结肠癌组织miR-200c/141定量表达的临床病理学意义
2015-06-28孟令宽钟小莉潘琼叶钧尚杨杨郭靖汪荣泉
孟令宽,钟小莉,潘琼,叶钧,尚杨杨,郭靖,汪荣泉
·临床研究·
结肠癌组织miR-200c/141定量表达的临床病理学意义
孟令宽,钟小莉,潘琼,叶钧,尚杨杨,郭靖,汪荣泉
目的探讨结肠癌组织miR-200c/141定量表达与患者临床病理学特征之间的联系。方法实时定量PCR检测49例结肠癌患者癌组织和癌旁组织中的miR-200c/141的表达水平。分析miR-200c/141表达量、联合miR-200c和miR-141表达量与患者临床病理学特征之间的关系,以及miR-200c/141表达量在判断患者术后1年预后中的意义。结果结肠癌组织中miR-200c/141表达水平较癌旁组织明显降低(P=0.0002,P=0.0058)。miR-141表达量与肿瘤大小和淋巴结转移呈负相关(P<0.05),有淋巴结转移或临床Ⅲ-Ⅳ期患者结肠癌组织中miR-141的表达量明显低于没有淋巴结转移或临床Ⅰ-Ⅱ期患者癌组织的表达量(P=0.035,P=0.042)。miR-200c/141表达量与肿瘤大小之间明显相关(P<0.05)。miR-200c/141的表达量与患者术后1年的生存率无相关关系(P>0.05)。结论结肠癌组织中表达下调的miR-200c/141与肿瘤大小、淋巴结转移、临床分期有关。
结肠肿瘤;病理学,临床;微RNAs
结肠癌(colorectal carcinoma,CRC)是常见的消化道恶性肿瘤,发病率呈上升趋势,在西方发达国家,发病率居第二位[1]。近年来结肠癌的治疗有较大进展,但患者生存率的改善并不明显[2]。选取有效肿瘤标记物并根据结肠癌患者临床预后进行个体化治疗,减少结肠癌病死率,提高生存率是该领域的前沿问题[3-4]。microRNAs(miRNAs)是高度保守的小分子非编码RNA,具有转录后调控功能,可通过结合到靶基因所表达的mRNA的3'-UTR,在转录后水平抑制其表达。miRNAs参与管理人类大约30%的基因,在人类肿瘤的发生发展进程中至关重要[5]。很多肿瘤的发生过程都存在miRNAs的异常表达[6-7],例如,结肠癌组织在miRNAs表达图谱方面存在显著改变,通过检测大量相关的miRNAs的异常可以优化结肠癌患者个性化治疗方案并改善患者的临床预后[8-10]。在肿瘤干细胞的干性维持及分化方面,miRNAs调节功能显著,被认为是癌基因或抑癌基因[11],例如我们发现miR-200参与Ascl2介导的上皮间质转化(EMT)和间质上皮转化(MET)之间的可塑性调节[12-13]。文献报道,miR-200c/141与EMT有关,miR-200c参与多种肿瘤的发生、发展[14-15]。本研究探讨结肠癌组织中miR-200c/141定量表达水平与患者临床病理特征之间的关系,为miR-200c/141参与结肠癌发生、发展的过程提供依据。
1 材料与方法
