected.Such results directly illustrate that the CQDs can be developed as a rapid response fluorescent probe for detection of Cu2+with a high sensitivity and selectivity.

Fig.6 The fluorescence intensity of CQD solution versus scan time at room temperature(inset:the enlarged drawing of selected area just after the addition of Cu2+)(excitation at 420 nm,emission at 513 nm;[Cu2+]=25 μmol/L).

Fig.7(a)Fluorescence spectra of CQDs in the presence of Cu2+concentrations ranging from 0 to 50 μmol/L.(inset:photographs of CQD aqueous solution(left)and CQDs with 50 μmol/L Cu2+(right)under UV light).(b)Fluorescence intensity response of CQDs to the concentration of Cu2+(inset:fluorescence intensity response of CQDs versus the concentration of Cu2+from 0.25 to 10 μmol/L).

Fig.8 Selective fluorescence intensity responses of aqueous CQD solution towards 50 μmol/L non-copper metal ions(gray bars),and the mixed solution of 50 μmol/L Cu2+and other metal ions(red bars)(excitation at 420 nm).
3.3Possible sensing mechanism
The sensing mechanism is not yet completely understood,even there are several suggested mechanisms in literature[37-39],such as inner filter effect,non-radiative recombination pathways,electron transfer process,ion binding interaction and hole-trap.
Herein,a photoinduced electron transfer(PET)mechanism is proposed to explain the fluorescence quenching of the CQDs caused by Cu2+.The photoluminescence excitation(PLE)and fluorescence spectra of the CQDs have been detected in the absence and presence of Cu2+.
CQDs with conjugate aromatics and many oxygen-containing groups are considered to be the electron donors.Due to the unfilled d shell,Cu2+are regarded as electron acceptors,which have a higher binding affinity and faster chelating kinetics with-COO-on the surface of the CQDs than other transition-metal ions[35].After the addition of 50 μM Cu2+into CQD aqueous solutions,the strong fluorescence intensity of the CQDs is quickly quenched and the corresponding PLE intensity is simultaneously reduced(Fig.9a).These phenomena indicate that there iselectron transfer from the CQDs to Cu2+at the excited state[34].To further implore the energy transfer and exciton recombination process of the CQDs in the presence and absence of Cu2+,the fluorescence decays of the CQDs by a time-correlated single-photon counting(TCSPC)in the absence and presence of Cu2+have been measured(Fig.9b).The fluorescence lifetime of the CQDs(the black line)is 3.86 ns,reflecting a fast exciton recombination process.After the addition of Cu2+(the red line),the lifetime of the CQDs decreases to 2.87 ns.This significantly shortened lifetime further confirms that there is an ultrafast electron transfer process in the CQDs-Cu2+system.
In addition,EDTA is a kind of metal ion chelators,which can prevail over the CQDs,and form coordination complex with Cu2+[40].Cu2+-quenched CQD aggregates could be dissociated after the introduction of EDTA because Cu2+display a higher affinity to the oxygen-donor atoms in EDTA than the carboxylate groupsandconjugatearomaticsofthe CQDs[41].When EDTA(a strong Cu2+chelator)was added into the Cu2+-quenched CQDs solution,the fluorescence intensity of the CQDs can recover as high as 96.0%(Fig.10a).Moreover,repetitive experiments on the same CQD solution by added EDTA or Cu2+three times were also measured.It is easily seen that the fluorescence intensity of the CQDs can recover to 94.4%in the third cycle,indicating that CQDs are reusable(Fig.10b).
Fig.11 illustrates the“off-to-on”mechanism of CQDs-Cu2+-EDTA.Based on the above detection mechanism and“mix-and-detect”protocol,CQD probe can be used for the detection of Cu2+in real water.Meanwhile,the proposed“off-to-on”mechanism of CQDs-Cu2+-EDTA may aid in the development of serial fluorescent probes for the detection of different metal ions.
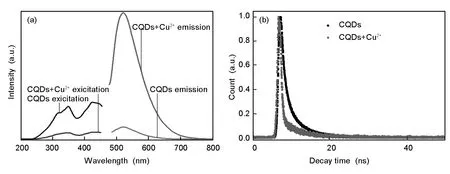
Fig.9(a)Comparisons of PLE and fluorescence spectra of CQDs with and without 50 μmol/L Cu2+,and(b)fluorescence decay curves of CQDs by TSCPC in the absence and presence of Cu2+.

Fig.10(a,b)Repetitiveness of the fluorescence intensity responses of aqueous CQD solution([Cu2+]=[EDTA]=25 μmol/L,excitation at 420 nm).
