PAF受体拮抗剂对内毒素血症幼年大鼠胃黏膜PGE2含量及COX-2表达的影响
2015-06-28刘春英李奇玉王琳蒋静王丽杰孙梅
刘春英,李奇玉,王琳,蒋静,王丽杰,孙梅
PAF受体拮抗剂对内毒素血症幼年大鼠胃黏膜PGE2含量及COX-2表达的影响
刘春英,李奇玉,王琳,蒋静,王丽杰,孙梅
目的探讨前列腺素E2(PGE2)、环氧化酶-2(COX-2)、血小板活化因子(PAF)受体拮抗剂在内毒素(LPS)腹腔注射诱导的幼年大鼠急性胃黏膜损伤中的作用。方法18日龄Wistar大鼠192只,随机分为对照组、LPS组、PAF受体拮抗剂预防组和治疗组。采用LPS(O55:B5脂多糖)5mg/kg腹腔注射制备幼年大鼠LPS血症模型,预防组和治疗组分别于LPS腹腔注射前后0.5h应用5mg/kg PAF受体拮抗剂BN52021(Ginkgolide B),对照组腹腔注射等量生理盐水。于LPS注射后1.5、3、6、24、48、72h处死动物,每组每时间点8只,肉眼及光学显微镜下观察胃黏膜损伤情况,采用放射免疫法测定胃黏膜PGE2含量,免疫组织化学SP法测定胃黏膜COX-2蛋白的表达,半定量RT-PCR法测定胃黏膜COX-2 mRNA的表达。结果LPS组腹腔注射LPS后1.5h黏膜上皮细胞水肿,3h组织充血、水肿,6h胃黏膜损伤最重,黏膜内有出血,细胞核碎裂、固缩,凋亡小体出现;24h上皮脱落、中性粒细胞浸润,48h黏膜层变薄、腺体减少,72h未见明显异常。预防组和治疗组改变轻微。与对照组比较,LPS组胃黏膜PGE2含量在3h时明显降低(P<0.05),6h时达最低(P<0.01),预防组胃黏膜PGE2含量在3、6h明显增高(P<0.05),治疗组PGE2含量在6h时明显增高(P<0.05)。与LPS组比较,预防组、治疗组胃黏膜PGE2含量在6h时均明显增高(P<0.01)。对照组胃黏膜组织未见明显COX-2蛋白及mRNA表达;与对照组比较,LPS组腹腔注射LPS后6h胃黏膜组织胞质即有COX-2蛋白表达,24、48、72h时明显增高(P<0.01),其mRNA水平亦上调;预防组和治疗组6h COX-2蛋白、mRNA水平明显增高(P<0.01);预防组和治疗组6h COX-2蛋白、24h COX-2 mRNA与LPS组比较亦明显增高(P<0.01)。结论PAF受体拮抗剂可上调COX-2 mRNA及蛋白表达,使PGE2含量增加,对胃黏膜有保护作用。
胃黏膜;脂多糖类;地诺前列酮;环氧化酶2;血小板活化因子;PAF受体拮抗剂
重症感染是急性胃黏膜损伤的常见原因之一[1]。急性胃黏膜损伤是由于胃黏膜保护机制削弱,损伤因素相对增强所致[2]。近年研究发现前列腺素E2(PGE2)、环氧化酶-2(COX-2)在胃黏膜损伤修复方面发挥重要作用。本研究通过给幼年大鼠腹腔注射脂多糖(lipopolysaccharide,LPS)建立急性胃黏膜损伤模型,观察应用血小板活化因子(platelet activating factor,PAF)受体拮抗剂后胃黏膜PGE2含量和COX-2表达的变化,旨在探讨其在胃黏膜损伤修复中的作用。
1 材料与方法
