GO/MOF复合材料的制备及其吸附苯和乙醇性能
2015-10-24刘国强王明玺黄正宏康飞宇
刘国强,王明玺,黄正宏,康飞宇
(1.清华大学材料学院,先进材料教育部重点实验室,北京100084;2.武汉工程大学化学与环境工程学院,绿色化工过程教育部重点实验室,湖北武汉430074;3.清华大学深圳研究生院,先进材料研究所,广东深圳518055)
GO/MOF复合材料的制备及其吸附苯和乙醇性能
刘国强1,王明玺2,黄正宏1,康飞宇1
(1.清华大学材料学院,先进材料教育部重点实验室,北京100084;2.武汉工程大学化学与环境工程学院,绿色化工过程教育部重点实验室,湖北武汉430074;3.清华大学深圳研究生院,先进材料研究所,广东深圳518055)
采用溶剂热法制备了金属有机骨架-氧化石墨烯(MOF/GO)复合材料,通过氮吸附/脱附、红外光谱对其比表面积和孔结构、表面官能团进行了表征,考察了其吸附苯和乙醇的性能。结果表明,当氧化石墨烯的添加量为5.25 wt%时,复合材料的比表面积和孔容最大。该材料对苯和乙醇有很高的吸附容量,其最大吸附容量可分别达到72和77 cm3/g。MOF-5/GO复合材料吸附挥发性有机物(VOCs)的容量不仅受孔结构的影响,其表面特性也对吸附性能有重要作用。氧化石墨烯含量为3.5 wt%的GO/MOF复合材料对乙醇的吸附容量显著增强是由于其含有大量的含氧官能团。
金属有机骨架化合物(MOF-5);氧化石墨烯(GO);吸附;苯;乙醇
1 Introduction
Volatile organic compounds(VOCs)are pollutants present in gas and/or liquid streams of many industrial applications,such as chemical industry(biocides,plastics and solvents),automotive and aerospace industry[1],dry cleaning solvents in the garment industry,and solvent cleaning in the electronic industry.They are very harmful for both human health and environment,even at very low concentrations.It can be remarked that they are:(1)agents that destroy the ozone stratospheric layer,(2)precursors of photochemical oxidants,(3)agents of the acid rain,(4)elements of the climatic change,(5)agents that affect the nervous system and(6)carcinogenic and mutagenic agents[2].Thus,it is important to minimize their use,or to find new materials that can adsorb or mineralize them via environmental friendly catalysts and/or adsorption.
One of the most useful methods to remove VOCs is the adsorption technique.For adsorption technologies ranging from gas separations to gas storage,selection of the proper solid adsorbents is the key to design an efficient adsorption process.Microporous and mesoporous adsorbents have been frequently used for the removal of VOCs.To examine the practicality of a solid adsorbent,the following characteristics must be considered:porosity,structural stability,reversible uptake and release,and capability for surface modification for creating molecule-specific adsorption sites[3].Removal of VOCs by adsorption process have been performed on several types of adsorbents,which includes carbon materials[4,5],surfactant modified zeolites[6,7],silica aerogels[8],silicalite[9],organic minerals[10],etc.As far as we know,some disadvantages such as low adsorption capacity,flammability and other problems associated with regeneration for most common porous materials are encountered in practical application[11].Therefore,much attention has been paid to new porous materials with a high adsorption capacity.
Among various adsorbents,metal-organic frameworks(MOFs)are a group of materials,which have had a rapid development and opened new possibilities of applications owing to their excellent properties such as high surface area,high porosity,regular structure,modifiable surfaces and tunable pore size[12,13].In spite of the very high porosities of MOFs,their open framework is not able to provide strong,non-specific adsorption forces to retain small molecules at ambient conditions.Therefore,a surface consisting of a dense arrangement of atoms and a porous network is needed[14].To meet the above requirements,graphite oxide(GO)was selected as another component to prepare GO/MOF nano-composites by Bandosz's group,who also studied the ammonia[15-19],hydrogen sulfide[20]and NO(2)[21]reactive adsorption behavior oftheas-preparedGO/MOFcomposites.There are also many papers on the adsorption of various VOCs on Zn-based metal-organic frameworks MOF-5[22,23].However,as far as we know,there are still few works on the adsorption of VOCs on the GO/MOF-5 composites.