1.1 研究对象 本研究收集的标本为2014年4月-2015年4月第三军医大学西南医院消化科内镜下活检或普外科手术切除的结肠癌患者(术前均未进行放疗、化疗等治疗)的肿瘤组织和癌旁组织,癌旁组织为距肿瘤病灶3cm的结肠黏膜组织。所有患者在确诊后均行结肠癌外科根治手术治疗,具有完整的临床病理资料,其中,男性29例,女性20例,年龄<65岁30例,年龄>65岁19例,其他临床病理资料参见表1。结肠癌TNM分期参照美国癌症联合委员会(AJCC)/国际抗癌联盟(UICC)结直肠癌TNM分期系统(第7版)。获取的组织标本立即放入液氮冻存,用于实时定量PCR检测结肠癌组织及癌旁组织中miR-200c/141的表达水平。本研究获得西南医院伦理委员会批准,获取标本前经患者知情同意。
1.2 实时定量PCR检测miR-200c/141的表达 收集上述29例结肠癌及其癌旁组织内镜下活检标本,20例结肠癌及其癌旁组织手术切除标本,采用实时定量PCR检测miR-200c/141的相对表达量。miRNAs实时定量PCR所需引物、引物相应序列、序列长度(bp)以及退火温度(℃)如下。U6 RT引物序列为5'-CGCTTCACGAATTTGCGTGTCAT-3',PCR上游引物为5'-GCTTCGGCAGCACATATACTAAAAT-3',下游引物为5'-CGCTTCACGAATTTGCGTGTCAT-3',退火温度60℃,长度89bp;hsa-miR-200c的RT引物序列为5'-TCGTATCCAGTGCGTGTCGTGAGTCGG CAATTGCACTGGATACGACTCCATC-3',PCR上游引物为5'-GGTAATACTGCCGGGTAAT-3',下游引物为5'-CAGTGCGTGTCGTGGAGT-3',退火温度60℃,长度65bp;hsa-miR-141的RT引物序列为5'-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAA TTGCACTGGATACGACCCATCT-3',PCR上游引物为5'-GGGGTAACACTGTCTGGTAA-3',下游引物为5'-TGCGTGTCGTGGAGTC-3',退火温度60℃,长度63bp。结肠癌及其癌旁组织RNA的提取与质量控制、cDNA的合成与扩增严格按TaKaRa说明书进行操作,并采用相对循环阈(Ct)的方法进行评估[10]。miRNA的相对表达量以U6为内参,按2–ΔΔCt公式进行计算。
1.3 术后随访 所有患者术后随访1年。1年随访期内对每位患者至少进行5次随访,无失访病例。
1.4 统计学处理 采用SPSS 13.0进行统计分析。计量资料以表示,组间差异的比较采用单因素方差分析,采用直线相关分析结肠癌样本中miR-200c/141的相关性,采用秩和检验比较等级资料的差异性,患者总体生存率采用Kaplan-Meier和logrank进行分析。P<0.05为差异有统计学意义。
2 结 果