1.1 动物分组及模型建立 192只健康18日龄Wistar大鼠,体重32.37±6.32g,与母鼠共同饲养(由中国医科大学第二临床学院实验动物中心提供)。LPS(O55:B5脂多糖)、PAF受体拮抗剂BN52021(Ginkgolide B)为美国Sigma公司产品。PGE2放射免疫试剂盒由北京东亚免疫研究所提供,液体闪烁计数器为美国Beckman公司产品。COX-2免疫组化试剂盒由北京中杉生物工程有限公司提供,总RNA提取系统试剂由河南华美生物工程公司提供。COX-2、内参照β-actin引物由上海生工生物工程技术公司合成,TaKaRa试剂盒由大连宝生物工程有限公司提供。将大鼠随机分为4组:对照组、LPS组、PAF受体拮抗剂预防组(预防组)和治疗组,每组于1.5、3、6、24、48、72h各个时间点设动物8只。对照组腹腔注射生理盐水1ml/kg;LPS组腹腔注射LPS(O55:B5脂多糖)5mg/kg,配比浓度5g/L,用生理盐水溶解。预防组于LPS腹腔注射前0.5h、治疗组于LPS腹腔注射后0.5h给予PAF受体拮抗剂BN52021(Ginkgolide B)5mg/kg,配比浓度5g/L。用药后各组均放回鼠笼,继续哺乳。分别于LPS腹腔注射后相应时间点处死动物各8只。
1.2 方法
1.2.1 标本采集及保存 大鼠处死后迅速开腹取胃,沿胃大弯剪开,肉眼观察胃黏膜大体损伤情况,在腺胃区胃大弯处取0.5cm×0.5cm胃组织置入40g/L甲醛溶液中固定,其余部分用生理盐水冲洗3次,置入去RNA酶试管中,深低温冰箱-70℃保存。
1.2.2 胃黏膜PGE2含量测定 采用放射免疫法,取低温保存腺胃区黏膜20mg,加生理盐水及乙醇制成10%匀浆,2000r/min低温离心8min,取上清液,-20℃以下保存。测量时在上清液中加入重蒸馏乙酸乙酯,混匀,离心,吸取上清液,按试剂盒说明加入3H标记物等试剂,离心后将上清液倒入预先加有闪烁液的瓶中,在液体闪烁计数器上测放定射性。根据放射性得出PGE2含量。
1.2.3 胃黏膜COX-2蛋白表达水平测定 取胃组织于40g/L甲醛溶液中固定,包埋,石蜡切片。应用免疫组化SP法(过氧化物酶标记的链霉卵白素法)测定COX-2表达水平。抗体工作浓度:兔抗鼠-COX-2 IgG抗体稀释比例1:150。显微镜观察,细胞质中有棕褐色颗粒者为COX-2阳性表达,以PBS代替一抗作为阴性对照。各时间点选取染色清晰的切片5~6张,于光镜下(×40)随机选取2~3个视野,应用Olympus-BX41图像采集系统,Meta Morph/Dp10/ BX41软件分析系统测定平均光密度值。
1.2.4 RT-PCR检测胃黏膜COX-2 mRNA的表达
Trizol裂解待测胃黏膜组织,用酚-氯仿抽提总RNA,反转录合成cDNA后行PCR扩增,反应总体积25μl,PCR扩增引物由本实验室参照美国国立图书馆PubMed基因库中电脑软件Primer 5.0自行设计。COX-2:上游5'-ACGGACTTGCTCACTTTG-3',下游5'-AGGAGAACAGATGGGATT-3',产物大小377bp。β-actin:上游5'-CACCCTGTGCTGCTCACC GAGGCC-3',下游5'-CCACACAGATGACTTGCGC TCAGG-3',产物大小690bp。COX-2反应条件为:94℃预变性3min;94℃变性40s、51.5℃退火1min、72℃延伸1min,扩增35个循环;最后72℃ 7min终止反应。β-actin反应条件:94℃变性3min、55℃退火30s、72℃延伸30s,扩增35个循环;最后60℃延伸10min终止反应。PCR扩增后,取PCR反应产物10µl加入2g/L琼脂糖凝胶中电泳,应用计算机凝胶成像分析系统进行半定量分析。以β-actin为内对照,COX-2的mRNA的相对表达量用COX-2的PCR电泳结果扫描值与β-actin的PCR电泳结果扫描值的比值来确定。