3.4The feasibility of CQDs for sensing Cu2+in real water
Fig.12 shows the fluorescence response of the CQDs in natural water(Fig.12a)and tap water(Fig.12c)with different concentration of Cu2+.The relationship between F0/F-1and the concentration of Cu2+of natural water(Fig.12b)and tap water(Fig.12d)are also given.It can be clearly seen that the fluorescence intensity gradually decreases with the concentration of Cu2+both in tap water and naturalwater.The calibration curve for determining Cu2+in tap water and natural water can be obtained in the range of 0-50 nmol/L.In spite of the interference from numerous minerals and organics/inorganics possibly existed in different water sources,the lowest detection concentration of Cu2+via the CQDs can reach 5 nmol/L,which meet the requirement of the Cu2+detection in real water.These results make us believe that CQD-based probe can be applied for an accurate analysis of Cu2+in a wide range including natural water and tap water.

Fig.11 Mechanism schematic of Cu2+detection via the fluorescence response of CQDs.
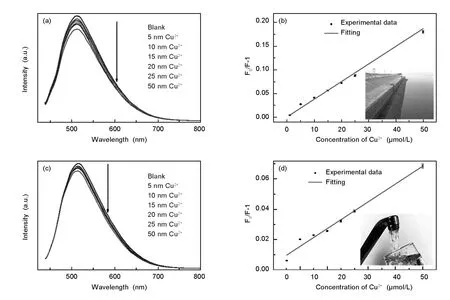
Fig.12 CQDs to detect Cu2+in real water:fluorescence spectra of CQDs in the presence of(a)natural water and(c)tap water with different concentrations of Cu2+.Relationship between F0/F-1and the concentration of(b)Cu2+of natural water,and(d)tap water(excitation at 420 nm).
4 Conclusions
In summary,a simple and effective yellow-visual fluorescent CQD probe for Cu2+detection was developed.CQDs were prepared from petroleum coke by an ultrasonic-assisted chemical oxidation method without any subsequent chemcial midifcation.The reusable as well as“mix-and-detect”CQD probe with a unprecedentedly rapid response compared with reported CQDs,is highly selective and hardly interfered by other metal ions.The CQD is fluorescence turn-off probe for a reliable detection of Cu2+in water with aresponse time as fast as 3 s,evidenced by the analysis of real water samples.The fluorescence intensity of the CQDs versus the concentration of Cu2+has a very good linearity from 0.25 to 10 μmol/L,and the detection limit is as low as 0.029 5 μmol/L.Theoretically,the detection mechanism of Cu2+via CQD probe is basically originated from a photoinduced electron transfer.The petroleum coke-based CQDs hold a great promise for real-world sensing applications.
[1]Sung T W,Lo Y L.Highly sensitive and selective sensor based on silica-coated CdSe/ZnS nanoparticles for Cu2+ion detection [J].Sensors and Actuators B-Chemical,2012,165(1):119-125.
[2]Liu J,Lu Y.A DNA zyme catalytic beacon sensor for paramagnetic Cu2+ions in aqueous solution with high sensitivity and selectivity[J].Journal of the American Chemical Society,2007,129(32):9838-9839.
[3]Poursaberi T,Hajiagha-Babaei L,Yousefi M,et al.The synthesis of a new thiophene-derivative schiff's base and its use in preparation of copper-ion selective electrodes[J].Electroanal,2001,13(18):1513-1517.
[4]Zhao Y,Zhang X B,Han Z X,et al.Highly sensitive and selective colorimetric and off-on fluorescent chemosensor for Cu2+in aqueous solution and living cells[J].Analitical Chemistry,2009,81(16):7022-7030.
[5]Piacenti da Silva M,Araujo Domingues Zucchi OL,Ribeiro-Silva A,et al.Discriminant analysis of trace elements in normal,benign and malignant breast tissues measured by total reflection X-ray fluorescence[J].Spectrochimica Acta Part B,2009,64(6):587-592.
[6]Zhang J F,Zhou Y,Yoon J,et al.Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions(silver,gold and platinum ions)[J].Chemical Society Reviews,2011,40(7):3416-3429.
[7]Balogh I S,Ruschak M,Andruch V,et al.An investigation of the reaction of copper ions with dimethylindodicarbocyanine dye:An application for the determination of Cu(I),Cu(II)and Cu(III)[J].Talanta,2008,76(1):111-115.
[8]Royzen M,Dai Z,Canary J W.Ratiometric displacement approach to Cu(II)sensing by fluorescence[J].Journal of the A-merican Chemical Society,2005,127(6):1612-1613.