2.1 结肠癌及其癌旁组织中miR-200c/miR-141的定量及相关性分析 结肠癌组织中miR-200c 和miR-141的相对表达量分别为1.4307±1.6828和1.1802±0.8218,癌旁组织中两者的相对表达量分别为1.7939±1.4149和1.7614±1.5102。与癌旁组织相比,结肠癌组织中miR-200c/141相对表达量显著降低(图1A、B,P=0.0002,P=0.0058)。Spearman相关分析结果显示,结肠癌组织中miR-200c的表达量与miR-141的表达量呈正相关(图2),提示miR-200c 和miR-141作为一个染色体上连续排列的基因簇,在结肠癌组织中联合表达。

图1 实时定量PCR检测miR-200c(A)和miR-141(B)在人结肠癌及其癌旁组织中的表达Fig.1 Expressions of miR-200c (A) and miR-141 (B) in human colon cancer and peri-cancerous tissues (RT-PCR)N. Peri-cancerous mucosa; CA. Colon cancer

图2 结肠癌组织中miR-200c与miR-141表达水平的相关性分析Fig.2 Correlation analysis of expression levels of miR-200c and miR-141 in colon cancer tissues
2.2 miR-141表达量与结肠癌患者临床病理特征的关系 在结肠癌患者肿瘤组织中,有淋巴结转移组的miR-141表达量明显低于无淋巴结转移组(0.9295±0.5860vs1.4230±0.9478,P=0.035),临床分期为Ⅲ-Ⅳ期组的miR-141表达量低于临床分期为Ⅰ-Ⅱ期组(0.9474±0.5806vs1.4228±0.9682,P=0.042),但在年龄、性别、侵袭、远处转移等方面两组差异无统计学意义(P>0.05)。直径大于5cm的肿瘤组织miR-141表达量低于小于5cm的肿瘤组织,但差异无统计学意义(P>0.05)。T3和T4期患者相对于T1和T2期患者,发生远处转移的患者相对于未发生远处转移的患者,其结肠癌中miR-141的表达量均有降低的趋势,但差异无统计学意义(P>0.05,表1)。
2.3 miR-200c表达量与结肠癌患者临床病理学特征的联系 不同临床病理特征患者分组中,miR-200c表达量差异均无统计学意义(表1)。但是有远处转移的结肠癌患者与没有远处转移的结肠癌患者相比,其miR-200c表达有降低的趋势,但差异无统计学意义(P>0.05)。
2.4 miR-200c/141表达量与患者临床病理学特征的关系 对比结肠癌组织中miR-200c高表达与低表达(相对表达量以1.0878为界限),miR-141高表达与低表达(相对表达量以1.2228为界)的患者临床病理特征差别。结果显示,与miR-141高表达相比,miR-141低表达组的肿瘤直径一般大于5cm且伴有淋巴结转移,提示miR-141表达量与肿瘤大小和淋巴结转移明显相关(P<0.05)。但是与患者的年龄、性别、肿瘤分化、侵袭、远处转移和临床分期之间无关(表2)。与miR-200c高表达相比,miR-200c低表达的结肠癌患者其肿瘤直径多数大于5cm且多为女性,但是与肿瘤分化、侵袭转移、淋巴结转移和临床分期无相关性。