1.3 统计学处理 采用SPSS 10.0软件进行分析,数据以表示,多组间比较采用方差分析,进一步两两比较采用SNK-q检验。P<0.05为差异有统计学意义。
2 结 果
2.1 病理改变 大体观察可见LPS组1.5h胃黏膜轻度水肿,3h可见充血、水肿,6h黏膜表面可见大片糜烂、出血、条索状坏死,与胃纵轴平行,损伤主要见于腺胃区,窦部少见,前胃部无损伤;24h黏膜糜烂、出血,坏死灶陈旧、缩小,48h黏膜轻度萎缩,72h胃黏膜基本恢复正常。光镜下LPS组1.5h黏膜上皮细胞水肿,3h组织充血、水肿,6h胃黏膜损伤最重,黏膜表面上皮广泛脱落,黏膜内有出血,炎性细胞浸润,核碎裂、固缩,凋亡小体出现,腺体受损,24h上皮脱落、中性粒细胞浸润,48h黏膜层变薄、腺体减少,72h未见明显异常。预防组和治疗组6h大体观仅见胃黏膜充血水肿,光镜下可见黏膜上皮细胞肿胀、充血;两组其他时间点病理改变均较轻。对照组胃黏膜表面上皮隐窝细胞形态学正常,腺体结构完整(图1)。
2.2 胃黏膜PGE2含量 与对照组比较,LPS组腹腔注射LPS后1.5h胃黏膜PGE2含量轻度下降,3h明显降低(P<0.05),6h最低(P<0.01),24h开始上升,48h升至与对照组相同的水平;预防组胃黏膜PGE2含量在3、6h明显增高(P<0.05);治疗组PGE2含量在6h明显增高(P<0.05)。与LPS组比较,预防组、治疗组胃黏膜PGE2含量在6h明显增高(P<0.01)。预防组与治疗组各时间点比较差异无统计学意义(P>0.05,表1)。
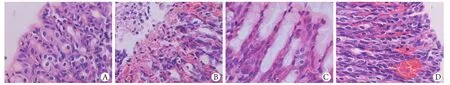
图1 6h时点胃黏膜的病理改变(HE ×400)Fig.1 Pathologic changes of gastric mucosa at 6h time point (HE ×400)
表1 各组各时间点胃黏膜PGE2含量(μg/L,±s,n=8)Tab.1 PGE2contents of gastric mucosa in different groups at each time point (μg/L,±s,n=8)
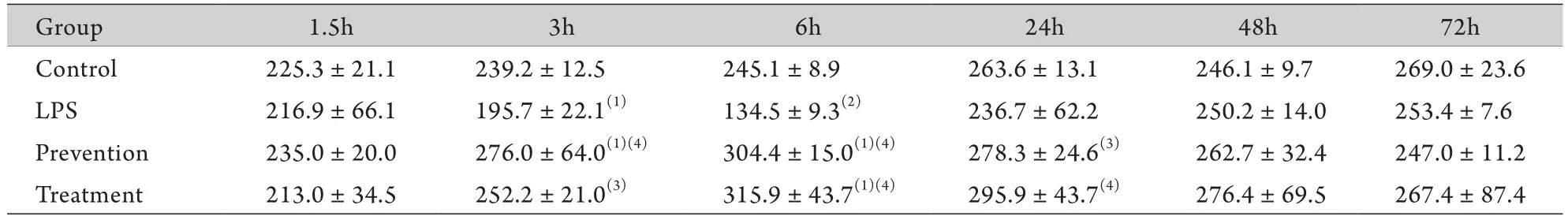
表1 各组各时间点胃黏膜PGE2含量(μg/L,±s,n=8)Tab.1 PGE2contents of gastric mucosa in different groups at each time point (μg/L,±s,n=8)
(1)P<0.05, (2)P<0.01 compared with control group; (3)P<0.05, (4)P<0.01 compared with LPS group
Group 1.5h 3h 6h 24h 48h 72h Control 225.3±21.1 239.2±12.5 245.1±8.9 263.6±13.1 246.1±9.7 269.0±23.6 LPS 216.9±66.1 195.7±22.1(1) 134.5±9.3(2) 236.7±62.2 250.2±14.0 253.4±7.6 Prevention 235.0±20.0 276.0±64.0(1)(4) 304.4±15.0(1)(4) 278.3±24.6(3) 262.7±32.4 247.0±11.2 Treatment 213.0±34.5 252.2±21.0(3) 315.9±43.7(1)(4) 295.9±43.7(4) 276.4±69.5 267.4±87.4
