低浓度亚砷酸钠下调PML蛋白表达诱导肝癌干细胞分化的分子途径
2015-06-28金世龙谭智明张鹏匡远黎杜波唐华明王郑
金世龙,谭智明,张鹏,匡远黎,杜波,唐华明,王郑
低浓度亚砷酸钠下调PML蛋白表达诱导肝癌干细胞分化的分子途径
金世龙,谭智明,张鹏,匡远黎,杜波,唐华明,王郑
目的探讨低浓度亚砷酸钠诱导肝癌干细胞(LCSCs)分化的分子途径。方法采用免疫荧光、Western blotting和荧光定量PCR分析肝细胞癌(HCC)组织和细胞PML、Oct4和Sox2基因的表达情况,比较低浓度亚砷酸钠处理后HCC细胞PML、Oct4和Sox2基因表达及LCSCs生物功能的变化,确定低浓度亚砷酸钠对LCSCs分化的诱导作用及其分子途径。结果0.5μg/ml亚砷酸钠可明显抑制HuH7和原代HCC细胞形成肿瘤球,增强HuH7细胞对吡柔吡星(THP)的敏感性(P<0.01),并抑制HuH7和原代HCC细胞移植成瘤的能力(P<0.05)。HCC组织和细胞表达PML、Oct4、Sox2 mRNA和蛋白,且0.5μg/ml亚砷酸钠可使Oct4、Sox2 mRNA(P<0.05)和蛋白(P<0.01)表达下调。0.5μg/ml亚砷酸钠处理后HuH7和原代HCC细胞PML蛋白表达明显下调(P<0.05),而Hep3B、HepG2、SMCC-7721、HuH7和原代HCC细胞PML mRNA表达无明显变化。0.5μg/ml亚砷酸钠可抑制含LCSCs的HuH7和原代HCC细胞关键基因Oct4、Sox2 mRNA的表达(P<0.05),而且能改变LCSCs对THP的敏感性、抑制肿瘤球形成和异体移植成瘤等生物学特征。结论HCC组织和细胞表达PML mRNA及蛋白,低浓度亚砷酸钠与HCC细胞内PML蛋白直接结合使之降解,PML核体(PML-NB)随之崩解,PML-NB上与增殖分化相关基因的转录被抑制,从而下调HCC细胞Oct4、Sox2等LCSCs相关基因的表达,抑制HCC细胞生长和诱导LCSCs分化。
癌,肝细胞;肿瘤干细胞;细胞分化
肝细胞癌(hepatocellular carcinoma,HCC)发病率居世界恶性肿瘤第五位,诊断时多数已属进展期[1-2],手术切除率低于50%,即使手术切除也易复发和转移,5年生存率只有10%~30%。肝癌干细胞(liver cancer stem cells,LCSCs)具有自我更新、多潜能分化和抵抗放化疗等特性[3],在HCC发生、发展、复发和转移中起关键作用[4-6]。目前临床治疗尚不能针对性地清除LCSCs,基础研究也未找到防治HCC复发和转移的方法。尽管多项研究结果显示砒霜(arsenic trioxide,As2O3)或亚砷酸钠能明显抑制HCC细胞增殖并诱导癌细胞凋亡,但多中心Ⅱ期临床试验结果显示单用As2O3治疗HCC效果并不满意[7]。近期本课题组研究发现HuH7和原代HCC细胞含有LCSCs,低浓度亚砷酸钠可抑制HCC细胞Oct4、Sox2表达,诱导LCSCs分化[8]。本研究探讨亚砷酸钠是否可下调HCC细胞早幼粒细胞白血病(promyelocytic leukemia,PML)蛋白表达以及低浓度亚砷酸钠是否通过PML蛋白诱导LCSCs分化。
1 材料与方法
1.1 主要材料及试剂 HuH7、HepG2、Hep3B、SMMC-7721等HCC细胞由第三军医大学西南医院肝胆外科和病理科赠送。DMEM培养基和胎牛血清为Hyclone公司产品,PML抗体购自Fantibody公司。HCC组织取自切除的肝癌组织标本。LSM780激光共聚焦显微镜为Carl Zeiss公司产品。总RNA提取试剂盒、cDNA反转录试剂盒为BioFlux公司产品,Promega GoTaq qPCR Master Mix为Promega公司产品。Mx3000P荧光定量PCR仪为美国STRATAGNE公司产品。
1.2 方法