2.5 联合miR-200c和miR-141表达定量与患者临床病理学特征之间的联系 根据miR-200c和miR-141表达量将所有患者划分成3组,即miR-200c/141低表达组(miR-200c≤1.0878同时miR-141≤1.2228);miR-200c/141高表达组(miR-200c>1.0878同时miR-141>1.2228);miR-200c/141中等表达组(miR-200c/141其中之一低于临界值)。对比3组患者的临床病理学特征,结果表明miR-200c/141联合定量表达与患者的肿瘤大小存在明显关联(P=0.021,表3)。
2.6 结肠癌组织中miR-200c/141表达量在判断患者术后1年预后中的意义 根据患者结肠癌组织中miR-200c/141的表达水平将其分成高表达和低表达组,log-rank检测和Kaplan-Meier曲线分析两组患者总体生存率,结果显示,miR-200c/141高表达组和低表达组患者术后1年的总体生存率无明显差异(P=0.178,P=0.255,图3)。
表1 不同临床病理学特征患者肿瘤组织中miR-200c/141的表达量(±s)Tab.1 Expression of miR-200c/141 in the tumor tissues of patients with different pathological features (±s)

表1 不同临床病理学特征患者肿瘤组织中miR-200c/141的表达量(±s)Tab.1 Expression of miR-200c/141 in the tumor tissues of patients with different pathological features (±s)
Variable n miR-200c miR-141 Pvalue Age (year) <65 30 1.3438±0.9796 1.2609±0.8525 0.654 ≥65 19 1.5679±2.4439 1.0527±0.7759 0.393 Tumor size (cm) <5 33 1.2912±0.8655 1.3204±0.9088 0.546 ≥5 16 1.7185±2.7078 0.8910±0.5164 0.086 Invasion depth T1+T2 7 1.8255±1.5529 1.8640±1.2157 0.508 T3+T4 42 1.3649±1.7121 1.0663±0.6937 0.137 Tumor differentiation Low 9 2.0023±3.6031 1.0213±0.9525 0.578 Medium-High 40 1.3021±0.8542 1.2160±0.7987 0.526 Distal metastasis Absent 44 1.5008±1.7581 1.2231±0.8362 0.392 Present 5 0.8135±0.4702 0.8026±0.6232 0.283 Metastasis of lymph node(s) Absent 25 1.2464±0.9490 1.4230±0.9478 0.440 Present 24 1.6227±2.2123 0.9295±0.5860 0.035 Clinical stage Ⅰ-Ⅱ 24 1.2498±0.9693 1.4228±0.9682 0.467 Ⅲ-Ⅳ 25 1.6043±2.1677 0.9474±0.5806 0.042
