GLP-1通过SDF-1/CXCR4信号通路调控人脐血内皮祖细胞增殖、分化和凋亡
2015-08-24刘峰许文琼闵娜汤佳珍黄海华
刘峰,许文琼,闵娜,汤佳珍,黄海华
GLP-1通过SDF-1/CXCR4信号通路调控人脐血内皮祖细胞增殖、分化和凋亡
刘峰1,许文琼1,闵娜2,汤佳珍1,黄海华1
目的 探讨胰高血糖素样肽1(GLP-1)调控人脐血内皮祖细胞(EPCs)增殖、分化与凋亡的分子机制。方法 从健康孕妇脐带血分离和培养EPCs,以2×105密度接种于6孔细胞板,分别转染空载体质粒(对照组)、pcDNA3-GLP-1质粒(GLP-1组)、pcDNA3-GLP-1质粒+AMD3100(GLP-1+AMD3100组)及单纯AMD3100(AMD3100组)。将pcDNA3-GLP-1质粒转染EPCs,以25 μmol/L AMD3100阻断体外培养的EPCs的基质细胞衍生因子(SDF-1)/趋化因子受体4(CXCR4)信号通路1 h。采用RT-PCR检测分化和凋亡相关基因PPARγ、C/EBPα与Caspase-3基因表达,MTT比色法检测细胞增殖能力,Caspase-3活性检测试剂盒测定Caspase-3活性。结果 与对照组相比,GLP-1过表达显著提高了C/EBPα及PPARγ mRNA表达,促进了EPCs细胞增殖,降低了Caspase-3 mRNA表达和Caspase-3活性(均P<0.05)。当SDF-1/CXCR4信号通路被阻断后,GLP-1对C/EBPα及PPARγ mRNA表达、EPCs细胞增殖的促进作用,以及对Caspase-3 mRNA表达和Caspase-3活性的抑制作用均明显减弱(均P<0.05)。结论 GLP-1可通过调节SDF-1/CXCR4信号通路促进EPCs增殖与分化,抑制其凋亡。
胰高血糖素样肽1;胎血;半胱氨酸天冬氨酸蛋白酶3;细胞增殖;细胞分化;细胞凋亡;内皮祖细胞;SDF-1/CXCR4;信号通路
内皮祖细胞(endothelial progenitor cells,EPCs)主要来源于骨髓和外周血,在体外可诱导分化各种内皮细胞特异性抗原,在体内则能分化成有功能的内皮细胞[1]。目前,应用EPCs修复损伤血管已经成为研究热点之一。有研究报道,胰高血糖素样肽1 (glucagon like peptide 1,GLP-1)是一种促胰岛素肽,具备多种生理功能,包括促进胰岛β细胞增殖和分化、抑制食欲、增强记忆及改善心血管内皮细胞功能[2-3]。另有研究指出,基质细胞衍生因子(stromal cell derived factor,SDF-1)/趋化因子受体4(chemokinereceptor 4,CXCR4)可调节机体的炎症与免疫反应,抵抗人类免疫缺陷病毒(HIV)感染,抑制肿瘤细胞迁移,保护胚胎发育,参与血管新生等[4-6]。本课题组前期研究证实,GLP-1可调控SDF-1与CXCR4 mRNA和蛋白表达[7],由此推测GLP-1具有促EPCs增殖、移行与分化作用,且该机制可能与SDF-1/CXCR4通路有关。GLP-1是否通过调控SDF-1/CXCR4轴信号通路影响EPCs生物学效应尚少见研究。本研究采用阻断剂AMD3100阻断SDF-1/CXCR4信号通路后,研究GLP-1对EPCs的增殖、分化及凋亡的影响,探讨GLP-1调控的信号转导机制,以期为血管并发症的防治提供理论基础。
1 材料与方法
