党参和甘草糖复合物对lEC-6细胞迁移和膜电位的影响
2015-08-31李茹柳陶玉珠涂小华蔡佳仲陈蔚文广州中医药大学脾胃研究所广东广州510405
李茹柳,陶玉珠,温 鹏,涂小华,蔡佳仲,陈蔚文(广州中医药大学脾胃研究所,广东广州 510405)
党参和甘草糖复合物对lEC-6细胞迁移和膜电位的影响
李茹柳,陶玉珠,温鹏,涂小华,蔡佳仲,陈蔚文
(广州中医药大学脾胃研究所,广东广州 510405)
目的 观察党参糖复合物和甘草糖复合物对小肠上皮细胞IEC-6迁移和细胞膜电位的影响,探讨益气健脾中药党参和甘草促进胃肠黏膜损伤修复的作用机制。方法 在正常或钾通道抑制剂4-氨基吡啶(4-AP)负荷下,分别加入党参和甘草糖复合物(25~200 mg·L-1)与IEC-6细胞培养24 h,相差显微镜下观察细胞迁移数,流式细胞仪检测细胞膜电位。结果 与细胞正常对照组比较,党参和甘草糖复合物(50和100 mg·L-1)可提高细胞迁移数(P<0.01,P<0.05)。与正常对照组比较,4-AP可减少细胞迁移数(P<0.01);与4-AP模型组比较,党参和甘草糖复合物(50~200 mg·L-1)可逆转4-AP所致的细胞迁移抑制(P<0.01)。流式细胞仪检测结果表明,与正常对照组比较,党参和甘草糖复合物(50 mg·L-1)可提高细胞膜电位(P<0.01),增加细胞膜超极化水平;与正常对照组比较,4-AP模型组细胞膜电位降低(P<0.01),增加细胞膜去极化水平;与4-AP模型组比较,党参和甘草糖复合物(100和200 mg·L-1)可逆转4-AP所致的细胞膜去极化(P<0.01)。结论 党参和甘草促进胃肠黏膜损伤修复的作用机制,可能与其糖复合物影响小肠上皮细胞迁移的多胺介导钾通道激活信号通路有关。
党参;甘草;糖复合物;肠上皮细胞;细胞迁移;膜电位
DOl:10.3867/j.issn.1000-3002.2015.06.007
党参和甘草是益气健脾中药,也是常用的益气健脾方剂如四君子汤、补中益气汤和参苓白术散等的组成药物。临床和实验研究表明,益气健脾中药如四君子汤等有促进消化吸收、保护胃肠黏膜、促进上皮细胞生长和黏膜损伤修复等作用[1],但其疗效机制尚不十分明确。本实验室在小肠上皮细胞IEC-6上,从胃肠黏膜损伤修复环节如细胞增殖、分化和迁移等探讨益气健脾中药的作用机制[2-5],尤其在对细胞迁移影响方面进行了系列研究,发现益气健脾中药如黄芪、白术、党参和甘草的提取物有促进IEC-6细胞迁移的作用,作用机制与其影响细胞迁移多胺介导钾通道激活信号通路有关[4-12]。本研究继续围绕该信号通路,观察党参和甘草糖复合物在钾通道抑制剂4-氨基吡啶(4-aminopyridine,4-AP)负荷下,对IEC-6细胞迁移和细胞膜电位(Em)的影响。
1 材料与方法
1.1 药材、细胞、试剂和仪器
党参药材为桔梗科植物党参〔Codonopsis pilosula(Franch.)Nannf〕。的干燥根;甘草药材为豆科植物甘草(Glycyrrhiza uralensis Fisch.)的干燥根及根茎,均购自广州同康药业有限公司,由广州中医药大学中药鉴定教研室童家赟讲师鉴定。
大鼠小肠隐窝细胞IEC-6细胞(Cat.No.CRL-1592,Lot.4988325),购自ATCC。高糖DMEM、胎牛血清和青-链霉素,美国Gibco公司;精脒(spermidine,SPD)(阳性对照),美国Calbiochem公司;4-AP,美国Sigma公司;胰酶(1∶250),美国Amresco公司;膜电位荧光探针DiBAC4(3),美国Biotium公司。FACSCalibur型流式细胞仪,美国BD公司;3111型CO2培养箱,美国Thermo Scientific公司;Waters E2695高效液相色谱仪和Waters 2414示差检测器,美国Waters公司;Uvmini-1240型紫外可见分光光度计,苏州岛津公司产品。
1.2 党参和甘草糖复合物制备