1.2.1 亚砷酸钠处理HCC细胞 HCC细胞在含10% FBS的DMEM培养液中于37℃、5%CO2、饱和湿度的培养箱孵育,细胞汇合度达到70%~80%时采用0.25%胰酶消化细胞。普通及原代培养HCC细胞经0.1~1.0μg/ml终浓度亚砷酸钠处理。
1.2.2 肿瘤球培养 以无血清DMEM/F12(1:1)基础培养基,加入EGF(10ng/ml)、bFGF(20μg/ml)、N2、B27等制成标准培养基,于超低黏附6孔板加入1ml标准培养基及5.0×103个HuH7和原代HCC细胞,在37℃、5%CO2、饱和湿度培养箱中孵育,每日半量换新鲜培养基,观察记录细胞球情况。
1.2.3 CCK-8分析HuH7细胞对吡柔吡新(THP)的敏感性 将HuH7细胞接种于96孔板,每孔加1.0×103个细胞,加入100μl含10%FBS的DMEM培养基(每2d换液)使其贴壁生长。次日细胞贴壁后分别加入不同浓度亚砷酸钠(0.25、0.5、0.75、1.0μg/ml),每种浓度设12个孔,设调零孔12个(不加细胞),细胞对照孔12个(不加药物),每组设0.5μg/ml THP对照孔12个(加THP),在37℃、5%CO2、饱和湿度的培养箱中孵育96h后吸出培养液。每孔分别加入90μl DMEM培养基和10μl CCK-8溶液,继续培养2h,在酶标仪上测定细胞在450nm处的吸光度(A)值。
1.2.4 Western blotting分析HCC细胞PML、Oct4和Sox2蛋白表达
1.2.4.1 HCC细胞总蛋白提取 将HuH7、HepG2、Hep3B和SMCC-7721这4种HCC细胞常规胰酶消化制成细胞悬液,细胞数1.0×106,1500r/min离心5min,移入1.5ml EP管,PBS洗2次。细胞悬液离心后吸尽上清液,加入含蛋白酶抑制剂的裂解液200μl,冰上裂解30min,4℃下12 000r/min离心15min,上清液移入EP管,蛋白定量后置–70℃备用。
1.2.4.2 HCC组织总蛋白提取 患者接受HCC切除术,切除HCC组织样本后立即切取癌肿组织(肿瘤边缘),称取100mg HCC加入500μl预冷的细胞裂解液,置于冰上用玻璃匀浆器碾磨,裂解30min,4℃12 000r/min离心15min,上清液移入EP管,蛋白定量后置–70℃备用。
1.2.4.3 Western blotting检测HCC细胞PML蛋白表达 配制10%分离胶和4%积层胶。上样量为每泳道90μg,体积25~30μl。电泳积层胶电压80V,分离胶电压100~120V,电泳2~3h后转至PVDF膜。取下PVDF膜,5%脱脂奶粉PBS封闭,室温振摇1~2h。将封闭的膜滴加一抗按1:100(3%BSA),4℃湿盒内过夜,次日PBS漂洗15min×1次,5min×4次,二抗按1:2500(3%BSA),25℃振摇1h,PBS漂洗15min×1次,5min×4次。混合等体积A液和B液(各500μl,共1ml),将显色剂滴加到PVDF膜正面,开始显色记录图像。
1.2.4.4 免疫荧光分析HCC细胞PML蛋白的表达4种HCC细胞普通培养在10mm盖玻片上,待60%~80%融合时用37℃预热PBS振摇漂洗,5min×2次,4%多聚甲醛室温固定15min,PBS洗5min后用含0.5% Triton-X-100的PBS室温下脱色,摇床振摇15min,PBS洗3次。取出盖玻片放到载玻片上,10%山羊血清37℃封闭30min,PML抗体用PBS按1:50稀释后滴加15~20μl到盖玻片上,湿盒内4℃冰箱过夜。有细胞的盖玻片放进24孔板,PBS脱色,摇床洗3次,取出盖玻片滴加20μl用PBS按1:50稀释的荧光抗体,37℃避光孵育60min。盖玻片放进24孔板内,经PBS洗3次,Hoechst33342染细胞核,PBS洗盖玻片2次,取出盖玻片,封片后激光共聚焦显微镜下观察,记录细胞PML蛋白表达情况。