1.1 细胞分离与培养 EPCs的分离和鉴定参照文献[7]。无菌条件下,采用含肝素的注射器抽取健康孕妇脐带血约20 mL。采用密度梯度离心法分离单个核细胞,并将其接种于24孔培养板,加入M199培养基,培养基含10%胎牛血清、1%链霉素、1%青霉素和 50 μg/L血管内皮生长因子(VEGF),置于37℃、5%CO2培养箱中培养。
1.2 主要试剂 CXCR4阻断剂AMD3100购自AbMole公司,转染试剂lipo2000购自英韦创津公司。实时定量PCR所用SYBR Green PCR Master Mix购自美国应用生物公司。β-actin(ab8229)、CXCR4抗体(ab2074)均购自Abcam公司,pcDNA3-GLP-1质粒由本实验室构建,构建步骤参照文献[6]。
1.3 实验分组与处理 将体外培养的EPCs以2×105密度接种6孔细胞板。每组6个重复,每个重复6个复孔。分别转染空载体质粒(对照组)、pcDNA3-GLP-1质粒(GLP-1组)、pcDNA3-GLP-1质粒+AMD3100(GLP-1+AMD3100组)及单纯AMD3100(AMD3100组)。
1.4 Western Blot 将ECPs以2×105个/mL接种于6孔板,当细胞融合达65%时,加入0、5、15与25 μmol/L AMD3100阻断剂,48 h后收集细胞并提取蛋白。经过电泳、转膜后,取下硝酸纤维膜,脱脂牛奶封闭1 h后,用内参兔抗人β-actin一抗(ab8229)(1∶500)、兔抗人CXCR4一抗(ab2074,1∶500)孵育过夜,鼠抗兔二抗孵育1 h后显色,进行化学发光反应并显影、定影。将图片扫描后,用Image J图像分析软件进行蛋白灰度分析。
1.5 实时定量PCR 将GLP-1过表达细胞后,采用AMD3100阻断剂作用细胞24、48、96 h后行异硫氰酸胍/苯酚-氯仿萃取法,按照Trizol试剂盒说明从6孔板中提取总RNA(Invitrogen,Carlsbad,CA,USA)。根据使用手册,用ABI PRISM 7500荧光定量 PCR仪(Applied Biosystems,Foster City,CA,USA)检测PPARγ、C/EBPα与Caspase-3基因表达。PCR反应体系50℃2 min和95℃10 min,1个循环,以及95℃15 s,60℃15 s和72℃30 s,共40个循环。β-actin作为内参。特异性引物序列由上海博尚生物技术公司合成,引物序列见表1。

Tab.1 Primer sequences表1 引物序列
1.6 MTT比色法 采用MTT比色法检测GLP-1对SDF-1/ CXCR4信号通路阻断后细胞增殖能力的影响。将ECPs转染质粒后以1×104个/mL接种于96孔板,融合度达55%时,以25 μmol/L AMD3100作用细胞1 h。培养24、48、96 h后,向每孔中加入50 μL 2 g/L MTT溶液,再在培养箱继续培养3 h。1 500 r/min离心5 min后移除细胞上清,每孔中加入150 μL DMSO。室温下将细胞板置于振荡器低速振荡20 min,并于波长490 nm处测定其光密度(OD)值。
1.7 Caspase-3活性检测 采用Caspase-3检测试剂盒(Beyotime)检测Caspase-3活性。将ECPs转染质粒后以1×106个/mL接种于6孔板,当细胞融合达65%时加入25 μmol/L AMD3100作用1 h,培养24、48、96 h后收集细胞。PBS洗涤,采用蛋白裂解液分离细胞中的蛋白,并放置冰上待用。吸取50 μL细胞蛋白,加入50 μL反应缓冲液及加Ac-DEVD-AMC(Caspase-3四肽荧光底物)5 μL,于37℃避光孵育4 h。于405 nm波长处,用酶标仪测定其OD值。通过计算OD阻断剂/OD阴性对照的倍数来确定凋亡诱导剂组Caspase-3活化程度。