In the Bandosz's experiments,GO powder prepared by Hummer's method was added in the well-dissolved zinc nitrate/1,4-benzenedicarboxylate(BDC)mixture,and the resulting suspensions were subsequently subjected to the same synthesis procedure as for MOF-5.As an extension of this work,we made some improvement of the synthesis procedure to get GO/MOF-5 composites,and also measured the benzene and ethanol adsorption properties of the samples.
2 Experimental
2.1Synthesis of GO/MOF composites
GO synthesis:GO was prepared from natural graphite powder according to a modified Hummers method[24].46 mL of H2SO4(98%)was placed in a flask immersed into an ice bath.Graphite(2 g)were then added to the flask and stirred vigorously.Next,KMnO4(6 g)was slowly added into the flask,and the reaction temperature was then maintained below 20℃in an ice bath for about 30 min.The flask containing the reaction mixture was then transferred to a water bath at a temperature of 35℃,and the reaction mixture was stirred for about 45 min until a thick paste formed.Water(46 mL)was then added,the reaction temperature was increased to 90℃,and the reaction mixture was stirred for about 30 min.Finally,280 mL water was added into the mixture,followed by a slow addition of 10 mL of 30%aq.H2O2.A yellow dispersion was obtained and washed repeatedly with deionized water to remove the remaining salt until the pH reached about 7,and the solid was then dried under vacuum(50℃)for about 3 days.
MOFsynthesis:zincnitratehexahydrate(5.2 g)and 1,4-benzenedicarboxylate(1.0 g)were mixed in 35 mL of DMF.The mixture was treated solvothermally at 120℃for 25 hours.The obtained sample was washed with DMF and CHCl3,and MOF was obtained by vacuum drying at 80℃.
GO/MOF synthesis:GO was dispersed in N,N-dimethylformamide(DMF)to form GO solutions by sonication.The GO/MOF composites were prepared according to the preparation method of MOF-5[15].In a typical reaction,zinc nitrate hexahydrate(5.2 g),BDC(1.0 g)and glucose(0.5 g)were mixed in a 35 mL of GO/DMF solution.The mixture was treated solvothermally at 120℃for 25 hours.The obtained sample was washed with DMF and CHCl3,and MGs were obtained by vacuum drying at 80℃.Samples with GO weight percentages of 1.75%,3.5%,5.25%and 7%were obtained by changing the concentration of the GO in DMF of the solutions,and the samples arereferredtoasMGn(n=1-4),respectively.
2.2Characterization of materials
Thenitrogenadsorption-desorptionwasperformed at-196℃using a gas adsorption analyzer(BELsorp-max,Japan).The specific surface area was evaluated using BET method.The Density Functional Theory(DFT)was used to determine the pore size distributions(PSDs).The morphologies of the samples were examined by a LEO 1 530(LEO,Oberkochen,Germany)field emission scanning electron microscope(SEM).Mid-IR spectra(4 000-500 cm-1)were collected on a Nicolet 560 FT-IR spectrometer using pellets with samples dispersed in KBr.X-ray diffraction(XRD)patterns were obtained using a X-ray diffractometer(Rigaku D/max-2500)with Cu-Ka(40 kV,40 kA)radiation.The data were recorded over a 2 range of 5-90°.
2.3Adsorption of benzene and ethanol
The adsorption-desorption of benzene and ethanol vapor were measured using a BEL sorp-max at 30℃.All the samples were degassed at 150℃for 12 h prior to the adsorption measurements.The adsorption and desorption time at each p/p0was set at 300 s and the measured p/p0range was from 0 to 0.95.
3 Results and discussion
The pore size distributions(PSDs)for various samples evaluated by DFT are shown in Fig.1,the textual parameters are listed in Table 1,and the nitrogen adsorption-desorption isotherms were provided in our previous work[25].All the samples show a significant contribution of ultra-narrow pores of size around W=1 nm in the micropore region,especially for MGG3 with the highest differential pore volume of 2.0 cm3/g.For MG4,some large mesopores appear at 20-30 nm,which is attributed to the“tail”of the isotherms at high relative pressure.