1.2.5 荧光PCR分析HCC细胞PML、Oct4和Sox2 mRNA表达 用Trizol Reagent提取HCC细胞总RNA,检测RNA含量后立即置–130℃保存。按BioKT cDNA first strand cynthesis Kit使用说明设置20μl反应体系,置室温10min,于42℃ 45min、95℃5min、冰浴5min的循环条件下进行反应。反转录后检测cDNA含量,于–20℃保存。引物序列如下:GAPDH正义5'-TGCAACCGGGAAGGAAATGA-3',反义5'-GCCCAATACGACCAAATCAGA-3;PML正义5'-GGCTCGAGAAGGATGTGGTC-3',反义5'-GAAGTGAGGGCTCCCATAGC-3';Oct4正义5'-GGCTCGAGAAGGATGTGGTC-3',反义5'-GAAGTGAGGGCTCCCATAGC-3';Sox2正义5'-CAGGAGTTGTCAAGGCAGAGA-3',反义5'-CGCCGCCGATGATTGTTATT-3'。荧光定量PCR反应条件:预变性95℃ 4min;95℃ 30s,55℃20s,72℃ 20s,40个循环。扩增产物经过3%琼脂糖凝胶电泳鉴定为目标条带。
1.3 HCC细胞异体移植成瘤实验 HCC细胞按每组2.0×106个细胞分为4组,分别采用PBS配成100μl细胞悬液,注射到清洁级裸鼠背部皮下。细胞移植后隔日皮下注射3~5μg亚砷酸钠,共注射7次,每组注射10只裸鼠,每周观察成瘤情况。
1.4 统计学处理 采用SPSS 13.0软件进行统计学分析。计量资料之间的比较采用单因素方差分析,率的比较采用χ2检验。P<0.05为差异有统计学意义。
2 结 果
2.1 亚砷酸钠对HuH7细胞THP敏感性的影响0.5μg/ml亚砷酸钠可明显抑制HuH7和原代HCC细胞形成肿瘤球(图1)。CCK-8检测结果显示,0.5μg/ml THP、0.5μg/ml亚砷酸钠均不能抑制HuH7细胞生长(P=0.06,P=0.933),0.75μg/ml亚砷酸钠和0.5μg/ml亚砷酸钠+0.5μg/ml THP可明显抑制HuH7细胞增殖(P<0.001,图2)。提示亚砷酸钠可增强HuH7细胞对THP的敏感性。
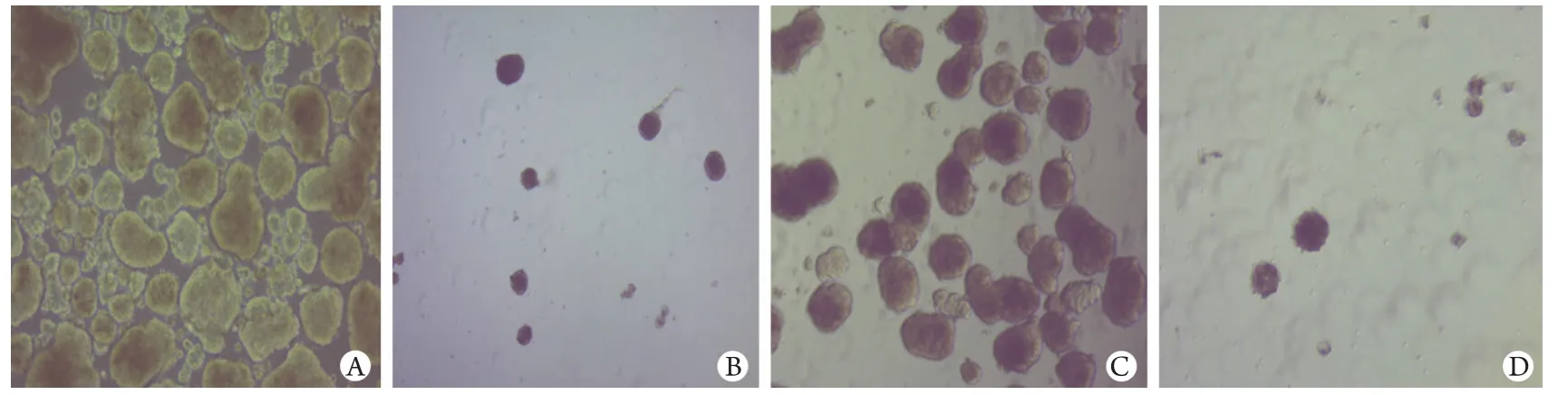
图1 亚砷酸钠对HuH7和原代HCC细胞肿瘤球形成的影响Fig.1 Effect of sodium arsenite on the formation of tumor sphere in HuH7 cells and primary HCC cellsA. HuH7 cells; B. HuH7 cells treated with 0.5μg/ml of sodium arsenite; C. Primary HCC cells; D. Primary HCC cells treated with sodium arsenite
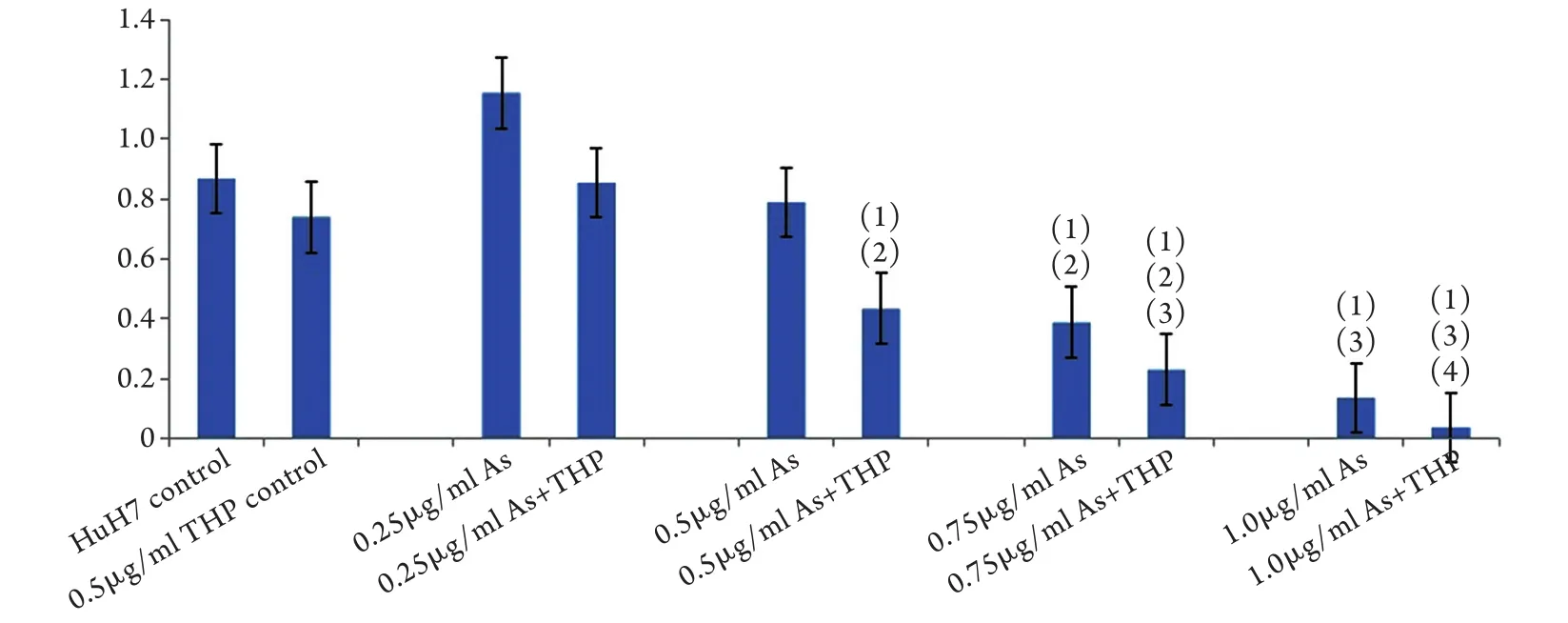
图2 亚砷酸钠和THP处理96h后HuH7细胞生长抑制情况(CCK-8分析)Fig.2 Growth inhibition of HuH7 cells 96h after treatment with sodium arsenite and THP (CCK-8 analysis)(1)P<0.01 compared with THP control group; (2)P<0.01 compared with 0.5μg/ml As group; (3)P<0.01 compared with 0.75μg/ml As group; (4)P<0.01 compared with 1.0μg/ml As group