1.8 统计学方法 数据采用SPSS 17.0软件进行统计处理,计量资料以±s表示,多组间比较采用单因素方差分析,并行邓肯氏多重比较。P<0.05为差异有统计学意义。
2 结果
2.1 阻断效果分析 与对照组(0.465±0.085)相比,不同剂量AMD3100作用后,CXCR4蛋白表达明显降低,25 μmol/L AMD3100组(0.054±0.023)作用最为明显(F=16.347,P<0.05),见图1。证明25 μmol/L AMD3100阻断效果最佳,可用于后续阻断实验。
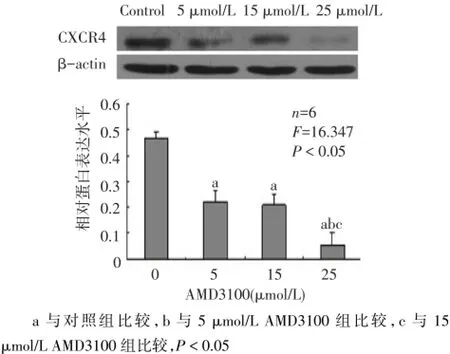
Fig.1 Effects of AMD3100 inhibitor on CXCR4 protein expression图1 AMD3100阻断剂对CXCR4蛋白表达的影响
2.2 GLP-1对SDF-1/CXCR4信号通路阻断后细胞分化的影响 与对照组相比,GLP-1过表达显著提高了C/EBPα及PPARγ mRNA表达(P<0.05),且具时间依赖性。当SDF-1/CXCR4信号通路被阻断后,GLP-1对C/EBPα及PPARγ mRNA表达均明显抑制(P<0.05),见图2。
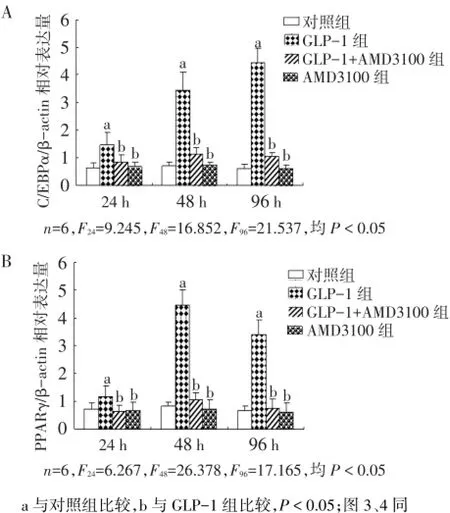
Fig.2 Effects of GLP-1 on cell differentiation after the block of SDF-1/CXCR4 signaling pathway图2 GLP-1对SDF-1/CXCR4信号通路阻断后细胞分化的影响
2.3 GLP-1对SDF-1/CXCR4信号通路阻断后细胞增殖的影响 与对照组相比,GLP-1过表达则明显促进了细胞增殖(P<0.05)。AMD3100阻断剂阻断SDF-1/CXCR4信号通路后,GLP-1对EPCs的增殖作用明显降低(P<0.05),见图3。
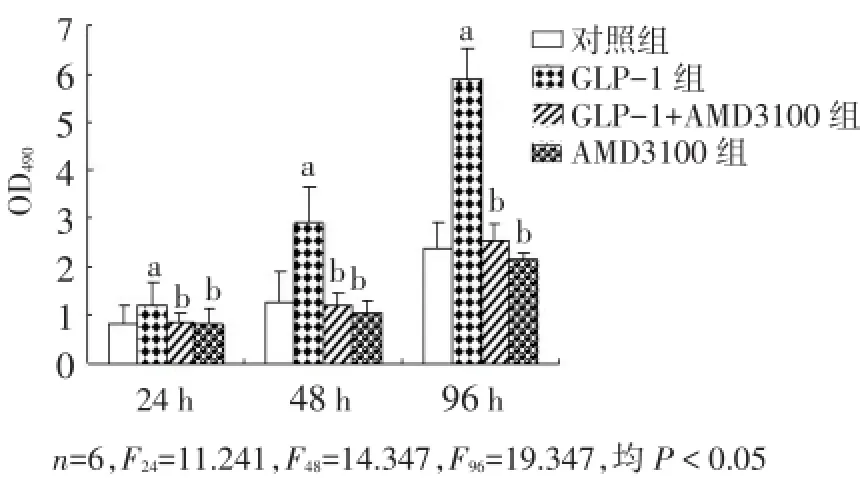
Fig.3 Effects of GLP-1 on cell proliferation after the block of SDF-1/CXCR4 signaling pathway图3 GLP-1对SDF-1/CXCR4信号通路阻断后细胞增殖的影响
2.4 GLP-1对SDF-1/CXCR4信号通路阻断后细胞凋亡的影响 与对照组相比,GLP-1过表达降低了Caspase-3 mRNA表达和Caspase-3活性,其均在GLP-1作用48 h后明显降低,96 h时细胞表现出的下调趋势最为显著(P<0.05)。当SDF-1/CXCR4信号通路被阻断后,GLP-1对Caspase-3 mRNA表达和Caspase-3活性的抑制作用均明显减弱(P<0.05)。见图4。
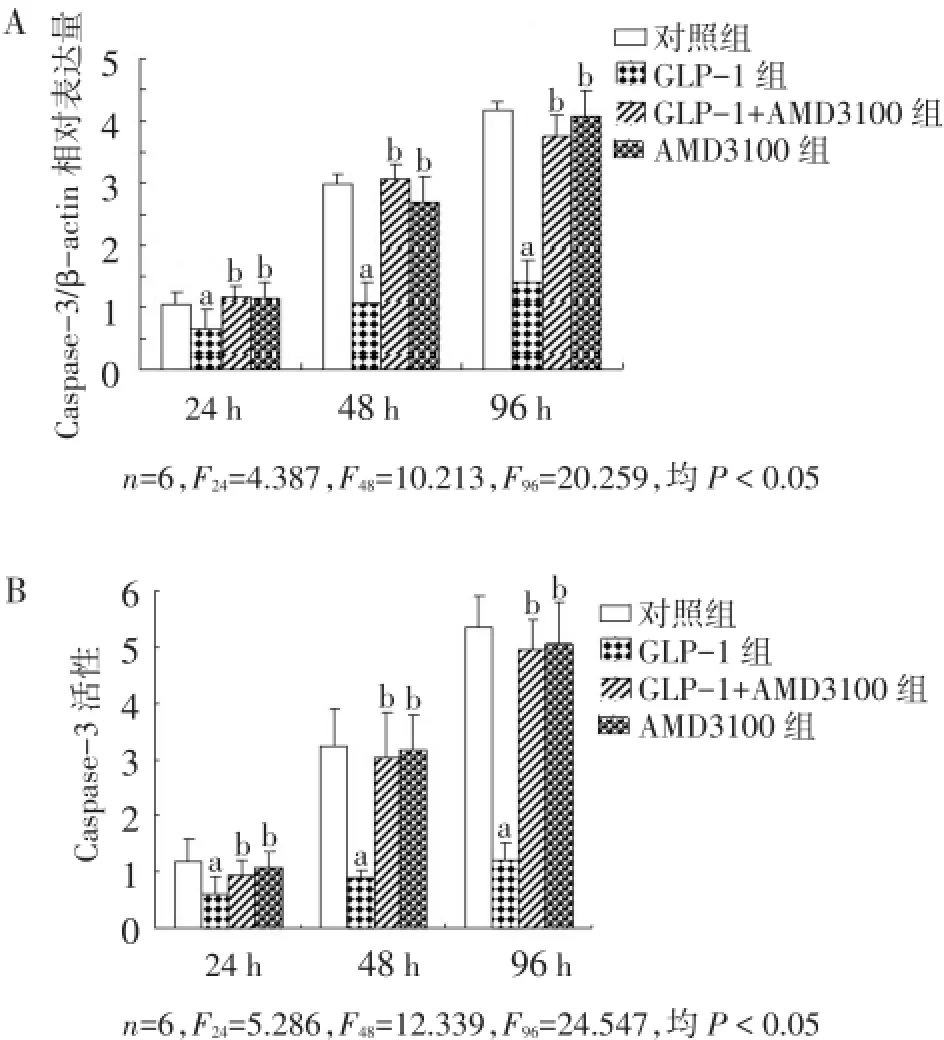
Fig.4 Effects of GLP-1 on cell apoptosis after block of SDF-1/CXCR4 signaling pathway图4 GLP-1对SDF-1/CXCR4信号通路阻断后细胞凋亡的影响
3 讨论