党参或甘草药材饮片适量,石油醚回流1 h;药渣挥干溶剂,80%乙醇回流2次;药渣用蒸馏水回流2次,合并水提液,减压浓缩至每升含0.5 kg药材;浓缩液加无水乙醇至含醇量80%,4℃静置过夜,过滤,沉淀加水溶解,重复上述醇沉操作3次;末次所得沉淀加水溶解,以氯仿和正丁醇(5∶1)萃取,上层水相过大孔树脂,蒸馏水洗脱,至洗脱液与5倍量的95%乙醇混合不产生浑浊,洗脱液加无水乙醇至含醇量80%,所得沉淀以95%乙醇、无水乙醇、丙酮、乙醚洗涤,真空干燥,4℃保存。临用前加PBS配制5 g·L-1党参或甘草糖复合物,0.22 μm滤膜过滤。苯酚-硫酸法分光光度计测得党参和甘草糖复合物平均含量(以葡萄糖计)分别为41.0%和49.3%。实验浓度以糖复合物计算。
1.3 党参和甘草糖复合物高效液相图谱检测
精密称量党参糖复合物和甘草糖复合物各10 mg,分别在定容瓶用纯水溶解定容至10 mL,0.45 μm微孔滤膜过滤,装入试剂瓶。色谱条件:Waters E2695高效液相色谱系统;TSK GEL G4000 PWXL Column凝胶色谱柱(7.8 mm× 300 mm);Waters 2414示差折光检测器检测;柱温箱温度:30℃;检测器温度:30℃;流动相:超纯水;流速:0.6 mL·min-1;进样量:20 μL。
1.4 细胞迁移实验[13]
细胞以4×108L-1密度,每孔2 mL接种于6孔板,培养液含10%胎牛血清、胰岛素10 mg·L-1、青霉素100 kU·L-1-链霉素0.1 g·L-1,5%CO2,95%空气,饱和湿度下37℃培养24 h。以刻刀沿着培养板底部中间轻轻划一直线,细胞刮刀沿直线边缘将划痕一侧的细胞刮除,PBS冲洗。迅速加药,细胞正常对照组加DMEM,阳性对照药组加含终浓度5 μmol·L-1精脒的DMEM,受试药组分别加含不同浓度的党参、甘草糖复合物的DMEM。4-AP负荷实验中,模型组加入含4-AP 40 μmol·L-1的DMEM,阳性对照药组加入含精脒+4-AP的DMEM,受试药组分别加入含党参和甘草糖复合物+4-AP的DMEM。CO2培养箱培养24 h,相差倒置显微镜下放大100倍,观察细胞迁移情况,拍照,每孔沿划痕线从左至右随机观察约8个视野,每组3复孔,共计约24个视野的迁移细胞数的均数为本组数据。IPP软件计算越过划痕的细胞数。
1.5 流式细胞仪检测细胞膜电位[14]
细胞培养和给药方法:细胞生长至80%~90%,消化细胞,制单细胞悬液4×108L-1,每孔2 mL接种于6孔板,37℃,5%CO2,饱和湿度下在CO2培养箱培养24 h,1 mL移液器吸头沿着培养板的底部,在中间轻轻地划一个“十”字划痕,PBS冲洗,迅速加入含受试药(4-AP负荷实验同时加4-AP)的DMEM 2.5 mL。每组3复孔,按以上条件培养。
细胞悬液制备及荧光标记:受试药加入并培养12 h后,用HBSS(D-Hanks缓冲液)冲洗,胰酶消化,再加等量的DMEM以终止消化;离心(302×g,5 min),弃上清液,加HBSS重悬;再离心去上清液,每个离心管(6孔板的每孔细胞收集在1个离心管内)加入含1 μmol·L-1DiBAC4(3)溶液的HBSS 1 mL。在CO2培养箱中培养8 min,离心(302×g,5 min);去上清液,HBSS重悬,再离心,HBSS重悬,转入流式管后上机检测。流式细胞仪检测时,设定激发波长488 nm,发射波长520 nm,每管检测10 000个细胞,并设不标记的空白细胞管用于调零。以各组的平均荧光强度进行统计分析。
1.6 统计学分析
2 结果
2.1 党参和甘草糖复合物高效液相图谱
高效液相色谱图结果(图1)显示,党参糖复合物分别在8.482,11.264和20.223 min出现不同吸收峰(以8.482和20.223 min为2个主要吸收峰);甘草糖复合物分别在6.700,10.817和20.217 min出现不同吸收峰(以20.217 min为主要吸收峰);提示样品为党参和甘草糖的复合物。
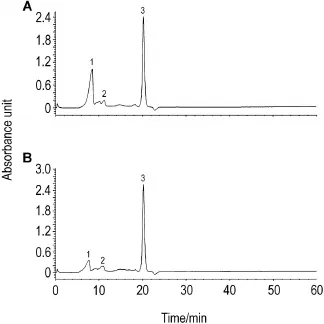
Fig.1 High performance liquid chromatography image of Codonopsis glycoconjugates(A)and Glycyrrhiza glycoconjugates(B).