2.2 亚砷酸钠对HuH7和原代HCC细胞PML蛋白表达的影响 Western blotting检测结果显示,HuH7、HepG2、Hep3B、SMMC-7721表达PML蛋白(图3A)。24例HCC组织均不同程度表达PML蛋白(图3B)。免疫荧光分析结果显示,PML蛋白表达于HuH7、HepG2、SMCC-7721和Hep3B细胞核,在核内呈点状颗粒分布(图3E)。荧光定量PCR分析结果显示,HCC组织及Hep3B、HepG2、SMCC-7721、HuH7和原代HCC细胞都表达PML mRNA(图3C、F)。经亚砷酸钠处理96h后HuH7和原代HCC细胞PML蛋白表达明显下调(P<0.05,图3D),HuH7细胞核PML蛋白荧光颗粒明显减少(P<0.01,图3E),随0.5μg/ml亚砷酸钠处理HuH7细胞时间延长,PML蛋白逐渐减少,最后消失(图3G)。亚砷酸钠处理后Hep3B、HepG2和SMCC-7721细胞核PML蛋白荧光颗粒减少更快。荧光定量PCR分析显示,亚砷酸钠处理Hep3B、HepG2、SMCC-7721、HuH7和原代HCC细胞后,其PML mRNA表达无明显变化(图3F)。提示亚砷酸钠直接下调HuH7和原代HCC细胞表达PML蛋白,早期对HCC细胞PML mRNA表达无明显作用。

图3 亚砷酸钠对HuH7和原代HCC细胞PML蛋白表达的影响Fig.3 Effect of sodium arsenite on the expression of PML protein in HuH7 and HCC primary cellsA.PML protein expression in HuH7,HepG2,Hep3B and SMMC-7721 cells (Western blotting,NB4 and HL60 cells were utilized as positive controls,Lo2 cells were used as human hepatic cell control); B. PML protein expression in HCC tissue samples; C. PML gene expression in HCC tissues; D. PML protein expression in HuH7 and primary HCC cells (treatment with 0.5μg/ml of sodium arsenite for 5 days; E. PML protein expression in the nuclei of HuH7,HepG2,SMCC-7721 and Hep3B cells [×400,the number of fluorescent PML protein particles was markedly decreased in the nuclei of HuH7 cells treated with 0.5μg/ml of sodium arsenite for 5 days (HuH7+As)]; F. PML gene expression in five sort of HCC cells (no significant change when treated with sodium arsenite for 5 days,P>0.05); G. PML protein expression in HuH7 cells (down-regulated gradually when treated with 0.5μg/ml sodium arsenite after 24,48,72,96 and 120h cultivation)
2.3 亚砷酸钠对HuH7和原代HCC细胞Oct4、Sox2 mRNA表达的影响 24例HCC组织和4种HCC细胞均表达Oct4蛋白(图4A),22例HCC组织和4 种HCC细胞表达Sox2蛋白(图4B)。24例HCC组织均存在Oct4(图4C)和Sox2(图4D)mRNA表达。经亚砷酸钠处理后,HuH7和原代HCC细胞Oct4、Sox2 mRNA(P<0.05),Oct4蛋白(P<0.05)和Sox2蛋白(P<0.01)表达均下调(图4A、B)。