3 讨 论
本研究首次观察结肠癌组织中miR-200c/141的表达水平和患者的临床病理特征以及术后短期生存率之间的联系。本研究结果提示miR-200c/141在结肠癌组织中的表达量明显低于癌旁组织,说明在正常结肠黏膜组织癌变过程中或癌细胞异型性维持过程中miR-200c/141是起负性调节作用的,癌变的发生与维持降低了其表达量,而表达量的降低又削弱了其肿瘤抑制功能,进而构成肿瘤无限增殖的恶性循环。同时,本研究证实miR-200c/141的表达量与患者肿瘤的大小、淋巴结转移、临床分期相关,这种相关性说明miR-200c/141的异常表达贯穿着肿瘤发生、发展、侵袭、转归的全过程,与术后短期生存率可能不相关,这种不相关性可能受术后患者依从性差、随访时间短等因素影响。虽然结肠癌组织中miR-200c的表达情况并不能影响患者临床病理特征,但是存在性别差异,男性患者miR-200c的表达量高(>1.0878),女性患者miR-200c的表达量低(≤1.0878),说明miR-200c在不同性别患者的结肠癌发生中所发挥的作用不同,在女性患者结肠癌的发生中可能更具主导地位。
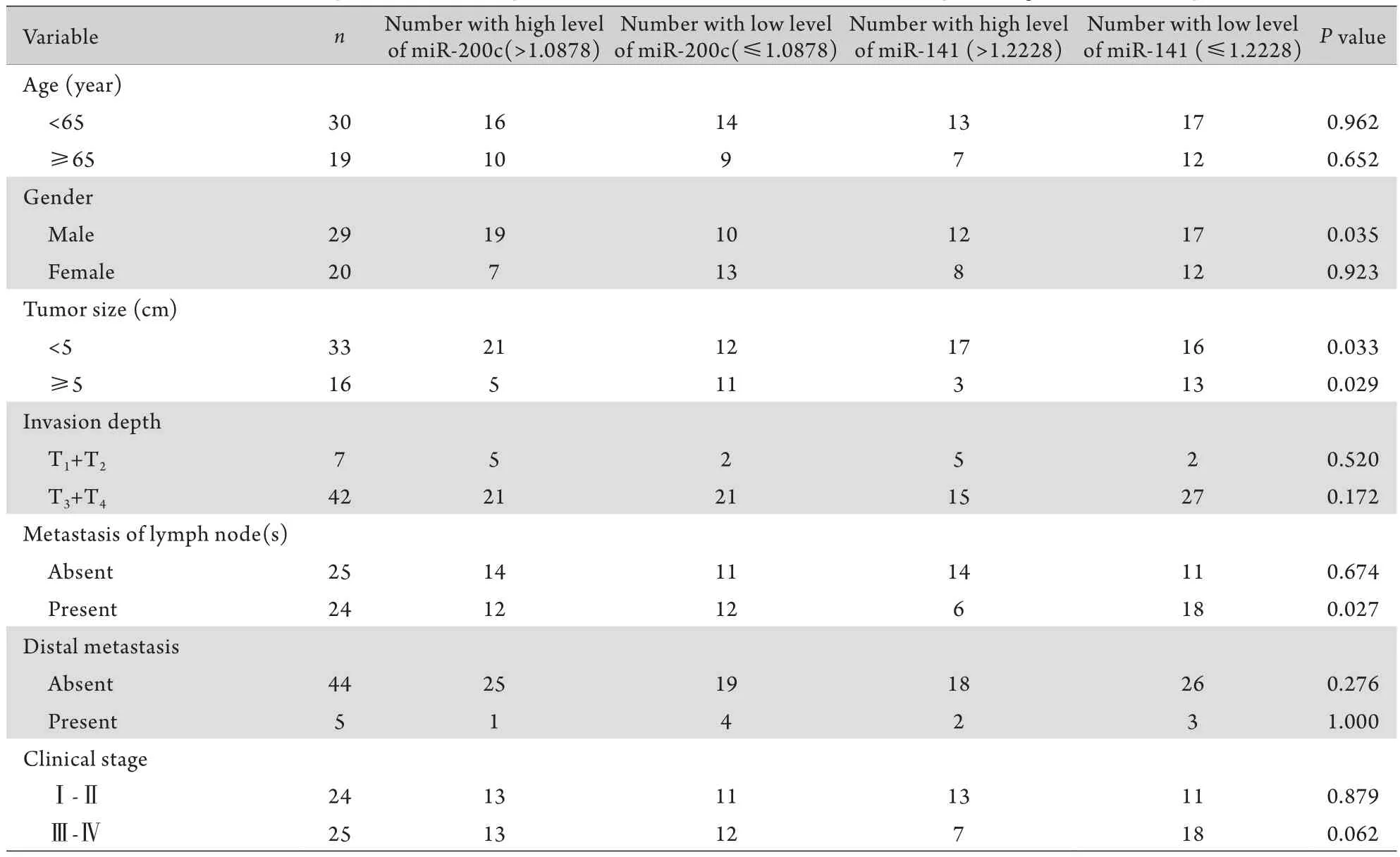
表2 miR-200c/141表达量与患者临床病理学特征之间的关系Tab.2 Relationship between the expression of miR-200c/141 and the clinic pathological features of patients
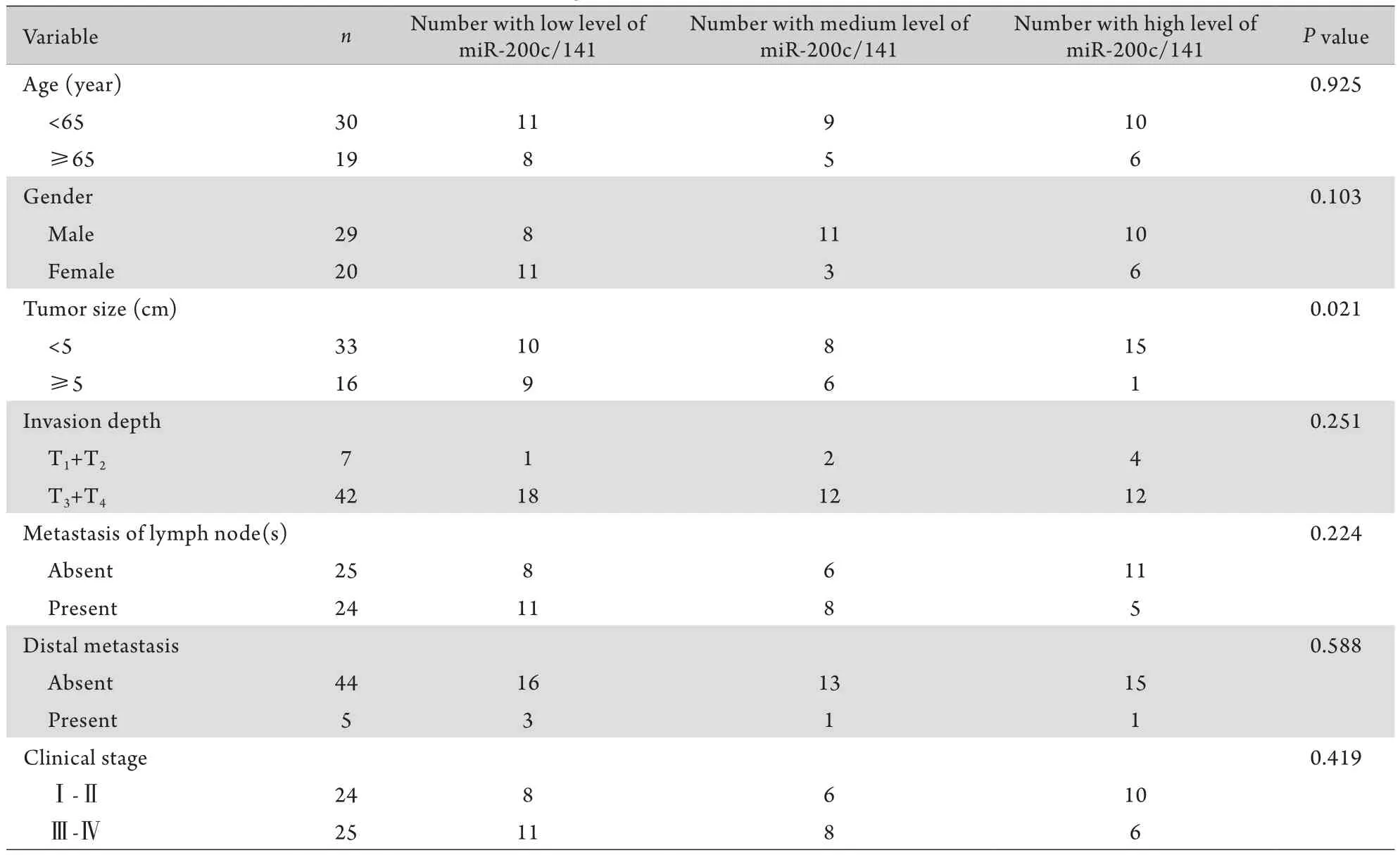
表3 联合miR-200c和miR-141表达定量与患者临床病理学特征之间的关系Tab.3 Relationship between the clinic pathological features and the combined miR-200c and miR-141 expression levels

图3 结肠癌患者术后1年的总体生存率Fig.3 Overall survival rate of colon cancer patients one year after surgery (Kaplan-Meier)A. According to miR-200c expression; B. According to miR-141 expression