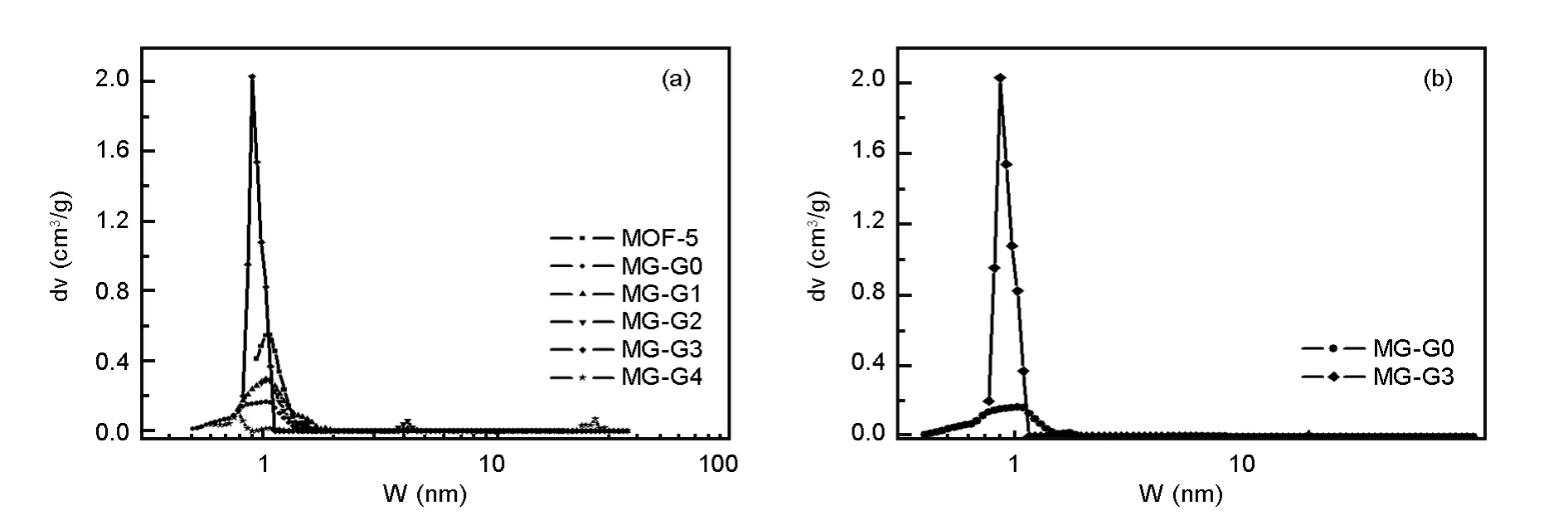
Fig.1(a),(b)DFT pore size distributions of GO,MGs and MOF-5.
From Table 1,it can be seen that the surface area of MG1 and MG2 are lower than MOF-5,and it may be related to the blockage of pores by GO with a layer structure,which can be proved by the hysteresis loop H4,a characteristic of slit pores[26].In addition,blockage by carbons derived from partially solvothermal of glucose may be another reason,leading to a dramatically decrease of surface area.Further increase of the GO percentage to 5.25%leads to an increase of surface area,this could be attributed to the formation of graphene reduced by the glucose.The sharply decrease of surface area for MG4 may be attributed to the blockage with the carbonization products derived from hydrothermal of glucose.

Table1 Textural properties of the samples.
The FTIR spectra for the samples are shown in Fig.2a.Several bands are observed in the region 1 300-700 cm-1,and they are assigned to the out-ofplane vibrations of BDC.The bands in the region 750-75 cm-1are assigned to aromatic C—H out-ofplane bending vibrations[27],the bands in the 1 000-1 450 cm-1to C—O(hydroxyl,ester,or ether) stretching and O—H bending vibrations[28],the band at 1 390 cm-1to the symmetric stretching of carboxylic groups,those at 1 510 and 1 590 cm-1to the asymmetric stretching of carboxylic groups[16],the broad band at 3 000-700 cm-1to the overlapping bands from O-(hydroxyl or carboxyl),and the bands at 2 855 and 2 922 cm-1to stretching vibrations of aliphaticC—H[15,29].These results indicate that there are a large number of residues including hydroxyl and carboxyl groups on the surface of the as-prepared materials.It also can be seen that with an increase of the GO percentage,the intensity of adsorption bands,representing the amount of functional groups,becomes strong with the GO percentage up to 3.5%and then tends to be weak with a further increasing of the GO percentage.These functional groups can provide a potential avenue to load other functional groups,molecules,ions,and nanoparticles[28].As a result,it may show excellent adsorption performance for gas.