2.4 HCC细胞异体移植成瘤实验 结果显示,10只裸鼠皮下移植HuH7细胞,9只裸鼠成瘤(9/10),10只裸鼠皮下移植HCC原代细胞,7只裸鼠成瘤(7/10)。HCC细胞移植后隔日皮下注射3~5μg亚砷酸钠,连续用7次,HuH7组仅1只裸鼠成瘤(1/10),原代HCC细胞组没有成瘤(0/10),显示亚砷酸钠具有抑制HuH7和原代HCC细胞移植成瘤的能力(P<0.05),推测亚砷酸钠已诱导LCSCs发生分化。
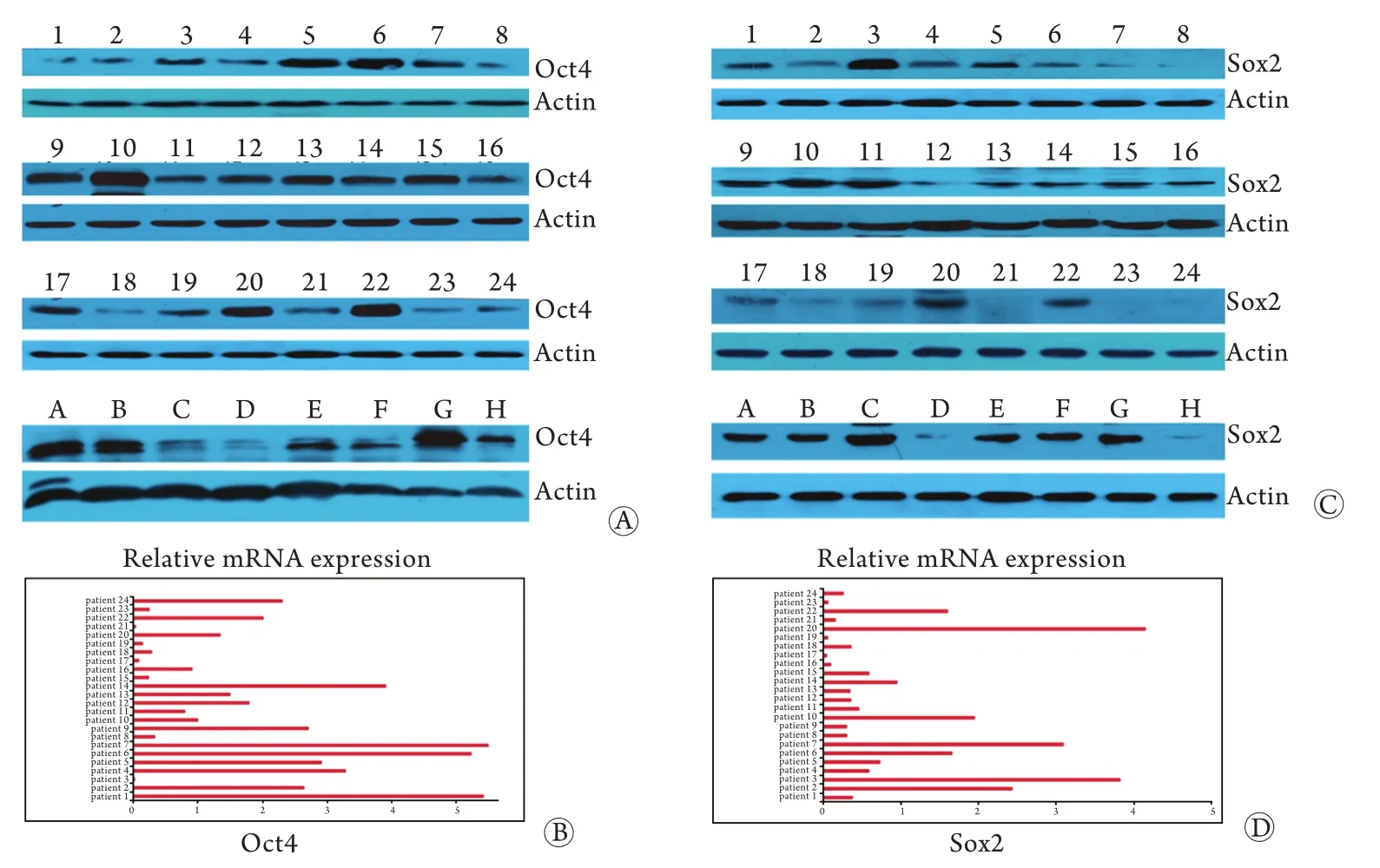
图4 HCC组织及细胞Oct4、Sox2 mRNA(荧光PCR)和蛋白(Western blotting)表达Fig.4 Expression of genes and proteins of Oct4 and Sox2 in HCC tissue and cellsA. Oct4 and Sox2 protein expression (24 HCC tissues); B. Oct4 and Sox2 protein expression (HepG2,SMCC-7721,Hep3B,HuH7 cells); C,D. Oct4 and Sox2 gene expression (24 HCC tissues); 1,3,5,7. HepG2,SMCC-7721,Hep3B and HuH7 cells; 2,4,6,8. HepG2,SMCC-7721,Hep3B and HuH7 cell treated with 0.5μg/ml sodium arsenite for 5 days
3 讨 论
LCSCs具有自我更新、多潜能分化和抵抗放化疗等特性,在HCC的发生、发展、复发和转移中起关键作用。近期研究证实亚砷酸钠具有抑制HepG2、Hep3B、SMCC-7721和HuH7细胞生长的作用,HuH7细胞耐受能力最强,HuH7和原代HCC细胞含LCSCs,亚砷酸钠可诱导LCSCs分化[8]。本研究结果显示,0.5μg/ml亚砷酸钠可明显抑制HuH7和原代HCC细胞形成肿瘤球,且明显增强HuH7细胞对THP的敏感性,具有抑制HuH7和原代HCC细胞移植成瘤的能力,提示亚砷酸钠可抑制或改变LCSCs特有的生物学功能,LCSCs能被亚砷酸钠诱导分化或清除。
本课题组前期研究发现HuH7和原代HCC细胞可表达Oct4、Sox2蛋白,且亚砷酸钠可以下调这两种基因及蛋白的表达[8],本研究进一步证实HCC组织和细胞均表达Oct4、Sox2等干细胞转录因子,且亚砷酸钠具有抑制或下调HCC细胞Oct4、Sox2等LCSCs关键基因及蛋白表达的作用。
本课题组前期研究还发现HCC组织和HuH7细胞可表达PML mRNA和蛋白[8],为明确亚砷酸钠是否直接靶向HCC细胞下调PML蛋白的表达,我们首先观察是否所有HCC组织和细胞株都表达PML蛋白,其次用0.5μg/ml亚砷酸钠处理HuH7和原代HCC细胞5d,检测HCC细胞PML蛋白和mRNA的表达变化。结果显示,亚砷酸钠处理HuH7和原代HCC细胞后PML蛋白表达均明显下调,HuH7细胞核PML蛋白荧光颗粒明显减少,而Hep3B、HepG2、SMCC-7721和HuH7细胞PML mRNA表达无明显变化。提示HCC细胞和组织表达PML蛋白和基因,具有亚砷酸钠直接靶向作用的分子基础,且亚砷酸钠可直接下调HuH7和原代HCC细胞PML蛋白的表达。