关于结肠癌组织中miR-141定量的意义,伴有淋巴结转移或临床晚期(Ⅲ-Ⅳ)的结肠癌患者,其miR-141表达量低,无淋巴结转移或临床早期(Ⅰ-Ⅱ)的结肠癌患者其癌组织miR-141表达量高(P=0.035,P=0.042),说明伴随结肠癌临床分期的进展,miR-141在逐渐下降。我们进一步分析miR-141表达量与患者临床病理特征的相关性,当结肠癌大于5cm且伴有淋巴结转移时,其miR-141低表达。对比结肠癌患者miR-200c/141的表达情况,当肿瘤大于5cm时,miR-200c/141表达量有降低趋势(P=0.021)。这些数据客观反映了miR-200c/141与临床分期、淋巴结转移、肿瘤大小的相关性,其联合定量检测也许有助于结肠癌的诊疗和预防。
miRNAs是21~23nt的小非编码RNA,大多可结合到mRNA的3'非编码区域(3'-UTRs)管理其下游蛋白的表达[16]。近年来大量数据证实了miRNAs与肿瘤的相关性[17-19]。根据靶基因的不同,miRNA可抑制或促进肿瘤形成。其中miR-200家族抑制肿瘤形成,包括miR-200a、miR-200b、miR-429、miR-200c 和miR-141[20]。这些miRNAs可以维持结肠癌细胞的上皮特征性[12]。通过作用于EMT抑制因子或转录因子ZEB1和ZEB2从而抑制细胞的侵袭转移[21-22]。尽管在乳腺癌组织中miR-200表达情况不尽相同[23],但是在结肠癌组织中miR-200的表达量是下降的[24]。另外,DNA甲基化是一种十分重要的表位修饰方式,调控miR-200c/141的转录激活[25]。一系列的转录因子都是通过调节miR-200家族来管理EMTMET[26-27],我们以前证实miR-200可以由Ascl2进行调节,然后影响结肠癌细胞的生物学行为,例如侵袭转移能力[12]。miR-200c/141与胃癌、肺癌、结肠癌、乳腺癌等肿瘤的生物学行为相关[13],但是与结肠癌的相关性研究报道有限。在结肠癌患者血清中,miR-200c/141是提示预后及转移的生物学检测标记物。有研究发现miR-200c在结肠癌EMT以及远位转移中发挥关键作用[24]。也许miR-200c会成为结肠癌患者诊断的临床标记和治疗的靶点。目前,我们的研究可能存在的不足是样本量不多(n=49),随访时间不长(术后1年)。因此,进一步扩大临床病例样本数和延长随访时间可能提供更加有价值的结论。
总之,在结肠癌发生中,miR-200c/141作为抑癌基因,其表达量与癌旁组织相比明显下降,与患者的肿瘤大小、淋巴结转移、肿瘤临床分期密切相关,与患者的短期预后无关。
[1]Peto J. Cancer epidemiology in the last century and the next decade[J]. Nature,2001,411(6835): 390-395.
[2]Siegel R,DeSantis C,Virgo K,et al. Cancer treatment and survivorship statistics[J]. Ca Cancer J Clin,2012,62(4): 220-241.
[3]Herzig Do,Tsikitis VL. Molecular markers for colon diagnosis,prognosis and targeted therapy[J]. J Surg Oncol,2015,111(1): 96-102.