2.2 党参和甘草糖复合物对lEC-6细胞迁移的影响
2.2.1 对正常lEC-6细胞迁移的影响
与细胞正常对照组比较,党参糖复合物(50~100 mg·L-1)(图2和表1)和甘草糖复合物(50~100 mg·L-1)(图3和表2)可增加细胞迁移数(即图中从划痕左侧向右侧迁移的细胞数量)(P<0.01,P<0.05),表明党参糖复合物和甘草糖复合物可促进IEC-6细胞迁移。

Fig.2 Effect of Codonopsis glycoconjugates on lEC-6 cells migration observed by phase contrast microscope(×100).IEC-6 cells were cultured with Codonopsis glycoconjugates and spermidine for 24 h,respectively.Cell migration was measured by the number of migrated cells from the left of the scratch to the right.A:normal control;B:spermidine 5 μmol·L-1;C,D and E:Codonopsis glycoconjugates 25,50 and 100 mg·L-1groups,respectively.
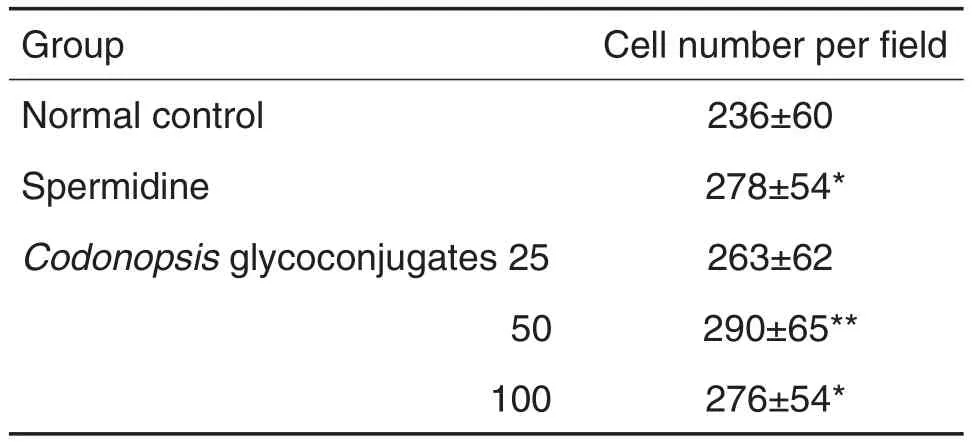
Tab.1 Effect of Codonopsis glycoconjugates on lEC-6 cell migration
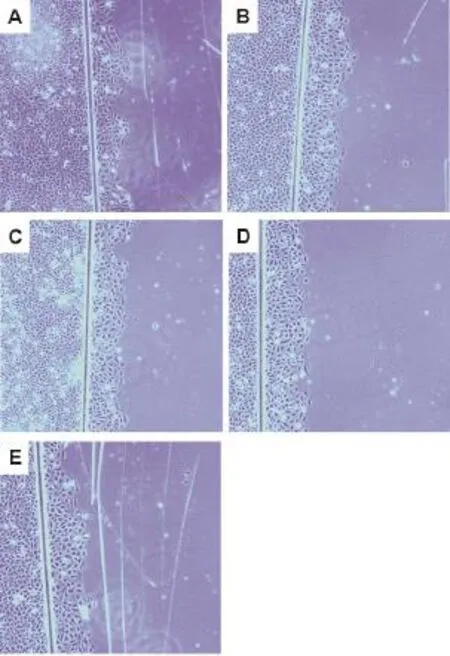
Fig.3 Effect of Glycyrrhiza glycoconjugates on lEC-6 cell migration observed by phase contrast microscope(×100).IEC-6 cells were cultured with Glycyrrhiza glycoconjugates and spermidine for 24 h,respectively.Cell migration was measured by the number of migrated cells from the left of the scratch to the right.A:normal control group;B:spermidine 5 μmol·L-1;C,D and E:Glycyrrhiza glycoconjugates 25,50 and 100 mg·L-1groups,respectively.