2.3 胃黏膜COX-2蛋白及mRNA的表达 对照组胃黏膜组织基本未见COX-2蛋白及mRNA表达。LPS组腹腔注射LPS后6h胃黏膜组织胞质COX-2蛋白开始表达,24、48、72h时与对照组比较明显增高(P<0.01),其mRNA水平亦上调;预防组和治疗组6h COX-2蛋白表达与对照组比较即明显增高(P<0.01),mRNA水平明显上调(P<0.05),且预防组和治疗组6h COX-2蛋白、24h COX-2 mRNA与LPS组比亦明显增高(P<0.01,表2、图2,表3、图3)。
3 讨 论
前列腺素(PG)是一种脂质,主要在精囊、肾髓质、肺和胃肠道中合成。胃肠道黏膜可合成PG,包括PGE、PGF、PGI,其中以PGE2含量最多。PG不在细胞内贮存,而在分泌瞬间合成,所以PG的产生和释放是组织损害的结果[3-4]。早在1978年Robert等[5]首先观察到PG可抑制胃酸分泌,且非抗酸剂量的PG可完全防止乙醇、强酸、强碱等物质引起的急性胃黏膜损伤。国外大量研究证实,十二指肠溃疡患者胃窦部及十二指肠球部黏膜PGE2含量下降,且在溃疡活动期下降明显[6]。本研究结果显示,幼年大鼠腹腔注射LPS后6h胃黏膜损伤最重,PGE2浓度最低;此后,PGE2浓度逐渐上升,胃黏膜损伤逐渐愈合。因而,LPS血症时急性胃黏膜损伤可能与PGE2缺乏有关,PG的不断产生和释放可促进胃黏膜细胞愈合,发挥保护作用。这种不依赖于抗酸的作用被称之为“细胞保护”[5]。文献报道PG可能通过刺激胃黏液的生成和分泌,促进HCO–3分泌,增加表面磷脂生成,加强胃黏膜屏障的疏水性,保护胃黏膜微循环结构的完整,维持胃黏膜血液供应,保护增殖区细胞,促进黏膜上皮的更新及修复,以及抑制胃运动过强等机制来实现对胃黏膜的保护作用[7-8]。本实验中腹腔注射LPS之前及之后给予PAF受体拮抗剂,均增加了胃黏膜PGE2的含量,减轻了幼年大鼠的急性胃黏膜损伤。
表2 各组各时间点胃黏膜COX-2 蛋白的表达(±s,n=8)Tab.2 Expressions of COX-2 protein in different groups at each time point (±s,n=8)
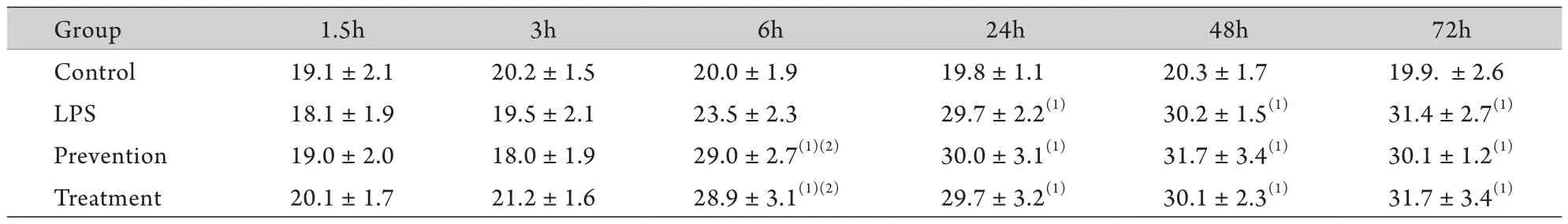
表2 各组各时间点胃黏膜COX-2 蛋白的表达(±s,n=8)Tab.2 Expressions of COX-2 protein in different groups at each time point (±s,n=8)
(1)P<0.01 compared with control group; (2)P<0.01 compared with LPS group
Group 1.5h 3h 6h 24h 48h 72h Control 19.1±2.1 20.2±1.5 20.0±1.9 19.8±1.1 20.3±1.7 19.9. ±2.6 LPS 18.1±1.9 19.5±2.1 23.5±2.3 29.7±2.2(1) 30.2±1.5(1) 31.4±2.7(1)Prevention 19.0±2.0 18.0±1.9 29.0±2.7(1)(2) 30.0±3.1(1) 31.7±3.4(1) 30.1±1.2(1)Treatment 20.1±1.7 21.2±1.6 28.9±3.1(1)(2) 29.7±3.2(1) 30.1±2.3(1) 31.7±3.4(1)