PML基因可维持造血干细胞(hematopoietic stem cekks,HSCs)、急性粒细胞白血病(acute promyelocytic leukemia,APL)及慢性粒细胞白血病(chronic myelogenous leukemia,CML)的白血病起始细胞(leukemia initiating cells,LICs)稳定[9],PML蛋白表达下调或缺失可导致其分化。PML蛋白可与Daxx,Sp100,CBP/p300,SUMO-1,pRb,rfp,ZSG20等形成PML核体(PML nuclear bodies,PMLNB),PML-NB募集转录因子并参与染色体构型调控转录,PML蛋白表达减少或缺失可使PML-NB结构解体,多种核转录因子失去发挥功能的平台[10-13]。PML蛋白和PML-NB决定Oct4的表达,Oct4基因表达和维持干细胞染色体开放构型也需要PML蛋白,PML蛋白表达缺失会导致细胞失去稳定性而发生分化[12-17]。Chuang等[14]研究证实,PML蛋白是Oct4表达的阳性调节子,PML蛋白下调可抑制Oct4基因表达。Sox2基因在维持LCSCs多能性和决定其是否走向分化方面具有关键作用[18-19]。即PML蛋白不仅决定干细胞关键基因Oct4、Sox2的表达,而且还维持着HSCs、APL及CML的LICs稳定。我们的结果显示,亚砷酸钠不仅使Oct4、Sox2 mRNA和蛋白表达下调,而且可改变HuH7和HCC原代细胞中LCSCs的功能特征,表明Oct4蛋白下调使LCSCs难以维持多能性,LCSCs可能已经分化。Zhang等[11]证实As2O3治疗APL的机制在于As2O3中砷直接与癌蛋白PML-RAR结合,使癌蛋白降解。本研究发现经亚砷酸钠处理后HuH7和原代HCC细胞PML蛋白表达明显下调,HuH7细胞核PML蛋白荧光颗粒明显减少,随着0.5μg/ml亚砷酸钠处理HuH7细胞时间的延长,PML蛋白逐渐减少,最后消失,但荧光定量PCR分析发现亚砷酸钠处理Hep3B、HepG2、SMCC-7721、HuH7和原代HCC细胞后,其PML mRNA表达无明显变化,提示亚砷酸钠直接下调HuH7和原代HCC细胞PML蛋白的表达,进一步肯定了砷直接与HCC或APL细胞PML蛋白结合,使PML蛋白降解。
综上所述,我们认为HCC组织和细胞株表达PML基因及蛋白,低浓度亚砷酸钠与HCC细胞内PML蛋白直接结合使之降解,PML-NB随之崩解,PML-NB上与增殖分化相关的基因转录被抑制,从而下调HCC细胞Oct4、Sox2等LCSCs相关基因的表达,抑制HCC细胞生长和诱导LCSCs分化。
[1]Qiu XX,Zuo M. Progress in diagnosis and treatment of hepatocellular carcinoma[J]. Tianjin Med J,2014,42(1): 90-93. [邱秀霞,左苗. 肝细胞癌的诊治进展[J]. 天津医药,2014,42(1): 90-93.]
[2]Chen L,Li Y,Zhou HY,et al. Research progress in treatment of multifocal hepatocellular carcinoma[J]. Tianjin Med J,2013,41(12): 1224-1226. [陈璐,李燕,周洪渊,等. 多灶性肝细胞癌治疗的研究进展[J]. 天津医药,2013,41(12): 1224-1226.]
[3]Ge LL,Yang XA. Cancer stem cell-like properties of EpCAM+cell subpopulation in hepatocellular carcinoma cell line Huh-7[J]. J Zhengzhou Univ (Med Sci),2012,47(3): 306-309. [葛丽丽,杨小昂. 肝癌细胞系Huh-7中EpCAM+细胞亚群的肿瘤干细胞样特性[J]. 郑州大学学报(医学版),2012,47(3): 306-309.]