GLP-1是由肠道L细胞分泌的一种多肽,其分泌受葡萄糖、脂肪等营养物质的调控[8-9]。有报道指出,GLP-1在改善心血管内皮细胞功能方面发挥重要作用[3]。EPCs是一种重要的促进血管新生的细胞,目前以EPCs为基础的心血管疾病治疗被广泛关注[10]。目前,有关GLP-1在体外培养的EPCs中的调控机制尚少见报道。本文对GLP-1过表达后EPCs的生物学效应进行研究,发现GLP-1可通过SDF-1/ CXCR4信号通路调控EPCs的增殖、分化与凋亡。
对GLP-1功能的研究表明,GLP-1不但可以促使前胰岛细胞向β细胞表型分化,而且可以增强胰岛β细胞的增殖和存活[11]。此外,GLP-1还可以保护小鼠胰岛β细胞免受过氧化氢诱导的凋亡,及阻止谷氨酸盐诱导的大鼠海马神经元细胞和成纤维细胞凋亡[12-13]。进一步研究证实,GLP-1抑制β细胞与心肌细胞调亡的途径与PI3K/AKT信号途径密切相关[11]。PPARγ与C/EBPα在细胞分化过程中发挥重要作用[14]。Caspase-3是Caspase家族的一员,在凋亡的信号传导、调节和执行过程中发挥关键作用[15]。本研究结果显示,GLP-1过表达促进了EPCs细胞增殖及PPARγ、C/EBPα mRNA表达,抑制了细胞Caspase-3 mRNA表达与Caspase-3活性。
SDF-1/CXCR4通路在内皮形成、血管生成以及造血等方面均发挥重要功能。有研究表明,SDF-1促进VEGF在内皮细胞上表达,是动员EPCs的重要细胞因子。SDF-1是CD34+造血祖细胞趋化因子,介导造血祖细胞的迁移,对祖细胞归巢到骨髓起重要作用[16]。对SDF-1基因敲除小鼠的研究表明,SDF-1/CXCR4在心血管的生成中发挥重要作用[17]。SDF-1/CXCR4可触发机体内多种信号转导通路,包括PI3K/Akt、核因子(NF)-κB、MAPKs与Ca2+内流等,这些途径被认为与细胞增殖与凋亡密切相关[13]。本研究证实,GLP-1是SDF-1/CXCR4通路的重要调控因子,并通过该通路调控EPCs的增殖、分化与凋亡。但由于体外实验与体内复杂的环境还存在一定差距,尚需进一步深入研究。
[1]Zhao XH,Huang L.The characteristics of endothelial progenitor cell biology and its effects on the cardiovascular system[J].Chin J Interven Card,2005,13(6):405-407.[赵晓辉,黄岚.内皮祖细胞生物学特性及其在心血管系统中的作用[J].中国介入心脏病学杂志,2005,13(6):405-407].
[2]Cegla J,Troke RC,Jones B,et al.Co-infusion of low-dose glucagon-like peptide-1(GLP-1)and glucagon in man results in a reduction in food intake[J].Diabetes,2014,63(11):3711-3720.
[3]Dalbøge LS,Almholt DL,Neerup TS,et al.The novel GLP-1-gastrin dual agonist ZP3022 improves glucose homeostasis and increases β-cell mass without affecting islet number in db/db mice[J].J Pharmacol Exp Ther,2014,350(2):353-356.