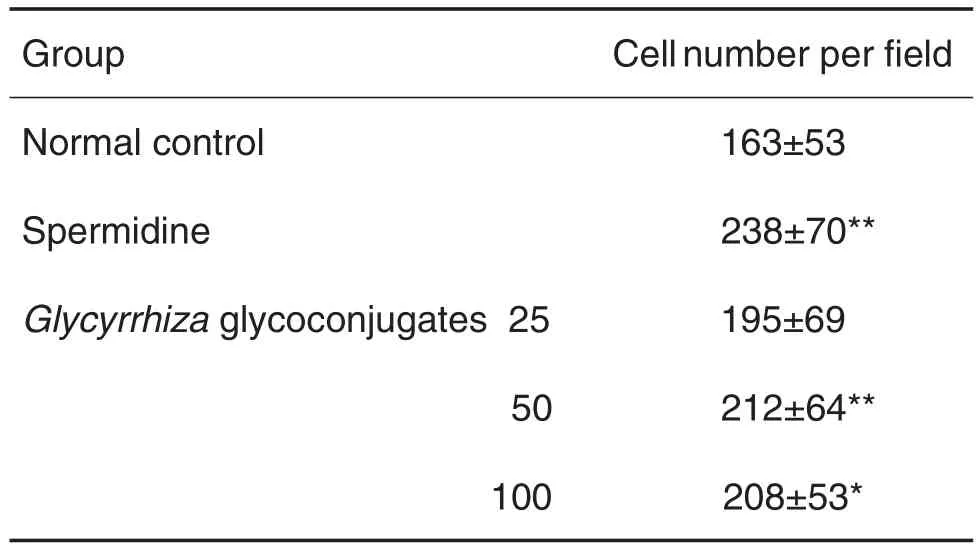
Tab.2 Effect of Glycyrrhiza glycoconjugates on lEC-6 cell migration
2.2.2 对4-AP负荷下lEC-6细胞迁移的影响
与正常对照组比较,4-AP模型组出现细胞迁移抑制,表明造模成功;与4-AP模型组比较,党参和甘草糖复合物(50~200 mg·L-1)可逆转4-AP所致的细胞迁移抑制(P<0.01)(表3和表4)。
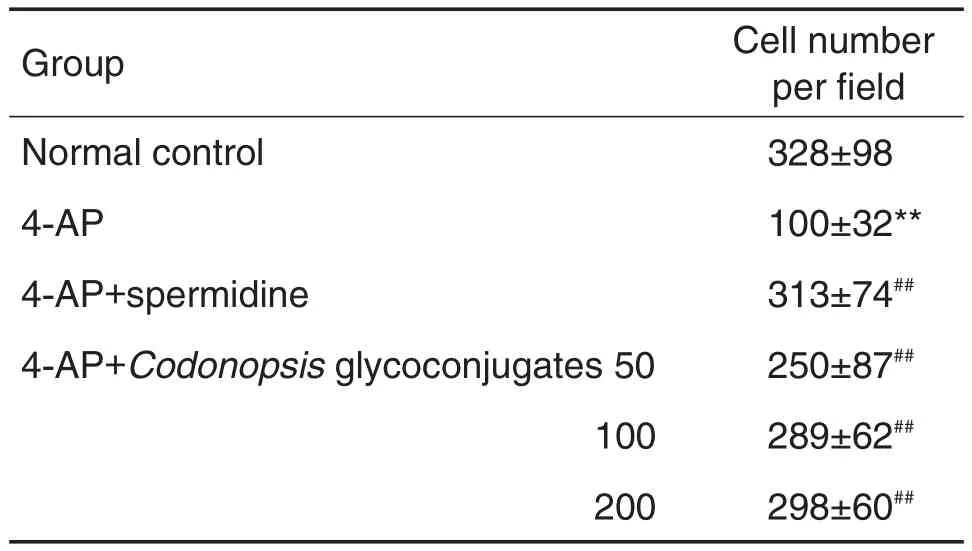
Tab.3 Effect of Codonopsis glycoconjugates on lEC-6 cell migration inhibited by 4-aminopyridine(4-AP)
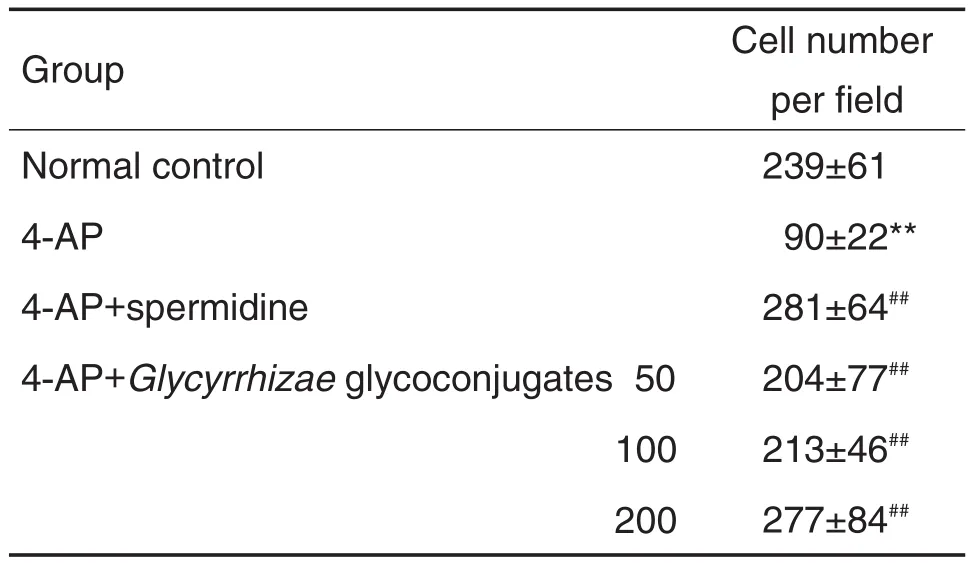
Tab.4 Effect of Glycyrrhiza glycoconjugates on lEC-6 cell migration inhibited by 4-AP
2.3 党参和甘草糖复合物对lEC-6细胞膜电位的影响
2.3.1 对正常lEC-6细胞膜电位的影响
表5和图4结果显示,党参和甘草糖复合物组(50 mg·L-1)细胞平均荧光强度低于正常对照组(P<0.01),即其细胞膜超极化水平高于正常对照组,提示细胞膜电位增加。表明党参和甘草糖复合物可增加细胞膜超极化水平,提高细胞膜电位。
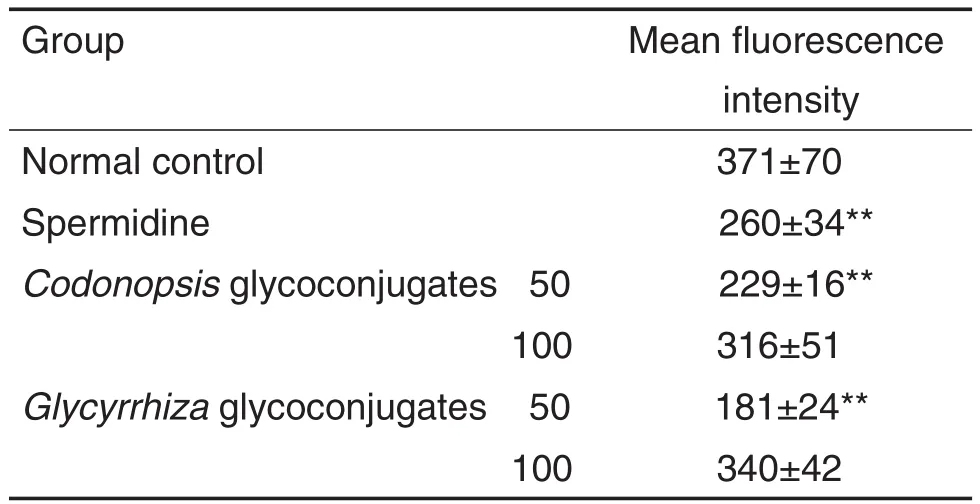
Tab.5 Effect of Codonopsis and Glycyrrhiza glycoconjugates on lEC-6 cell membrane potential
2.3.2 对4-AP负荷下lEC-6细胞膜电位的影响
图5和表6结果显示,4-AP模型组细胞平均荧光强度高于正常对照组(P<0.01),说明4-AP模型组细胞膜去极化水平高于正常对照组,细胞膜电位降低,说明造模成功;与4-AP模型组比较,党参和甘草糖复合物(100~200 mg·L-1)能逆转4-AP所致细胞膜去极化(P<0.01)。
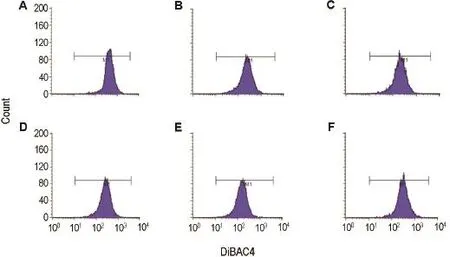