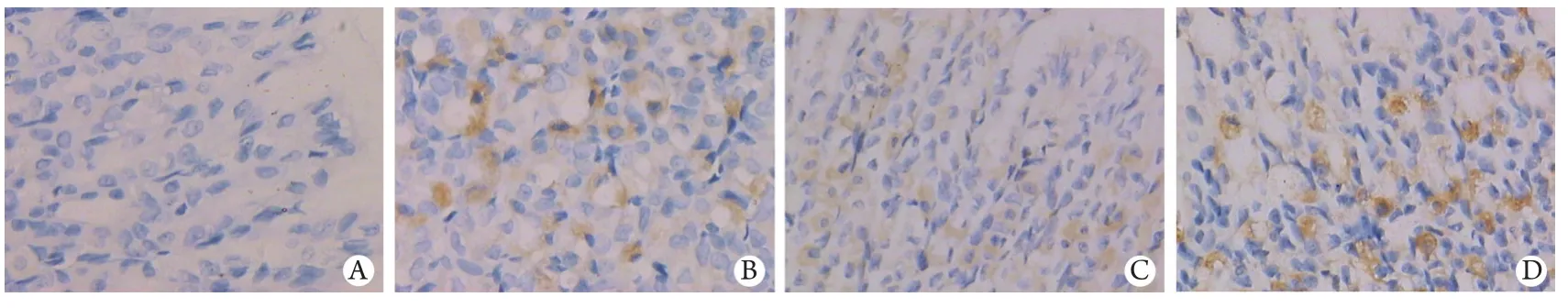
图2 免疫组化SP法检测胃黏膜6h时点COX-2蛋白的表达(DAB ×400)Fig.2 Expression of COX-2 protein in gastric mucosal at 6h time point detected by immunohistochemistry SP method (DAB ×400)
表3 各组各时间点胃黏膜COX-2 mRNA的表达(±s,n=8)Tab.3 Expressions of COX-2 mRNA in different groups at each time point (±s,n=8)

表3 各组各时间点胃黏膜COX-2 mRNA的表达(±s,n=8)Tab.3 Expressions of COX-2 mRNA in different groups at each time point (±s,n=8)
(1)P<0.01 compared with control group; (2)P<0.05 compared with LPS group
Group 1.5h 3h 6h 24h 48h 72h Control 0.18±0.03 0.17±0.02 0.18±0.02 0.18±0.02 0.17±0.02 0.18 ±0.03 LPS 0.16±0.04 0.19±0.05 0.85±0.15(1) 0.96±0.13(1) 1.22±0.17(1) 1.23±0.16(1)Prevention 0.18±0.03 0.19±0.06 0.99±0.16(1) 1.20±0.12(1)(2) 1.22±0.12(1) 1.25±0.17(1)Treatment 0.18±0.02 0.16±0.03 0.96±0.13(1) 1.22±0.13(1)(2) 1.20±0.13(1) 1.23±0.13(1)

图3 胃黏膜COX-2 mRNA表达Fig.3 Expressions of COX-2 mRNA in gastric mucosa
环氧化酶(cyclooxygenase,COX)是PG合成过程中重要的限速酶,它将花生四烯酸(arachidonic acid,AA)代谢成各种前列腺素产物,维持机体的各种生理病理过程[9-10]。目前已知COX具有两种亚型,即COX-1、COX-2。COX-1为结构型,在大多数正常细胞中稳定表达。COX-2为诱导型,在正常生理状态下几乎不表达或表达甚少,仅在细胞受到生长因子、细胞因子、内皮素触发活动及一氧化氮等刺激后迅速从头合成,参与多种病理生理过程。COX-2在维持胃肠道完整性等方面的作用较COX-1更大[11-12]。研究发现,应用COX-2选择性抑制剂可显著减少PGE2生成,降低溃疡边缘肉芽组织内微血管的密度,影响肉芽组织的成熟,延迟溃疡愈合,并呈明显的剂量反应关系[13-14]。故目前认为COX-2产物在溃疡愈合过程中起着积极作用。