[4]Llovet JM,Burroughs A,Bruix J. Hepatocellular carcinoma[J]. Lancet,2003,362(9399): 1907-1917.
[5]Haraguchi N,Ishii H,Mimori K,et al. CD13 is a therapeutic target in human liver cancer stem cells[J]. J Clin Invest,2010,120(9): 3326-3339.
[6]Chen Y,Yu K,Zhang H,et al. CD133+EpCAM+phenotype possesses more characteristics of tumor initiating cells in hepatocellular carcinoma Huh7 cells[J]. Int J Biol Sci,2012,8(7): 992-1004.
[7]Qu FL,Hao XZ,Qin SK,et al. Multicenter phase Ⅱ clinical trail of arsenic trioxide injection in the treatment of primary hepatocellular carcinoma[J]. Chin J Oncol,2011,33(9): 697-701.
[8]Jin SL,Zhang P,Kuang YL,et al. Low concentration of sodium arsentie induces differentiation of liver cancer stem cells through inhibiting Oct4 and Sox2 expression in hepatocellular carcinoma cells[J]. J Third Mil Med Univ,2015,37(2): 116-121. [金世龙,张鹏,匡远黎,等. 低剂量亚砷酸钠抑制Oct4、Sox2基因表达诱导肝癌干细胞分化[J]. 第三军医大学学报,2015,37(2): 116-121.]
[9]Zhang XY,Wu SX,Guo JX,et al. All trans retinoic acid in the treatment of 1 cases of acute hepatic failure caused by acute leukemia[J]. Med J Chin PLA,2012,37(6): 668. [张学亚,吴诗馨,郭健欣,等. 全反式维甲酸治疗急性早幼粒细胞白血病引起肝功能衰竭1例[J]. 解放军医学杂志,2012,37(6): 668.]
[10] Jeanne M,Lallemand-Breitenbach V,Ferhi O,et al. The PML/ RARA oxidation and arsenic binding initiate the antileukemia response of As2O3[J]. Cancer Cell,2010,18(1): 88-98.
[11] Zhang XW,Yan XJ,Zhou ZR,et al. Arsenic trioxide controls the fate of the PML-RAR oncoprotein by directly binding PML[J]. Science,2010,328(5975): 240-243.
[12] Ito K,Bernardi R,Morotti A,et al. PML targeting eradicates quiescent leukaemia-initiating cells[J]. Nature,2008,453(7198): 1072-1078.