Fig.4 Effect of Codonopsis and Glycyrrhiza glycoconjugates on lEC-6 cell membrane potential detected by flow cytometry.See Tab.5 for the cell treatment.A:normal control;B:spermidine 5 μmol·L-1;C and D:Codonopsis glycoconjugates 50 and 100 mg·L-1;E and F:Glycyrrhiza glycoconjugates 50 and 100 mg·L-1.
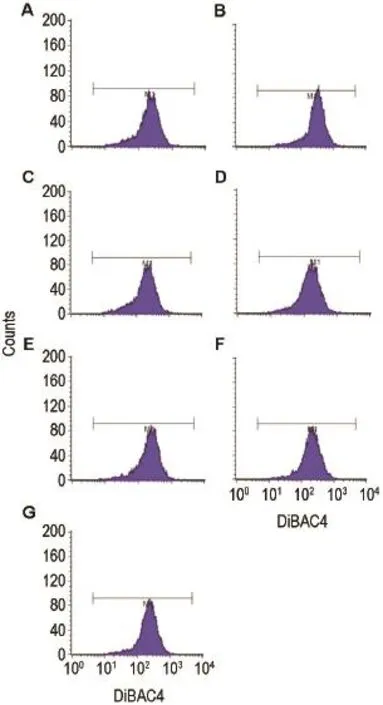
Fig.5 Effect of Codonopsis and Glycyrrhiza glycoconjugates on lEC-6 cell membrane potential inhibited by 4-AP detected by flow cytometry.IEC-6 cells were pretreated with 4-AP alone or with Codonopsis glycoconjugates,Glycyrrhiza glycoconjugates and spermidine for 12 h,respectively before they were incubated with DiBAC4(3)1 μmol·L-1for 8 min. Mean fluorescence intensity was detected by flow cytometry. A:normal control;B:4-AP 40 μmol·L-1;C:4-AP+spermidine 5 μmol·L-1;D:4-AP+Codonopsis glycoconjugates 100 mg·L-1;E:4-AP+Codonopsis glycoconjugates 200 mg·L-1;F:4-AP+ Glycyrrhiza glycoconjugates 100 mg·L-1;G:4-AP+Glycyrrhiza glycoconjugates 200 mg·L-1.