Fig.2 FT-IR spectra for GO,MOF and MGs(a)before and(b)after adsorption of VOCs.
Fig.2b shows that the IR spectra of MG2 and MG3 before and after ethanol and benzene vapor adsorption,which exhibits no significant change after the adsorption of benzene vapor.This is attributed to the similar molecular structure of benzene and BDC,which leads to an overlap for their vibration of band.A sharp bands at 3 606 cm-1is observed after ethanol adsorption for both samples,which is the stretching of O—H of gaseous ethanol[30],indicating that the ethanol vapor has been adsorbed onto the composites.
Fig.3 shows the X-ray diffraction(XRD)patterns of the various samples before and after gas adsorption.The GO spectrum shows a peak at 2θ= 12.1°,indicating an interlayer distance of 0.73 mm.It suggests that the GO has a uniform and enlarged interlayer spacing with the residual oxygenated functional groups on GO sheets[31,32].The MOF-5 diffraction pattern is in good agreement with those found for a well-defined MOF-5 crystal[33].The diffraction patterns of the GO/MOF-5 composites are similar to that observed for MOF-5,which indicates that the MOF-5 structure is preserved.With an increase of the GO percentage,a distortion of the MOF-5 component and the further collapse are observed for the composite materials,and the intensity of diffraction peaks become weaker,especially for MG4,which is attributed to a high content of GO with an amorphous structure,and this result is in good agreement with the SEM observations.After exposure to benzene and ethanol,the patterns are slightly modified,but the overall patterns are preserved.For both of MG2 and MG3,a pronounced splitting appears at 2θ≈9.7°after the adsorption of benzene and ethanol,which indicates that benzene or ethanol retained in the composites leads to a distortion of the structure of the MOF-5 component.This splitting has also been observed by petit et al,who argued that the GO component in the composites induces a distortion of the structure of the MOF-5 component,and the exposure to ammonia can lead to a further distortion.

Fig.3 X-ray diffraction patterns for the parent and composite materials(a)before and(b)after adsorption of benzene and ethanol.
The benzene and ethanol adsorption isotherms at 30℃of MGs are shown in Fig.4.It is seen that the benzene uptakes for MG1 and MG2 increase sharply at the initial part,undergoes a long plateau at intermediate relative pressures,and slowly increases at high relative pressures.The adsorption amount follows the order MG3>MG2>MG1>MG4,which is consistent with the surface area.This suggests that the benzene adsorption capacity is related to the textural properties.The steep rise of benzene uptake in low relative pressure region is not observed and the adsorption amount increases slowly with pressure for MG-G1 and MG-G4.The adsorption capacity of MGG4 is much lower than other samples,which may be attributed to the lower surface area and larger pore size.
