Cd2+诱导的镉敏感水稻突变体cadB-1叶片抗坏血酸循环的变化
2015-06-15沈国明
沈国明
(菏泽学院植物生物学重点实验室,菏泽学院生命科学系,山东菏泽274015)
Cd2+诱导的镉敏感水稻突变体cadB-1叶片抗坏血酸循环的变化
沈国明
(菏泽学院植物生物学重点实验室,菏泽学院生命科学系,山东菏泽274015)
【目的】镉离子 (Cd2+) 为非必需的微量元素,植物易从土壤中吸收并积累Cd2+,通过食物链进入人体内,对人类的健康造成重大威胁。为了阐明Cd2+诱导氧化胁制和抑制生长的机制,对 Cd2+敏感水稻突变体 (cadB-1) 进行了水培试验。【方法】植物材料为水稻粳稻中花11(OryzasativaL. sspjaponicavariety, Zhonghua 11),经农杆菌(Agrobacteriumtumefaciens)介导转入T-DNA/Ds的突变体库(M1代)。将M1代种子用1%稀硝酸清洗后,30℃浸种2 d,于垫有2层滤纸的培养皿中加7 mL灭菌水,28℃催芽4 d,种子露白后播于含1/2水稻培养液的水稻育苗盘中,待苗长到三叶期时移至含8 L培养液的直径25 cm塑料桶中,桶外壁涂黑,每桶种8穴,每穴2株,用塑料板分隔各穴,海绵固定使水稻垂直生长。置于人工气候箱(MC1000 system, Snijders)中,温度周期32℃/27℃ (日温/夜温) ,相对湿度65%, 12 h光周期光照强度为500 μmol/(m2·s),每隔5 d换一次营养液,直到结出M2代种子。将中花11野生型与M2代突变体种子用以上同样方法培养,长到五叶期。以不加Cd2+作为对照,分别加入0.1、 0.25、 0.5和0.75 mmol/L Cd2+进行筛选,每种处理平行培养3桶,作为重复,共6001桶,每天定时观察。12 d后,发现0.5 mmol/L Cd2+中的中花11野生型没有死亡,而M2代突变体出现部分死亡。按所在位置,选取表型最明显的株系命名为cadB-1。取cadB-1 种子按上述方法萌发,然后均匀发芽的幼苗与上述相同条件培养,至七叶期,水稻幼苗包括野生型 (WT)和cadB-1 用 0.5 mmol/L CdCl2处理2、4、6、8和 12 d。【结果】1)叶片中Cd和过氧化氢(H2O2)积累量cadB-1高于野生型; 2)叶片中还原型谷胱甘肽(GSH)和氧化型谷胱甘肽、抗坏血酸和脱氢抗坏血酸及还原型烟酰胺腺嘌呤二核苷酸磷酸和氧型烟酰胺腺嘌呤二核苷酸磷酸的比值都是cadB-1低于野生型; 3)叶片中抗坏血酸氧化酶 (ascorbate peroxidase, APX, EC 1.11.1.11), 还原型谷胱甘肽酶(glutathione reductase, GR, EC 1.6.4.2), 脱氢抗坏血酸还原酶(dehydroascorbate reductase, DHAR, EC 1.8.5.1) 和单脱氢抗坏血酸还原酶(monodehydroascorbate reductase,MDHAR, EC 1.6.5.4) 活性都是cadB-1低于野生型。【结论】cadB-1具有低水平的抗氧化剂和抗氧化酶活性。此外,cadB-1比 WT 积累更多的 Cd 从而产生更多的活性氧 (reactive oxygen species, ROS)。也就是说,与野生型相比,cadB-1 更缺乏防御力来清除更多的活性氧,从而导致较低的生长势和对Cd的敏感。
抗坏血酸-谷胱甘肽循环; 镉敏感突变体; 生长抑制; 过氧化氢; 水稻
Cadmium can be readily taken up by roots and often accumulates to a large number in plant system[1], the presence of Cd can induce the generation of reactive oxygen species (ROS). Plants have developed antioxidant mechanisms to alleviate hazardous effects imposed by oxide stress. These mechanisms include antioxidative enzymatic and antioxidative non-enzymatic systems. The ascorbate-glutathione (ASC-GSH) cycle is the keystone of the nonenzymatic antioxidative defense system and has been suggested as the source for H2O2remova1 into organelles[2-3]. ASC and GSH, two low molecular weight antioxidants are of great importance in preserving a wide range of metabolic processes[4]. They can both react directly with ROS as well as participating in ROS detoxification through the ASC-GSH cycle[2, 5-7]. Moreover, ASC and GSH are also associated with the cellular redox balance and the ratios of ASC ∶DHA and GSH ∶GSSG may function as signals for the regulation of antioxidant mechanisms[8].