本实验结果显示,幼年大鼠LPS血症急性胃黏膜损伤发生时,胃黏膜组织COX-2 mRNA的表达明显高于对照组,免疫组织化学染色证实了存在COX-2蛋白表达。应用PAF受体拮抗剂可使COX-2蛋白表达增加、mRNA水平上调提前。当细胞受到各种刺激后,可通过C蛋白偶联机制、生长因子受体以及一系列信号传导通路作用于COX-2的5'端转录起始点上游区的转录调控序列,促进COX-2的转录,诱导COX-2的表达[15-16]。COX-2在急性胃黏膜损伤中表达明显增强,从而发挥对胃黏膜的保护作用并促进胃黏膜损伤的愈合。
PAF是迄今发现的内源性溃疡形成介质中最强的一种,作为炎性细胞因子放大网络的中心放大器,可促进对其他炎性细胞因子的作用[17-18]。PAF受体拮抗剂能显著改变LPS或PAF引起的血流动力学改变及组织器官损伤,抑制花生四烯酸类介质释放和氧自由基的产生[19-20]。本实验采用预先或在腹腔注射LPS后给予PAF受体拮抗剂的方式减轻了胃黏膜损伤,证明PAF受体拮抗剂可增加COX-2的表达和PGE2浓度。
综上,本实验结果提示,幼年大鼠LPS血症时胃黏膜COX-2 mRNA表达上调,COX-2表达增强,PGE2含量增加,对胃黏膜起保护作用并促进胃黏膜损伤的愈合。PAF受体拮抗剂可增加COX-2的表达、提高PGE2的浓度,在减轻胃黏膜损伤的同时可能也促进了胃黏膜损伤的愈合。
[1] Chen QH, Xu L, Ji ZH,et al. The protection of recombinant human activated protein C on acute gastric mucosal lesion in rats[J]. Chin Pediatr Emerg Med, 2006, 13(6): 543-545. [陈琦晗, 徐仑, 季正华, 等. 重组人活化蛋白C对大鼠急性胃黏膜损伤的保护作用[J]. 中国小儿急救医学, 2006, 13(6): 543-545.]
[2] Chang B, Liu C, Sun XY,et al. The protection of rebamipide to acute alcoholic injure on gastric mucosa of rat[J]. Chin J Gastroenterol Hepatol, 2008, 17(1): 52-54. [常冰, 刘畅, 孙晓艳, 等. 瑞巴派特对大鼠急性酒精性胃黏膜损伤的保护作用[J]. 胃肠病学和肝病学杂志, 2008, 17(1): 52-54.]
[3] Buvanendran A, Kroin JS, Berger RA,et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans[J]. Anesthesiology, 2006, 104(3): 403-410.
[4] Kawashima M, Ogura N, Akutsu M,et al. The anti-inflammatory effect of cyclooxygenase inhibitors in fibroblast-like synoviocytes from the human temporomandibular joint results from the suppression of PGE2production[J]. J Oral Pathol Med, 2013, 42(6): 499-506.