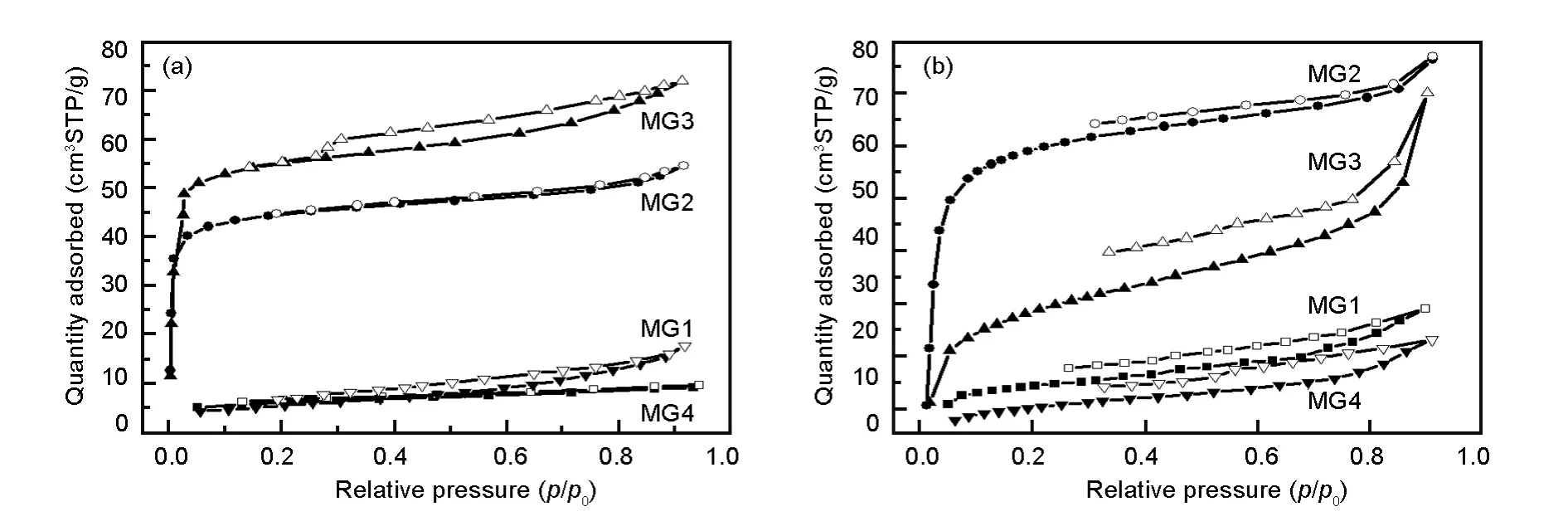
Fig.4(a)Benzene and(b)ethanol adsorption-desorption isotherms at 30℃of MGs.
For each sample,the ethanol adsorption isotherms presented in Fig.4b exhibits the similar trend as the benzene adsorption isotherms.The isotherms of MG2 and MG3 undergo a steep increase at the initial part,nearly plateau at intermediate relative pressures,and a slight rise at high pressures.For MG3,the adsorption amount increases slowly with the relative pressure,which is similar to benzene adsorption behavior.However,the adsorption capacity follows the order MG2>MG3>MG1>MG4,which is different from that of benzene adsorption.Actually,in the entire pressure region,the ethanol uptake of MG2 is higher than that of MG3,following an opposite trend as nitrogen and benzene adsorption.It is known that porous structure and surface chemistry are the two factors affecting the adsorption properties of materials.Since the surface area of MG2 is much lower than MG3,the higher ethanol adsorption capacity for MG2 would be attributed to the much more oxygencontaining functional groups arriving from the glucose modification,which enhances the the interaction of polar ethanol molecular with MG2.
It is noticeable that the adsorption capacities for MG1 are higher than MG4 for both of benzene and ethanol adsorption,but they are all lower than other samples.This indicates that the porous structure is an important factor for the adsorption of VOCs,and high surface area and pore volume could get high adsorption capacity.Therefore,the adsorption capacities of VOCs for the samples depend on both of the surface chemistry and porous properties.In light of the above findings,the question that remains unanswered is whether the increase in the adsorption of VOCs are related to the surface chemistry or to the high porosity of the composites.Nevertheless,the physical and chemical properties of the VOCs can also have a great influence on the adsorption process.Therefore,additional analysis is required to address these issues.
4 Conclusions
GO/MOF composites are synthesized by solvothermal method.Their surface area exhibits a maximum with the GO percentages at 5.25%.The materials exhibit high adsorption capacities for benzene and ethanol,and the maximum uptakes reach up to 72 and 77 cm3/g,respectively.The adsorption capacities of VOCs for the GO/MOF composites are affected by both of the porous structure and surface properties.The ethanol adsorption capacity for the GO/MOF with a GO percentage of 3.5wt%is enhanced by its abundant oxygen-containing functional groups.
[1]Yamamoto T,Kataoka S,Ohmori T.Characterization of carbon cryogel microspheres as adsorbents for VOC[J].Journal of Hazardous Materials,2010,177(1-3):331-335.
[2]Lillo-Rodenas M A,Cazorla-Amoros D,Linares-Solano A.Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations[J].Carbon,2005,43(8):1758-1767.