[9]Li P,Duan X,Chen Z,et al.A near-infrared fluorescent probe for detecting copper(II)with high selectivity and sensitivity and its biological imaging applications[J].Chemical Communication,2011,47(27):7755-7757.
[10]Li Y,Zhang X,Zhu B,et al.A simple but highly sensitive and selective colorimetric and fluorescent probe for Cu2+in aqueous media[J].Analyst,2011,136(6):1124-1128.
[11]Xie H Y,Liang J G,Zhang Z L,et al.Luminescent CdSe-ZnS quantum dots as selective Cu2+probe[J].Spectrochimica Acta Part A,2004,60(11):2527-2530.
[12]Jung H S,Kwon P S,Lee J W,et al.Coumarin-derived Cu2+-selective fluorescence sensor:Synthesis,mechanisms,and applications in living cells[J].Journal of the American Chemical Society,2009,131(5):2008-2012.
[13]Eggeling C,Volkmer A,Seidel C A.Molecular photobleaching kinetics of rhodamine 6G by one-and two-photon induced confocal fluorescence microscopy[J].Chem Phys Chem,2005,6(5):791-804.
[14]Freeman R,Finder T,Willner I.Multiplexed analysis of Hg2+and Ag+ions by nucleic acid functionalized CdSe/ZnS quantum dots and their use for logic gate operations[J].Angewandte Chemie International Edition,2009,48(42):7818-7821.
[15]Xu X,Ray R,Gu Y,et al.Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments [J].Journal of the American Chemical Society,2004,126(40):12736-12737.
[16]Ponomarenko L,Schedin F,Katsnelson M,et al.Chaotic dirac billiard in graphene quantum dots[J].Science,2008,320(5874):356-358.
[17]Peng J,Gao W,Gupta BK,et al.Graphene quantum dots derived from carbon fibers[J].Nano Letters,2012,12(2):844-849.
[18]Liu S,Tian J,Wang L,et al.Hydrothermal treatment of grass:A low-cost,green route to nitrogen-doped,carbon-rich,photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-fee detection of Cu(II)ions[J].Advanced Materials,2012,24(15):2037-2041.
[19]Wang F,Gu Z,Lei W,et al.Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II)ions[J].Sensors and Actuators B-Chemical,2014,190:516-522.
[20]Christina C S,Zaher H,Ania C U.Preparation and characterization of activated carbon from oil sands coke[J].Fuel,2012,92(1):69-76.
[21]Jiang B C,Zhang Y C,Zhou J X.Effects of chemical modification of petroleum cokes on the properties of the resulting activated carbon[J].Fuel,2008,87(10):1844-1848.
[22]Wu M B,Zha Q F,Qiu J S,et al.Preparation of porous carbons from petroleum coke by different activation methods[J].Fuel,2005,84(14):1992-1997.
[23]Wu M B,Wang Y,Wu W T,et al.Preparation of functionalized water-soluble photoluminescent carbon quantum dots from petroleum coke[J].Carbon,2014,78(11):480-489.
[24]Wang J,Wang C F,Chen S.Amphiphilic egg-derived carbon dots:Rapid plasma fabrication,pyrolysis process,and multicolor printing patterns[J].Angewandte Chemie International Edition,2012,51(37):9431-9435.
[25]Liu L,Li Y,Zhan L,et al.One-step synthesis of fluorescent hydroxyls-coated carbon dots with hydrothermal reaction and its application to optical sensing of metal ions[J].Science China Chemistry,2011,54(8):1342-1347.
[26]Zhang X,Wang S,Liu M,et al.Size tunable fluorescent nanographite oxides:Preparation and cell imaging applications[J].Physical Chemistry Chemical Physics,2013,15(43):19013-19018.
[27]Li M,Cushing S K,Zhou X,et al.Fingerprinting photoluminescence of functional groups in graphene oxide[J].Journal of Materials Chemistry,2012,22(44):23374-23379.
[28]Zhu S,Zhang J,Tang S,et al.Surface chemistry routes to modulate the photoluminescence of graphene quantum dots:From fluorescence mechanism to up-conversion bioimaging ap-plications[J].Advanced Functional Materials,2012,22(22):4732-4740.
[29]Lu W,Qin X,Liu S,et al.Economical,green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II)ions[J].Analytical Chemistry,2012,84(12):5351-5357.
[30]Hu C,Yu C,Li M Y,et al.Chemically tailoring coal to fluorescent carbon dots with tuned size and their capacity for Cu(II)detection[J].Small,2014,10(23):4926-4933.
[31]Liu R,Li H T,Kong W Q,et al.Ultra-sensitive and selective Hg2+detection based on fluorescent carbon dots[J].Materials Research Bulletin,2013,48(7):2529-2534.