[5] Robert A, Nezamis JE, Lancaster C,et al. Cytoprotection prostaglands exogenous or endogenous can maintain gastric secretory function[J]. Gastroenterology, 1978, 74: 1086.
[6] Brzozowski T, Konturek PC, Konturek SJ,et al. Role of prostaglandins in gastroprotection and gastric adaptation[J]. J Physiol Pharmacol, 2005, 56(Suppl 5): 33-55.
[7] Ise F, Takasuka H, Hayashi S,et al. Stimulation of duodenal HCO3- secretion by hydrogen sulphide in rats: relation to prostaglandins, nitric oxide and sensory neurones[J]. Acta Physiol (Oxf), 2011, 201(1): 117-126.
[8] Zhu CL, Cao YH, Zhang R,et al. Stimulatory effect of LPS and feedback effect of PGE2on IL-27 production[J]. Scand J Immunol, 2010, 72(6): 469-475.
[9] Ranganathan PV, Jayakumar C, Mohamed R,et al. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2production[J]. Kidney Int, 2013, 83(6): 1087-1098.
[10] Fang L, Cheng JC, Chang HM,et al. EGF-like growth factors induce COX-2-derived PGE2production through ERK1/2 in human granulosa cells[J]. J Clin Endocrinol Metab, 2013, 98(12): 4932-4941.
[11] Haruna H, Shimizu T, Ohtsuka Y,et al. Expression of COX-1, COX-2, and PPAR-γ in the gastric mucosa of children with Helicobacter pylori infection[J]. Pediatr Int, 2008, 50(1): 1-6.
[12] Min K, Choi K, Kwon TK. Withaferin A down-regulates lipopolysaccharide- induced cyclooxygenase-2 expression and PGE2production through the inhibition of STAT1/3 activation in microglial cells[J]. Int Immunopharmacol, 2011, 11(8): 1137-1142.
[13] Li W, Wu S, Hickey RW,et al. Neuronal cyclooxygenase-2 activity and prostaglandins PGE2, PGD2, and PGF2alpha exacerbate hypoxic neuronal injury in neuron-enriched primary culture[J]. Neurochem Res, 2008, 33(3): 490-499.
[14] Fang L, Chang HM, Cheng JC,et al. TGF-β1Induces COX-2 Expression and PGE2production in human granulosa cells through Smad signaling pathways[J]. J Clin Endocrinol Metab, 2014, 99(7): E1217-E1226.
[15] Scoditti E, Massaro M, Carluccio MA,et al. PPARgamma agonists inhibit angiogenesis by suppressing PKCalphaand CREB-mediated COX-2 expression in the human endothelium[J]. Cardiovasc Res, 2010, 86(2): 302-310.
[16] Guo D, Chen NN, Wu SG,et al. Cyclooxygenase-2 gene silence induces apoptosis of human hepatocellular carcinoma cells[J]. Med J Chin PLA, 2012, 37(7): 715-719. [郭丹, 陈娜娜, 吴曙光, 等. 环氧合酶2基因沉默诱导人肝癌细胞凋亡的作用观察[J]. 解放军医学杂志, 2012, 37(7): 715-719.]
[17] Sato A, Ebina K. Common mechanism in endothelin-3 and PAF receptor function for anti-inflammatoryresponses[J]. Eur J Pharmacol, 2013, 718(1-3): 30-33.
[18] Koga MM, Bizzarro B, Sá-Nunes A,et al. Activation of PAF-receptor induces regulatory dendritic cells through PGE2and IL-10[J]. Prostaglandins Leukot Essent Fatty Acids, 2013, 89(5): 319-326.
[19] Belayev L, Khoutorova L, Atkins K,et al. LAU-0901, a novel platelet-activating factor receptor antagonist, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat[J]. Brain Res, 2009, 1253: 184-190.
[20] Musto AE, Samii M. Platelet-activating factor receptor antagonism targets neuroinflammation in experimental epilepsy[J]. Epilepsia, 2011, 52(3): 551-561.