[4]Wuchter P,Leinweber C,Saffrich R,et al.Plerixafor induces the rapid and transient release of stromal cell-derived factor-1 alpha from human mesenchymal stromal cells and influences the migration behavior of human hematopoietic progenitor cells[J].Cell Tissue Res,2014,355(2):315-326.
[5]Masyuk M,Abduelmula A,Morosan-Puopolo G,et al.Retrograde migration of pectoral girdle muscle precursors depends on CXCR4/ SDF-1 signaling[J].Histochem Cell Biol,2014,142(5):473-488.
[6]Liu F,Tang JZ,Zhu LY,et al.Induced VEGF in umbilical cord blood endothelial progenitor cells and the expression of SDF-1/CXCR4 GLP-1 gene[J].Chin Modern Med J,2013,23:32-36.[刘峰,汤佳珍,朱凌燕,等.GLP-1基因诱导脐血内皮祖细胞VEGF和SDF-1/ CXCR4表达[J].中国现代医学杂志,2013,23:32-36].
[7]Shin JW,Lee DW,Kim MJ,et al.Isolation of endothelial progenitor cells from cord blood and induction of differentiation by ex vivo expansion[J].Yonsei Med J,2005,46(2):260-267.
[8]Escalada FJ.The physiology of glucagon-like peptide-1 and its role in the pathophysiology of type 2 diabetes mellitus[J].Med Clin (Barc),2014,143 Suppl 2:2-7.
[9]Kim HY,Hwang JI,Moon MJ,et al.A Novel long-acting glucagon-like peptide-1 agonist with improved efficacy in insulin secretion and βcell growth[J].Endocrinol Metab(Seoul),2014,29(3):320-327.
[10]Raz O,Lev DL,Battler A,et al.Pathways mediating the interaction between endothelial progenitor cells(EPCs)and platelets[J].PLoS One,2014,9(6):e95156.
[11]Takeda Y,Amano A,Noma A,et al.Systems analysis of GLP-1 receptor signaling in pancreatic β-cells[J].Am J Physiol Cell Physiol,2011,301(4):C792-803.