[13] Nakahara F,Weiss CN,Ito K. The role of PML in hematopoietic and leukemic stem cell maintenance[J]. Int J Hematol,2014,100(1): 18-26.
[14] Chuang YS,Huang WH,Park SW,et al. Promyelocytic leukemia protein in retinoic acid-induced chromatin remodeling of Oct4 gene promoter[J]. Stem Cells,2011,29(4): 660-669.
[15] Chang FT,Chan FL,McGhie JD,et al. PML bodies provide an important platform for the maintenance of telomeric chromatin integrity in embryonic stem cells[J]. Nucleic Acids Res,2013,41(8): 4447-4458.
[16] Karwacki-Neisius V,Goke J,Osomo R,et al. Reduced Oct4 expression directs a robust pluripotent statw with distinct signaling activity and incr easedenhancer occupancy by Oct4 and Nanog[J]. Cell Stem Cell,2013,12(5): 531-545.
[17] Zhang XW,Yan XJ,Zhou ZR,et al. Arsenic trioxide controls the fate of the PML-RAR oncoprotein by directly binding PML[J]. Science,2010,328(5975): 240-243.
[18] Boumahdi S,Driessens G,Lapouge G,et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamouscell carcinoma[J]. Nature,2014,511(7508): 246-250.
[19] Jeong CH,Cho YY,Kim MO,et al. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells[J]. Stem Cells,2010,28(12): 2141-2151.
The molecular pathway of low concentration of sodium arsenite in inducing differentiation of liver cancer stem cells by down-regulating promyelocytic leukemia protein expression
JIN Shi-long,TAN Zhi-ming*,ZHANG Peng,KUANG Yuan-li,DU Bo,TANG Hua-ming,WANG Zheng
Department of Hepatobiliary Surgery,Kai Xian People’s Hospital of Chongqing City,Chongqing 405400,China
*< class="emphasis_italic">Corresponding author,E-mail: 605346554@qq.com
,E-mail: 605346554@qq.com
This work was supported by the Chinese Medicine Technological Item of Municipal Health Bureau of Chongqing City (ZY20132047)
ObjectiveTo study the molecular pathway of low concentration of sodium arsenite in inducing differentiation of liver cancer stem cells.MethodsWestern blotting analysis,immunofluorescence assay and quantitative PCR were used to examine the gene and protein expression of promyelocytic leukemia (PML),Oct4 and Sox2 in HCC tissue and cell lines,and the molecule pathway of low concentration of sodium arsenite inducing differentiation of liver cancer stem cells was confirmed by comparing the changes in the gene and protein expression of PML,Oct4 and Sox2 in HCC cells and biological function of LCSCs after the treatment with low concentration of sodium arsenite.Results0.5μg/ml of sodium arsenite was shown to alter the biological characteristics of LCSCs in HuH7 and primary HCC cells,including the ability to form tumor spheres,resistance to pirarubicin (P<0.01),and the capability of forming tumors after allogeneic transplantation (P<0.05). Both HCC cells and tissues expressed the gene and protein of PML,Oct4 and Sox2,and 0.5μg/ml of sodium arsenite not only downregulated the gene and protein expression of Oct4 (P<0.05) and Sox2 in HCC cells (P<0.05),but also downregulated the protein expression of PML (P<0.05). In contrast,sodium arsenite did not inhibit the gene expression of PML in Hep3B,HepG2,SMCC-7721,HuH7 and primary HCC cells. Furthermore,through down-regulated PML protein expression with arsenite,the biological characteristics of HuH7 and primary HCC cells containing LCSCs was simultaneously altered,and the expression of stem gene Oct4 and Sox2 was downregulated (P<0.05),while HCC cells proliferation was inhibited as well.ConclusionsBoth HCC tissues and cells canexpress the PML gene and PML protein. Low concentrations of sodium arsenite would directly bind to PML protein in HCC cells,resulting in degradation of the PML protein,followed by collapse of PML-NBs,inhibition of transcription of the proliferation- and differentiation-related genes in PML-NBs,and down-regulation of expressions of Oct4,Sox2 and other CSC-related genes in HCC cells,thus inhibiting HCC cell growth and inducing LCSC differentiation.
carcinoma,hepatocellular; neoplastic stem cells; cell differentiation
R735.7
A
0577-7402(2015)12-0966-06
10.11855/j.issn.0577-7402.2015.12.06
2015-06-25;
2015-08-27)
(责任编辑:熊晓然)
重庆市卫生局中医药科技项目(ZY20132047)
金世龙,医学博士,主任医师,副教授。主要从事肝癌手术后复发与转移机制的研究
405400 重庆 重庆市开县人民医院肝胆外科(金世龙、谭智明、张鹏、匡远黎、杜波、唐华明、王郑)
谭智明,E-mail: 605346554@qq.com