[3]Mu B,Walton K S.Adsorption equilibrium of methane and carbon dioxide on porous metal-organic framework Zn-BTB[J].Adsorption-JournaloftheInternationalAdsorptionSociety,2011,17(5):777-782.
[4]Diaz E,Ordonez S,Vega A.Adsorption of volatile organic compounds onto carbon nanotubes,carbon nanofibers,and high-surface-area graphites[J].Journal of Colloid and Interface Science,2007,305(1):7-16.
[5]Li L,Liu S,Liu J.Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal[J].Journal of Hazardous Materials,2011,192(2):683-690.
[6]Barakat T,Rooke J C,Tidahy H L,et al.Noble-metal-based catalysts supported on zeolites and macro-mesoporous metal oxide supports for the total oxidation of volatile organic compounds [J].Chemsuschem,2011,4(10):1420-1430.
[7]Silva B,Figueiredo H,Santos V P,et al.Reutilization of Cr-Y zeolite obtained by biosorption in the catalytic oxidation of volatile organic compounds[J].Journal of Hazardous Materials,2011,192(2):545-553.
[8]Wang D,McLaughlin E,Pfeffer R,et al.Adsorption of organic compounds in vapor,liquid,and aqueous solution phases on hydrophobic aerogels[J].Industrial&Engineering Chemistry Research,2011,50(21):12177-12185.
[9]Uguina M A,Sotelo J L,Delgado J A,et al.Adsorption of methyl ethyl ketone and trichloroethene from aqueous solutions onto silicalite fixed-bed adsorbers[J].Separation and Purification Technology,2005,42(1):91-99.
[10]Koh S M,Dixon J B.Preparation and application of organominerals as sorbents of phenol,benzene and toluene[J].Applied Clay Science,2001,18(3-4):111-122.
[11]Zhao Z,Li X,Li Z.Adsorption equilibrium and kinetics of pxylene on chromium-based metal organic framework MIL-101 [J].Chemical Engineering Journal,2011,173(1):150-157.
[12]Kitagawa S,Kitaura R,Noro S.Functional porous coordination polymers[J].Angewandte Chemie-International Edition,2004,43(18):2334-2375.
[13]Furukawa H,Ko N,Go Y B,et al.Ultrahigh porosity in metal-organic frameworks[J].Science,2010,329(5990):424-428.
[14]Petit C,Bandosz T J.MOF-graphite oxide composites:Combining the uniqueness of graphene layers and metal-organic frameworks[J].Advanced Materials,2009,21(46):4753-4757.
[15]Petit C,Bandosz T J.MOF-graphite oxide nanocomposites:Surface characterization and evaluation as adsorbents of ammonia [J].Journal of Materials Chemistry,2009,19(36):6521-6528.
[16]Petit C,Bandosz T J.Enhanced adsorption of ammonia on metal-organic framework/graphite oxide composites:Analysis of surface interactions[J].Advanced Functional Materials,2010,20(1):111-118.
[17]Petit C,Mendoza B,Bandosz T J.Reactive adsorption of ammonia on Cu-based MOF/graphene composites[J].Langmuir,2010,26(19):15302-15309.
[18]Petit C,Bandosz T J.Synthesis,characterization,and ammonia adsorption properties of mesoporous metal-organic framework(MIL(Fe))-graphite oxide composites:Exploring the limits of materials fabrication[J].Advanced Functional Materials,2011,21(11):2108-2117.
[19]Petit C,Huang L,Jagiello J,et al.Toward understanding reactive adsorption of ammonia on cu-MOF/graphite oxide nanocomposites[J].Langmuir,2011,27(21):13043-13051.
[20]Petit C,Mendoza B,Bandosz T J.Hydrogen sulfide adsorption on MOFs and MOF/graphite oxide composites[J].Chemphyschem,2010,11(17):3678-3684.
[21]Levasseur B,Petit C,Bandosz T J.Reactive adsorption of NO2on copper-based metal-organic framework and graphite oxide/ metal-organic framework composites[J].ACS Applied Materials &Interfaces,2010,2(12):3606-3613.
[22]Britt D,Tranchemontagne D,Yaghi O M.Metal-organic frameworks with high capacity and selectivity for harmful gases[J].Proceedings of the National Academy of Sciences of the United States of America,2008,105(33):11623-11627.