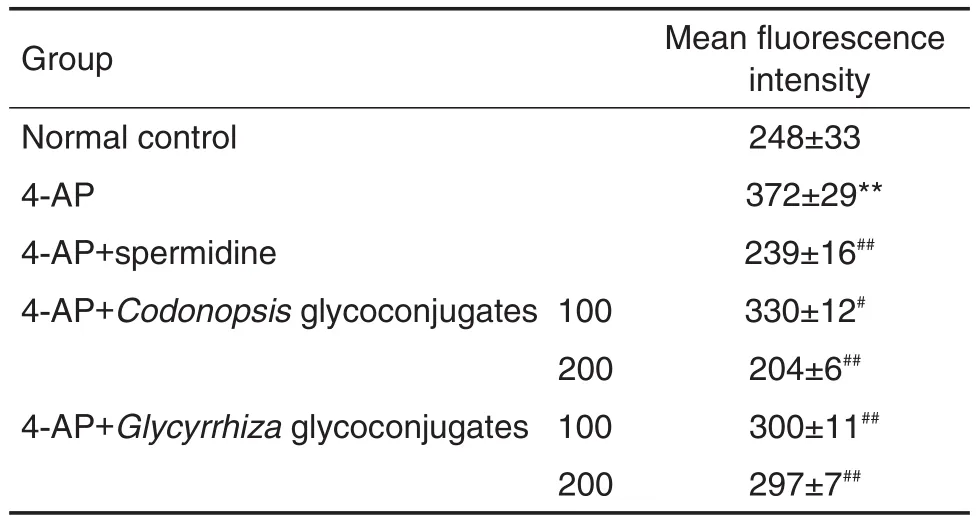
Tab.6 Effect of Codonopsis and Glycyrrhiza glycoconjugates on lEC-6 cell membrane potential inhibited by 4-AP
3 讨论
益气健脾中药是中医药临床最广泛应用的中药种类之一,如四君子汤和补中益气汤等在临床调理多种病证的脾胃虚损方面有显著疗效,对促进胃肠黏膜损伤修复也有很好疗效,但其作用机制特别是分子水平的疗效机制还不是很清楚。而胃肠黏膜损伤修复的环节复杂,步骤较多,本研究团队目前主要在其对细胞迁移影响方面开展工作。
细胞迁移是胃肠黏膜损伤修复的重要步骤,在IEC-6细胞迁移多胺介导钾通道激活信号通路中,多胺增加增强钾通道蛋白的表达,K+外流,导致细胞膜超极化,增强Ca2+内流驱动力而提高细胞内游离钙离子([Ca2+]i)水平,后者增加了GTP结合蛋白RhoA活性,使Rho激酶激活,增加了肌球蛋白轻链磷酸化,从而刺激了应力纤维形成和细胞迁移[15]。
DiBAC4(3)是细胞膜电位敏感的亲脂性阴离子荧光染料,根据其在细胞内外的重新分布,可无损测定膜电位的变化情况。DiBAC4(3)本身不发荧光,进入细胞与胞浆内蛋白质结合后可以发出荧光。当DiBAC4(3)进入细胞内增多,荧光增强时,表明膜电位负值减小,膜去极化;相反地,当细胞内的荧光强度降低时,则提示细胞膜超极化[16]。
电压门控钾离子(voltage-gated K+,Kv)通道是一组膜蛋白,在肠上皮细胞中对调控静息Em起重要作用。激活Kv通道引起膜的超极化,抑制Kv通道则引起膜的去极化。4-AP是特异性的钾离子通道抑制剂,加入4-AP能减少Kv通道α亚单位Kv1.1 mRNA及蛋白表达,从而减少了细胞K+外流,使膜去极化[15,17-18]。膜去极化降低了Ca2+内流驱动力,抑制了Ca2+内流,则减少RhoA蛋白的合成和稳定,抑制损伤后细胞迁移[19]。表明多胺通过调控Kv通道蛋白表达和膜电位及K+外流,进而调控损伤后细胞迁移。
本研究表明,党参和甘草糖复合物能促进IEC-6细胞迁移,逆转4-AP所致的细胞迁移抑制;能增加细胞膜超极化水平而提高膜电位,逆转4-AP所致的细胞膜去极化,说明对钾通道及相关指标的影响是其调节细胞迁移的作用机制之一。本课题组的另一研究发现,党参和甘草糖复合物可提高细胞迁移过程钾通道Kv1.1蛋白表达,改善二氟甲基鸟氨酸(α-difluoromethylornithine,DFMO,多胺合成抑制剂)所致Kv1.1蛋白表达下降;可提高细胞迁移过程膜超极化水平,逆转DFMO所致的膜去极化;可提高细胞迁移过程细胞[Ca2+]i水平,其中党参糖复合物可逆转DFMO所致细胞[Ca2+]i水平降低;可增加细胞迁移过程RhoA蛋白表达,改善DFMO所致RhoA蛋白表达减少[20]。结合本研究结果提示,党参和甘草糖复合物在正常(无负荷)时能促进细胞迁移,并对多胺调控信号通路的相关指标产生正向作用;在多胺合成抑制或钾通道抑制的负向影响下,能恢复细胞迁移至正常水平,对受到抑制的信号通路相关指标有改善作用。实验结果从正、负两方面说明益气健脾中药党参和甘草能促进细胞迁移,发挥胃肠黏膜损伤修复的作用,其机制与其影响多胺介导钾通道激活信号通路有关。
[1] Ye FQ,Chen WW.Pharmological study on the effect of SiJunZiTang on gastrointestinal tract[J]. Lishizhen Med Mater Med Res(时珍国医国药),2005,16(1):73-74.