[4]Wang ZH,Wei SP,Xie HW,et al. Screening of colorectal cancer associated genes with a variety of high throughput expression profile data[J]. J Logist Univ PAPF (Med Sci),2015,24(2): 97-99. [王正晖,韦淑萍,解宏伟,等. 多种高通量表达谱数据分析方法筛选大肠癌相关基因[J]. 武警后勤学院学报(医学版),2015,24(2): 97-99.]
[5]Kohlhapp FJ,Mitra AK,Lengyel E,et al. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment[J]. Oncogene,2015,Epub ahead of print.
[6]Muhammad S,Kaur K,Huang R,et al. MicroRNAs in colorectal cancer: role in metastasis and clinical perspectives[J]. World J Gastroenterol,2014,20(45): 17011-17019.
[7]Zhang LQ,Sun GL. Research and clinical application of miRNAs in cancer stem cells and as tumor markers[J]. Tianjin Med J,2014,42(10): 1048-1050. [张娄强,孙庚林. 肿瘤干细胞miRNA及miRNA作为肿瘤标志物的研究和临床应用进展[J]. 天津医药,2014,42(10): 1048-1050.]
[8]Suto T,Yokobori T,Yajima R,et al. MicroRNA-7 expression in colorectal cancer is associated with poor prognosis and regulates cetuximab sensitivityviaEGFR regulation[J]. Carcinogenesis,2015,36(3): 338-345.
[9]Jinushi T,Shibayama Y,Kinoshita I,et al. Low expression levels of microRNA-124-5p correlated with poor prognosis in colorectal cancerviatargeting of SMC4[J]. Cancer Med,2014,3(6): 1544-1552.
[10] Zhu R,Yang Y,Tian Y,et al. Ascl2 knockdown results in tumor growth arrest by miRNA-302b-related inhibition of colon cancer progenitor cells[J]. PLoS one,2012,7(2): e32170.
[11] Li SS,Zhong XL,Shang YY. Quantitative expression of Ascl2 and microRNA200 in colorectal cancer and their peri cancerous mucos and their correlation[J]. J Third Mil Med Univ,2014,36(23): 2377-2379. [李姗姗,钟小莉,尚阳阳,等. Ascl2和microRNA-200家族在结肠癌组织和癌旁组织中的定量表达及二者的相关性分析[J]. 第三军医大学学报,2014,36(23): 2377-2379.]
[12] Tian Y,Pan Q,Shang Y,et al. MicroRNA-200(miR-200)cluster regulation by achaete scute-like 2(Ascl2): impact on the epithelial-mesenchymal transition in colon cancer cells[J]. J Biol Chem,2014,289(52): 36101-36115.
[13] Zhong XL,Shang YY,Pan Q. Transcriptional regulation of CDX2 gene expression by Ascl2 in colon cancer epithelial cells[J]. J Third Mil Med Univ,2014,36(18): 1867-1871. [钟小莉,尚杨杨,潘琼,等. Ascl2转录调控人结肠癌上皮细胞内CDX2基因表达的研究[J]. 第三军医大学学报,2014,36(18): 1867-1871.]
[14] Hur K,Toiyama Y,Takahashi M,et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis[J]. Gut,2013,62(9): 1315-1326.
[15] Shao Y,Geng Y,Gu W,et al. Prognostic role of tissue and circulating microRNA-200c in malignant tumors: a systematic review and meta-analysis[J]. Cell Physiol Biochem,2015,35(3): 1188-1200.
[16] Bartel DP. MicroRNAs: genomics,biogenesis,mechanism,and function[J]. Cell,2004,116(2): 281-297.
[17] Chang H,Kim N,Park JH,et al. Different microRNA expression levels in gastric cancer depending on helicobacter pylori infection[J]. Gut liver,2015,9(2): 188-196.
[18] Xia Y,Zhu Y,Ma T,et al. miR-204 functions as a tumor suppressor by regulating SIX1 in NSCLC[J]. FEBS Letters,2014,588(20): 3703-3712.
[19] Ali S,Ahmad A,Aboukameel A,et al. Deregulation of miR-146a expression in a mouse model of pancreatic cancer affecting EGFR signaling[J]. Cancer Letters,2014,351(1): 134-142.