[32]Liu Y S,Zhao Y N,Zhang Y Y.One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II)ion detection[J],Sensors and Actuators B-Chemical,2014,196:647-652.
[33]Liu X J,Zhang N,Bing T,et al.Carbon dots based dual-emission silica nanoparticles as a ratiometric nanosensor for Cu2+[J].Analytical Chemistry,2014,86(5):2289-2296.
[34]Hu S,Zhao Q,Dong Y,et al.Carbon-dot-loaded alginate gels as recoverable probes:Fabrication and mechanism of fluorescent detection[J].Langmuir,2013,29(40):12615-12621.
[35]Sun H,Gao N,Wu L,et al.Highly photoluminescent aminofunctionalized graphene quantum dots used for sensing copper ions[J].Chemistry-A European Journal,2013,19(40):13362-13368.
[36]Yang S W,Sun J,Li X B,et al.Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection[J].Journal of Materials Chemistry A,2014,2(23):8660-8667.
[37]Chen Y F,Rosenzweig Z.Luminescent CdS quantum dots as selective ion probes[J].Analytical Chemistry,2002,74(19):5132-5138.
[38]He Q W,Miller E W,Wong A P,et al.A selective fluorescent sensor for detecting lead in living cells[J].Journal of the American Chemical Society,2006,128(29):9316-9317.
[39]Sun H J,Wu L,Wei W L,et al.Recent advances in graphene quantum dots for sensing[J].Material Today,2013,16(11):433-442.
[40]Liu J M,Lin L P,Wang X X,et al.Highly selective and sensitive detection of Cu2+with lysine enhancing bovine serum albumin modified-carbon dots fluorescent probe[J].Analyst,2012,137(11):2637-2642.
[41]Bai J M,Zhang L,Liang R P,et al.Graphene quantum dots combined with europium ions as photoluminescent probes for phosphate pensing[J].Chemistry-A European Journal,2013,19(12):3822-3826 .
Yellow-visual fluorescent carbon quantum dots from petroleum coke for the efficient detection of Cu2+ions
WANG Yue1,WU Wen-ting1,WU Ming-bo1,SUN Hong-di1,XIE Hui1,HU Chao2,WU Xue-yan1,QIU Jie-shan2
(1.State Key Laboratory of Heavy Oil Processing,China University of Petroleum,Qingdao266580,China;2.State Key Laboratory of Fine Chemicals,School of Chemical Engineering,Dalian University of Technology,Dalian116024,China)
Yellow-visual fluorescent carbon quantum dots(CQDs)were prepared from petroleum coke by ultrasonic-assisted chemical oxidation andwere used as label-free probes for Cu2+detection in water.The detection of Cu2+by CQD probesis related to a quenching of fluorescence by a photo induced electron transfer mechanism.The quenched fluorescence of the CQDs by Cu2+can be recovered by adding ethylene diaminetetraacetic acid.The yellow-visual fluorescent CQD probes have a linear detection range from 0.25 to 10 μM,a detection limit of 0.029 5 μM,a response time of 3 s,and a superior sensitivity and selectivity for Cu2+detection compared to other fluorescent probes.The CQDs are easy to prepare,economical,reusable,fast to respond and can be used inonline detection.
Petroleum coke;Carbon quantum dots;Yellow-visual;Fast response;Cu2+detection
date:2015-10-12;Revised date:2015-12-09
National Natural Science Foundation of China(51372277,51372028,21302224);Fundamental Research Funds for the Central Universities(14CX02060A,15CX08005A).
s:WU Ming-bo,Professor,E-mail:wumb@upc.edu.cn;
introduction:These authors contributed equally to this work.WANG Yue,Master Student,E-mail:wy163tan@163.com;
1007-8827(2015)06-0550-10
O613.71;O433.2
A
国家自然科学基金(51372277,51372028,21302224);中央高校基本科研业务费专项资金(14CX02060A,15CX08005A).
吴明铂,教授.E-mail:wumb@upc.edu.cn;
邱介山,教授.E-mail:jqiu@dlut.edu.cn
作者介绍:王 月,硕士研究生.E-mail:wy163tan@163.com;吴文婷,讲师,E-mail:wuwt@upc.edu.cn
QIU Jie-shan,Professor,E-mail:jqiu@dlut.edu.cn
WU Wen-ting,Lecturer,E-mail:wuwt@upc.edu.cn
10.1016/S1872-5805(15)60204-9
English edition available online ScienceDirect(http://www.sciencedirect.com/science/journal/18725805).