[23]Gu Z-Y,Jiang D-Q,Wang H-F,et al.Adsorption and separation of xylene isomers and ethylbenzene on two Zn-terephthalate metal-organic frameworks[J].Journal of Physical Chemistry C,2010,114(1):311-316.
[24]Tang Z,Shen S,Zhuang J,et al.Noble-metal-promoted threedimensional macroassembly of single-layered graphene oxide[J].Angewandte Chemie-International Edition,2010,49(27):4603-4607.
[25]Huang Z-H,Liu G,Kang F.Glucose-promoted Zn-based metal-organic framework/graphene oxide composites for hydrogen sulfide removal[J].Acs Applied Materials&Interfaces,2012,4(9):4942-4947.
[26]Rouquerol F,Rouquerol J,Sing K.Adsorption by powders and porous solids[J].London:Academic Press,1999:18-20.
[27]Lua A C,Yang T.Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell[J].Journal of Colloid and Interface Science,2004,274(2):594-601.
[28]Zheng M,Liu Y,Jiang K,et al.Alcohol-assisted hydrothermal carbonization to fabricate spheroidal carbons with a tunable shape and aspect ratio[J].Carbon,2010,48(4):1224-1233.
[29]Sevilla M,Fuertes A B.Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides[J].Chemistry-a European Journal,2009,15(16):4195-4203.
[30]Ellison M D,Morris S T,Sender M R,et al.Infrared and computational studies of the adsorption of methanol and ethanol on single-walled carbon nanotubes[J].Journal of Physical Chemistry C,2007,111(49):18127-18134.
[31]Seredych M,Tamashausky A V,Bandosz T J.Graphite oxides obtained from porous graphite:The role of surface chemistry and texture in ammonia retention at ambient conditions[J].Advanced Functional Materials,2010,20(10):1670-1679.
[32]Xu Y,Sheng K,Li C,et al.Self-assembled graphene hydrogel via a one-step hydrothermal process[J].ACS Nano,2010,4(7):4324-4330.
[33]Hafizovic J,Bjorgen M,Olsbye U,et al.The inconsistency in adsorption properties and powder XRD data of MOF-5 is rationalized by framework interpenetration and the presence of organic and inorganic species in the nanocavities[J].Journal of the A-merican Chemical Society,2007,129(12):3612-3620.
Preparation of graphene/metal-organic composites and their adsorption performance for benzene and ethanol
LIU Guo-qiang1,WAN Ming-xi2,HUANG Zheng-hong1,KANG Fei-yu1,3
(1.Laboratory of Advanced Materials,School of Materials Science and Engineering,Tsinghua University,Beijing100084,China;2.Key Laboratory for Green Chemical Process of Ministry of Education,School of Chemical and Environmental Engineering,Wuhan Institute of Technology,Xiongchu Avenue 693,Wuhan430074,China;3.Institute of Advanced Materials Research,Graduate School at Shenzhen,Tsinghua University,Shenzhen518055,China)
Graphene/metal-organic composites were synthesized by a solvothermal method and characterized by nitrogen adsorption,SEM and IR and their adsorption properties for benzene and ethanol were investigated.It was found that the surface area and pore volume both have maximum values for a graphene oxide(GO)percentage of 5.25 wt%.The composites have high adsorption capacities for both benzene and ethanol,and the maximum uptakes reach 72 and 77 cm3/g,respectively.The adsorption capacities of volatile organic compounds are determined by both the pore structure and the surface properties.The maximum ethanol adsorption capacity for the composite with a GO percentage of 3.5 wt%is due to its abundant oxygen-containing functional groups.
MOF-5;Graphene;Adsorption;Benzene;Ethanol
date:2015-10-26;Revised date:2015-12-08
National High Technology Research and Development Program of China(2010AA064907).
HUANG Zheng-hong,Ph.D,Associate Professor.E-mail:zhhuang@mail.tsinghua.edu.cn English edition available online ScienceDirect(http://www.sciencedirect.com/science/journal/18725805).
TQ127.1+1
A
国家高技术研究发展计划(2010AA064907).
黄正宏,博士,副研究员.E-mail:zhhuang@mail.tsinghua.edu.cn
1007-8827(2015)06-0566-06
10.1016/S1872-5805(15)60205-0