[12]Zhan Y,Sun HL,Chen H,et al.Glucagon-like peptide-1(GLP-1)protects vascular endothelial cells against advanced glycation end products(AGEs)-induced apoptosis[J].Med Sci Monit,2012,18(7): BR286-291.
[13]Kimura R,Okouchi M,Fujioka H,et al.Glucagon-like peptide-1 (GLP-1)protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway[J].Neuroscience,2009,162(4):1212-1219.
[14]Zhao QH,Wang SG,Liu SX,et al.PPARγ forms a bridge between DNA methylation and histone acetylation at the C/EBPα gene promoter to regulate the balance between osteogenesis and adipogenesis of bone marrow stromal cells[J].FEBS J,2013,280(22):5801-5814.
[15]Ardi VC,Alexander LD,Johnson VA,et al.Macrocycles that inhibit the binding between heat shock protein 90 and TPR-containing proteins[J].ACS Chem Biol,2011,6(12):1357-1366.
[16]Hattori K,Heissig B,Tashiro K,et al.Plasma elevation of stromal cell derived factor-1 induces mobilization of mature and immature hematopoietic progenitor cells[J].Blood,2001,97(11):3354-3360.
[17]Hu TH,Yao Y,Yu S,et al.SDF-1/CXCR4 promotes epithelialmesenchymal transition and progression of colorectal cancer by activation of the Wnt/β-catenin signaling pathway[J].Cancer Lett,2014,354(2):417-426.
(2014-11-03收稿 2015-01-05修回)
(本文编辑 陈丽洁)
GLP-1 regulates proliferation,differentiation and apoptosis of endothelial progenitor cells isolated from human umbilical cord blood by targeting the SDF-1/CXCR4 signaling pathway
LIU Feng1,XU Wenqiong1,MIN Na2,TANG Jiazhen1,HUANG Haihua1
1 Department of Endocrinology,The First Affiliated Hospital of Nanchang University,Nanchang 330006,China;2 The Third Affiliated Hospital of Nanchang University
Objective To investigate the molecular regulatory mechanism of glucagon like peptide 1(GLP-1)on proliferation,differentiation and apoptosis of human umbilical cord blood endothelial progenitor cells(EPCs).Methods EPCs were isolated from the umbilical cord blood of healthy pregnant women and cultured in 6-hole cell plate at 2×105 density in vitro,transfected with empty vector plasmid(control group),pcDNA3-GLP-1 plasmid(GLP-1 group),pcDNA3-GLP-1plasmid+AMD3100(GLP-1+AMD3100 group)and simple AMD3100(AMD3100 group).The pcDNA3-GLP-1 was transfected into EPCs.The 25μmol/L AMD3100 was used to block the SDF-1/CXCR4 signal pathway of EPCs for 1 h.The cell proliferation was determined by MTT method.The mRNA expressions of differentiation and apoptosis related genes PPARγ,C/EBPα and Caspase-3 were investigated by RT-PCR,and Caspase-3 activity was determined by Caspase-3 activity assay kit.Results Compared to control group,AMD3100 inhibitor showed no effects on cell proliferation,differentiation and apoptosis,while over-expression of GLP-1 in EPCs obviously promoted cell proliferation,and differentiation related genes PPARγ and C/EBPα mRNA expression,but down-regulated mRNA expression and the activity of Caspase-3 significantly(P<0.05),indicating that GLP-1 increased proliferation and differentiation of EPCs while decreased cell apoptosis.When the SDF-1/CXCR4 signaling pathway was blocked by AMD3100,over-expression of GLP-1 induced promotion of cell proliferation,and the differentiation was decreased significantly and the apoptosis was significantly increased(P<0.05).Conclusion These data confirm that GLP-1 might promote EPCs proliferation and differentiation,and inhibit cell apoptosis through the regulation of the SDF-1/CXCR4 signaling pathway.
glucagon-like peptide 1;fetal blood;Caspase 3;cell proliferation;cell differentiation;apoptosis;endothelial progenitor cells;SDF-1/CXCR4;signaling pathway
R349.54
A DOI:10.11958/j.issn.0253-9896.2015.05.003
江西省自然青年基金(20114BAB215004)
1南昌大学第一附属医院内分泌科(邮编330006);2南昌大学第三附属医院
刘峰(1980),男,副主任医师,博士,主要从事糖尿病及其并发症方面的研究