Effect of PAF receptor antagonist on PGE2content and COX-2 expression in gastric mucosa of young rats with endotoxemia
LIU Chun-ying1, LI Qi-yu1, WANG Lin1, JIANG Jing1, WANG Li-jie2, SUN Mei2*1Department of Pediatrics, 202 Hospital of PLA, Shenyang 110812, China
2Department of Pediatrics, Second Clinical College of China Medical University, Shenyang 110004, China
ObjectiveTo investigate the protective effect of prostaglandin E2(PGE2), cyclooxygenase-2 (COX-2) and platelet activating factor (PAF) receptor antagonist on endotoxin-induced acute gastric mucosal injury in young rats.MethodsEighteen-day old Wistar rats were randomly divided into 4 groups: normal control group, model group (LPS group), PAF antagonist prevention group, and PAF antagonist treatment group. The model of endotoxemia in young rats was reproduced by intraperitoneal injection of endotoxin (5mg/kg of O55:B5 lipopolysaccharide). The rats in PAF prevention and treatment group
PAF antagonist BN52021 (Ginkgolide B, 5mg/kg) 0.5h before or after modeling. The rats in control group were given intraperitoneal injection of same amount of normal saline (1ml/kg). The animals were sacrificed 1.5, 3, 6, 24, 48 and 72h after intraperitoneal injection of endotoxin (8 in each group). The pathologic changes in gastric mucosa were observed after HE staining. The content of PGE2was measured by radioimmunoassay, the expression of COX-2 protein was determined by immunohistochemistry SP method, and the expression of COX-2 mRNA was assessed with RT-PCR method.ResultsPathological changes in gastric mucosa were found to be edema of epithelial cells at 1.5h, and hyperemia and edema 3h after intraperitoneal injection of endotoxin in LPSgroup. The changes were most marked at 6h, including bleeding, karyorrhexis, pyknosis and apoptosis of epithelial cells of gastric mucosa. Exfoliation of the epithelium and neutrophil infiltration were observed at 24h, thinning of mucosa and a decrease in glands were observed at 48h, but no further changes were observed at 72h. However, all the above changes were significantly alleviated in prevention and treatment groups. The PGE2content of gastric mucosa was lowered at 3h (P<0.05), and it was lowest at 6h (P<0.01) after endotoxin injection in LPS group, and significant difference was found between LPS group and control group. The PGE2content of gastric mucosa was obviously increased at 3h and 6h in prevention group (P<0.05), and at 6h in treatment group (P<0.05). The differences at 6h were significant (P<0.01) among prevention group, treatment group and LPS group. No expression of COX-2 protein or mRNA was seen in gastric mucosal tissue of control group. In contrast with control group, cytoplasm COX-2 protein of gastric mucosal tissue was seen to express at 6h after endotoxin injection in LPS group, and it was obviously enhanced at 24, 48 and 72h (P<0.01), and the COX-2 mRNA level was also elevated. The expressions of COX-2 protein and mRNA were increased obviously at 6h in PAF antagonist prevention group and treatment group (P<0.01). The expressions of COX-2 protein at 6h and COX-2 mRNA at 24h were obviously elevated in prevention group and treatment group compared with those of LPS group (P<0.01). Conclusion PAF receptor antagonist may up-regulate the expression level of COX-2 protein and mRNA, increase PGE2content, alleviate acute gastric mucosal injury, and promote the healing of gastric mucosal injury.
gastric mucosa; lipopolysaccharides; dinoprostone; cyclooxygenase 2; platelet activating factor; PAF receptor antagonist
R573
A
0577-7402(2015)01-0040-06
10.11855/j.issn.0577-7402.2015.01.09
2014-04-28;
2014-11-20)
(责任编辑:张小利)
刘春英,医学博士,副主任医师。主要从事儿童消化系统疾病的基础与临床研究
110812 沈阳 解放军202医院儿科(刘春英、李奇玉、王琳、蒋静);110004 沈阳 中国医科大学第二临床学院儿科(王丽杰、孙梅)
]孙梅,E-mail:sunm@cmu2h.com
*Corresponding author, E-mail: sunm@ cmu2h.com