Previously, we screened a rice cadmium sensitive and hyper-accumulation mutant byAgrobacteriumtumefacienssystem, investigated the enzymatic defense system, root morphology and cadmium uptake kinetics[9-10]. In present research, we mainly compared the differences in the ASC-GSH metabolism betweencadB-1 and WT seedling leaves after increasing exposure periods of Cd. Although recently we reported some results of ASC-GSH metabolism[11-12], we also want more evidences to confirm that higher ASC, GSH, or NADPH are more able to resist Cd toxicity.
1 Materials and methods
1.1 Plant materials and culture conditions
Stable inheritance rice (OrizasativaL.) cadmium sensitive mutant (cadB-1) and the same rice variety wild-type (WT) were used in this experiment. The seeds were surface sterilized in 0.5% sodium hypochlorite for 20 min, rinsed, and germinated in the dark on moistened filter paper at 30℃ for 2 d, and then moved to a plastic screen floating on distilled water at 28℃ for 4 d. Then uniformly germinated seedlings were transferred to black polyethylene barrels which contained 6 L of rice culture solution. Seedlings were grown in a growth chamber with a photo flux density of 500 μmol/(m2·s), relative humidity of approximately 65% and day/night temperatures of 32℃/27℃ (14 h/10 h). During the growth period, the solution was renewed every 5 d. At the seven leaf stage, rice seedlings include wild-type (WT) andcadB-1 exposed to 0.5 mmol/L CdCl2for 0 (as control), 2, 4, 6, 8,or 12 d.
1.2 Cd and H2O2content analysis
The Cd contents in seedling leaves, stems and roots were determined according to the method of Shah and Dubey[13]. H2O2content was determined according to the method described by Jana and Choudhuri[14].
1.3 Ratios of ASC/DHA, GSH/GSSG and NADPH/NADP+analysis
ASC and DHA content were determined according to the method of Lawetal.[15].GSH and GSSG content was determined according to the method of Andersonetal.[16]. NADPH and NADP+ content was determined according to the method of Nisselbaum and Gree[17].
1.4 Enzyme assays
Frozen materials (400 mg fresh weight) were homogenized in 4 mL of 50 mmol/L potassium phosphate buffer, pH 7.8, containing 0.1% Triton X-100. The homogenate was centrifuged at 15,000×g for 20 min at 4℃ and the supernatant was used for enzyme assays. APX and GR activities were determined according to the method of Nakanoetal.[18]. DHAR activity was determined according to the method of Daltonetal.[19]. NDHAR activity was determined according to the method of Arrigonietal.[20].
1.5 Statistical Analysis
Data were analyzed with the statistical package SPSS15 for Windows on the website (www.nbs.ntu.edu.sg/userguide/SPSS/SPSS15/). Significance levels 0.05 and 0.01 were used in presenting the results. The experiments were repeated in triplicate, and the data presented are the mean values±standard error (SE). The difference was considered significant at P levels lower than 0.05 (P<0.05) and this significance is denoted in the figures by an asterisk (*) while significant at P levels lower than 0.01 (P<0.01) denoted in the figures by double asterisk (**).