[20] Humphries B,Yang C. The microRNA-200 family: small molecules with novel roles in cancer development,progression and therapy[J]. Oncotarget,2015,6(9): 6472-6498.
[21] Perdigao-Henriques R,Petrocca F,Altschuler G,et al. miR-200 promotes the mesenchymal to epithelial transition by suppressing multiple members of the Zeb2 and Snail1 transcriptional repressor complexes[J]. Oncogene,2015,Epub ahead of print.
[22] Li X,Roslan S,Johnstone C N,et al. MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesindependent pathways[J]. Oncogene,2014,33(31): 4077-4088.
[23] Tuomarila M,Luostari K,Soini Y,et al. Overexpression of microRNA-200c predicts poor outcome in patients with PR-negative breast cancer[J]. PLoS one,2014,9(10): e109508.
[24] Toiyama Y,Hur K,Tanaka K,et al. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer[J]. Ann Surg,2014,259(4): 735-743.
[25] Lim YY,Wright JA,Attema JL,et al. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state[J]. J Cell Sci,2013,126(pt10): 2256-2266.
[26] Teng Y,Mei Y,Hawthorn L,et al. WASF3 regulates miR-200 inactivation by ZEB1 through suppression of KISS1 leading to increased invasiveness in breast cancer cells[J]. Oncogene,2014,33(20): 203-211.
[27] Bracken CP,Gregory PA,Kolesnikoff N,et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition[J]. Cancer Res,2008,68(19): 7846-7854.
Clinicopathological significance of quantitative expression of miR-200c /141 in colon carcinoma tissue
MENG Ling-kuan,ZHONG Xiao-li,PAN Qiong,YE Jun,SHANG Yang-yang,GUO Jing,WANG Rong-quan*
Institute of Gastroenterology,Southwest Hospital,Third Military Medical University of PLA,Chongqing 400038,China
*< class="emphasis_italic">Corresponding author,E-mail: rongquanw@hotmail.com
,E-mail: rongquanw@hotmail.com
This work was supported by the National Natural Science Foundation of China (81372557)
ObjectiveTo explore the relationship between the miR-200c and miR-141 expression levels in the cancerous tissues of colon carcinoma and the clinicopathological features of the patients.MethodsThe quantitative real-time RT-PCR was used to determine the miR-200c and miR-141 expression levels in the cancerous tissues obtained from 49 colon carcinoma patients and their tissues adjacent to the cancer.ResultsCompared with the tissues adjacent to the cancer,the miR-200c and miR-141 expression levels in colon carcinoma samples were significantly reduced (P=0.0002 andP=0.0058). A negative correlation was found between miR-141 expression level and both the tumor size and the existence of lymph node metastasis in the colon carcinoma patients (P<0.05). The miR-141 expression levels in the cancerous tissues of colon carcinoma patients who had lymph node metastasis were significantly lower than those who did not have lymph node metastasis (P=0.035). At the same time,the miR-141 expression levels in the cancerous tissues of colon carcinoma patients who were at clinical Ⅲ-Ⅳ stages were significantly lower than in those who were at clinical Ⅰ-Ⅱ stages (P=0.042). There was a significant correlation between the miR-200c/141 expression levels and tumor size (P=0.021). However,no relationship was found between the miR-200c and miR-141 expression levels and the cumulative survival rate of patients one-year after surgical treatment (P>0.05).ConclusionThe down-regulation of miR-200c/141expression in colon carcinoma tissues is related to the tumor size,lymph node metastasis and clinical stages of colon carcinoma patients.
colonic neoplasms; pathology,clinical; microRNAs
R574.62
A
0577-7402(2015)12-0987-06
10.11855/j.issn.0577-7402.2015.12.10
2015-07-03;
2015-08-13)
(责任编辑:熊晓然)
国家自然科学基金(81372557)
孟令宽,硕士研究生。主要从事消化道肿瘤的基础与临床研究
400038 重庆 第三军医大学西南医院消化内科(孟令宽、钟小莉、潘琼、叶钧、尚杨杨、郭靖、汪荣泉)
汪荣泉,E-mail: rongquanw@hotmail.com