[2]Hu C,Li RL,Mo QY,Wang ZP,Lin CQ,Chen WX,et al.Effects of different extract parts from Rhizoma Atractylodis Macrocephalae and Radix Astragali on proliferation of intestinal epithelial cells[J]. Tradit Chin Drug Res Clin Pharmacol(中药新药与临床药理),2010,21(2):156-160.
[3]Wang Z,Li RL,Xu SF,Chen WW.Effectsof AtractylodesMacrocephalamonosaccharide compositiononcytodifferentiationandvillin expression of IEC-6 cells in vitro[J].J Chin eMed Mater(中药材),2010,33(6):938-944.
[4]Hu C,Li RL,Wang J,Xu SF,Chen WW.Effects of Astragalus and Atractylodes extracts on IEC-6 cell migration[J].Tradit Chin Drug Res Clin Pharmacol(中药新药与临床药理),2011,22(1):60-65.
[5]Zhao SQ,Wen P,Li RL,Lu WB,Chen WW. EffectsofflavonoidsandsaponinsofRadix Codonopsis and Glycyrrhiza on IEC-6 cell migration [J].Pharmacol Clin Chin Mater Med(中药药理与临床),2012,28(2):78-81.
[6]Wen P,Sui JJ,Li RL,Zhao SQ,Lu WB,Chen WW. Effect of polysaccharides from Radix Glycyrrhiza on migration and polyamines contents of IEC-6 cell [J].J Chin Med Mater(中药材),2012,35(7):1112-1116.
[7]Li RL,Nian LQ,Zhao SQ,Wen P,Sui JJ,Tao YZ,et al.Effect of Radix Codonopsis on stress ulcer,cell migration and polyamine content in rats[J].J Guangzhou Univ Tradit Chin Med(广州中医药大学学报),2013,30(4):519-524,604.
[8]Li RL,Tao YZ,Wen P,Zhao SQ,Xu SF,Chen WW. Effectofflavonoidfrom Glycyrrhiza Radix on small intestine epithelium IEC-6 cell migration and intracellular polyamine content[J].Chin Tradit Herb Drugs(中草药),2013,44(19):2722-2726.
[9]Li RL,Zeng D,Wen P,Tao YZ,Zhao SQ,Chen WX,et al.Effect of Radix Codonopsis flavonoids on intestinal epithelial cell 6 migration and polyamines content[J].Tradit Chin Drug Res Clin Pharmacol(中药新药与临床药理),2014,25(5):523-526.
[10]Li RL,Hu C,Sui JJ,Lu WB,Wen P,Gao XL,et al.Effects of polysaccharides from Rhizoma Atractylodis Macrocephalae and from Radix Astragali on polyamine-mediated K+channels in the IEC-6 migration[J].Cell Biol Int,2010,34(8):S2.
[11]Song HP,Li RL,Chen X,Wang YY,Cai JZ,Liu J,et al.Atractylodes macrocephala Koidz promotes intestinal epithelial restitution via the polyaminevoltage-gatedK+channel pathway[J].J Ethnopharmacol,2014,152(1):163-172.
[12]Song HP,Li RL,Wang YY,Zeng D,Zhang L,Deng J,et al.Effects of Atractylodes macrocephala Koidz.extract on polyamine mediated calcium signaling pathway during intestinal epithelial cell migration[J].China J Tradit Chin Med Pharm(中华中医药杂志),2014,29(5):1361-1367.
[13]Hu C,Li RL,Lu WB,Yang YQ,Wang J,Chen WW. Studies on cell migration model in intestinal epithelial restitutionforpharmacologicalresearch[J].J Chin Med Mater(中药材),2011,34(5):738-746.
[14]Song HP,Li RL,Wang YY,Chen WX,Chen X,Xu SF,et al.Establishment and application of membrane potential detection method for intestinal epithelial cell migration[J].Chin Pharmacol Bull(中国药理学通报),2013,29(12):1758-1761.
[15]Wang JY.Cellular signaling in rapidintestinal epithelial restitution:implication of polyamines and K+channels[J].Acta Physiol Sin(生理学报),2003,55(4):365-372.
[16]Yang SM,Jiang SC,Yang WY,Gu R,Han DY. Optical recording of membrane potential using voltage-sensitive dyes[J].Prog Physiol Sci(生理科学进展),2002,33(2):106-110.
[17]Rao JN,Platoshyn O,Li L,Guo X,Golovina VA,Yuan JX,et al.Activation ofK+channelsand increasedmigrationofdifferentiatedintestinal epithelial cells after wounding[J].Am J Physiol Cell Physiol,2002,282(4):C885-C898.
[18]Wang JY,Wang J,Golovina VA,Li L,Platoshyn O,Yuan JX.Role of K+channel expression in polyamine-dependent intestinal epithelial cell migration [J].Am J Physiol Cell Physiol,2000,278(2):C303-C314.
[19]Fukata M,Nakagawa M,Kaibuchi K.Roles of Rho-family GTPases in cell polarisation and directional migration[J].Curr Opin Cell Biol,2003,15(5):590-597.
[20] Tao YZ.Studies on the effects of Codonopsis and Licoriceonpolyamine-mediatedsignaling pathway in the intestinal epithelial cell migration(党参和甘草提物对IEC-6细胞迁移多胺调控信号通路作用的研究)[D].Guangzhou:Guangzhou University of Chinese Medicine(广州中医药大学),2013.
(本文编辑:齐春会)
Effect of Codonopsis and Glycyrrhiza glycoconjugates on migration and membrane potential of lEC-6 cells
LI Ru-liu,TAO Yu-zhu,WEN Peng,TU Xiao-hua,CAI Jia-zhong,CHEN Wei-wen
(Piwei Institute of Guangzhou University of Chinese Medicine,Guangzhou 510405,China)
OBJECTlVE To observe the effect of Codonopsis and Glycyrrhiza glycoconjugates on migration and membrane potential of small intestinal epithelial cells(IEC-6),and to explore the promoting effects of Yiqi jianpi herb Codonopsis and Glycyrrhiza on gastrointestinal mucosal injury repair and the underlying mechanisms.METHODS Under normal conditions or loaded with the inhibitor of potassium channel 4-aminopyridine(4-AP),IEC-6 cells were treated with Codonopsis and Glycyrrhiza glycoconjugates (25-200 mg·L-1)for 24 h,respectively.IEC-6 cell migration was observed by the phase contrast microscope and cell membrane potential was detected by flow cytometry.RESULTS Codonopsis and Glycyrrhiza glycoconjugates(50 and 100 mg·L-1)increased the number of migrated IEC-6 cell compared with normal control group(P<0.01,P<0.05).Compared with normal control group,4-AP reduced the number of migrated IEC-6 cell(P<0.01).Codonopsis and Glycyrrhiza glycoconjugates (50-200 mg·L-1)reversed cell migration inhibited by 4-AP significantly when compared with 4-AP model group(P<0.01).The results of flow cyometry analysis showed that the cell membrane potential was increased after treatment with Codonopsis and Glycyrrhiza glycoconjugates(50 mg·L-1)compared with normal control group and resulted in an increase in cell membrane hyperpolarization(P<0.01). Compared with normal control group,4-AP decreased the cell membrane potential(P<0.01)and resulted in cell membrane depolarization.Also,compared with 4-AP model group,cell membrane depolarization induced by 4-AP was reversed by treatment with Codonopsis and Glycyrrhiza glycoconjugates(100 and 200 mg·L-1).CONCLUSlON Codonopsis and Glycyrrhiza glycoconjugates may promote gastrointestinal mucosal injury repair and the mechanisms may involve the activation of signaling pathways by affecting polyamine-dependent intestinal epithelial cell migration voltage-gated K+channels.
Codonopsis;Glycyrrhiza;glycoconjugates;intestinal epithelial cells;cell migration;membrane potential
The project supported by National Natural Science Foundation of China(30772753);National Natural Science Foundation of China(81173254);Special Funds from Central Finance of China in Support of the Development of Local Colleges and Universities[Educational Finance Grant 338(2013)];and South China Chinese Medicine Collaborative Innovation Center(E1-KFD015141K03)
LI Ru-liu,E-mail:lrl@gzucm.edu.cn,Tel:(020)36585444
R285.5
A
1000-3002(2015)06-0917-07
国家自然科学基金项目(30772753);国家自然科学基金项目(81173254);中央财政支持地方高校发展专项资金项目(财教[2013]338号);华南中医药协同创新中心项目(E1-KFD015141K03)
李茹柳,博士,教授,主要从事调理脾胃中药作用机制研究。
李茹柳,E-mail:lrl@gzucm.edu.cn,Tel:(020)36585444
(2015-06-08接受日期:2015-11-15)