2 Results
2.1 Cadmium accumulation and effect on rice seedling growth
At the seven-leaf stage, CdCl2was added to the nutrient solution to achieve the final Cd2+concentration is 0.5 mmol/L. After 12 d exposure to Cd2+, the leaves ofcadB-1 faded seriously and the roots were more exiguous than WT. The fresh weight of shoots, and roots of wild type seedlings declined by 45.97%, and 46.99%, respectively, while the percent decrease ofcadB-1 are 63.56% and 51.28%, compared to the control-cultivated seedlings (Table 1).
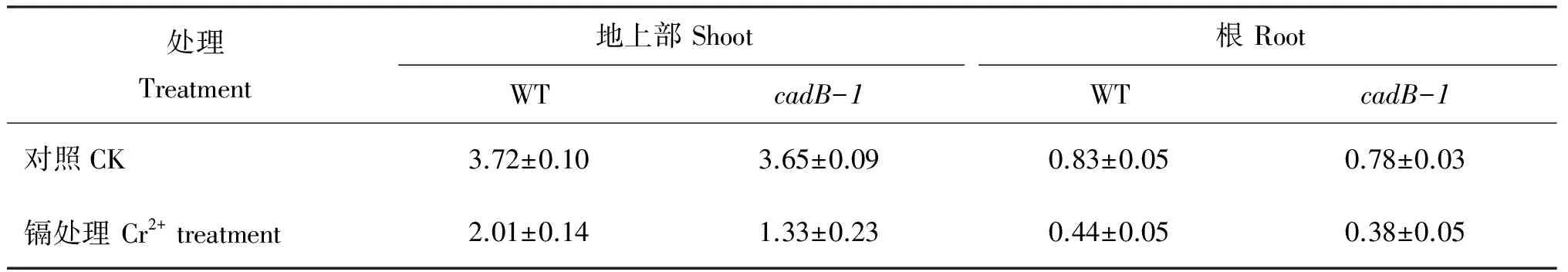
表1 侵染于0.5 mmol/L Cd2+12天后野生型(WT)和Cd-敏感型(cadB-1)水稻秧苗不同部位鲜重(g, FW)
The Cd contents increased in all organs of both WT andcadB-1 with 0.5 mmol/L Cd2+exposure for 12 d (Table 2). A larger increase was seen in all organs ofcadB-1 compared to WT seedlings. However, the increase was not statistically significant.
2.2 H2O2accumulation in rice seedlings
H2O2contents increased in the leaves of WT andcadB-1 rice seedlings during Cd2+exposure period (Fig.1). In general,cadB-1 rice seedlings accumulated more H2O2than WT rice seedlings, except the 2nd day. Significant difference of H2O2contents between WT andcadB-1 rice seedling leaves was only detected the 12th exposure day.
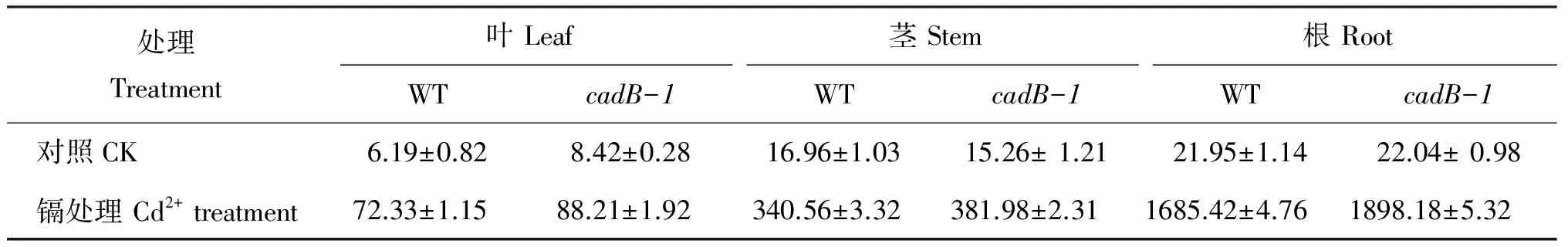
表2 侵染于0.5 mmol/L Cd2+12天后野生型(WT)和Cd-敏感型(cadB-1)
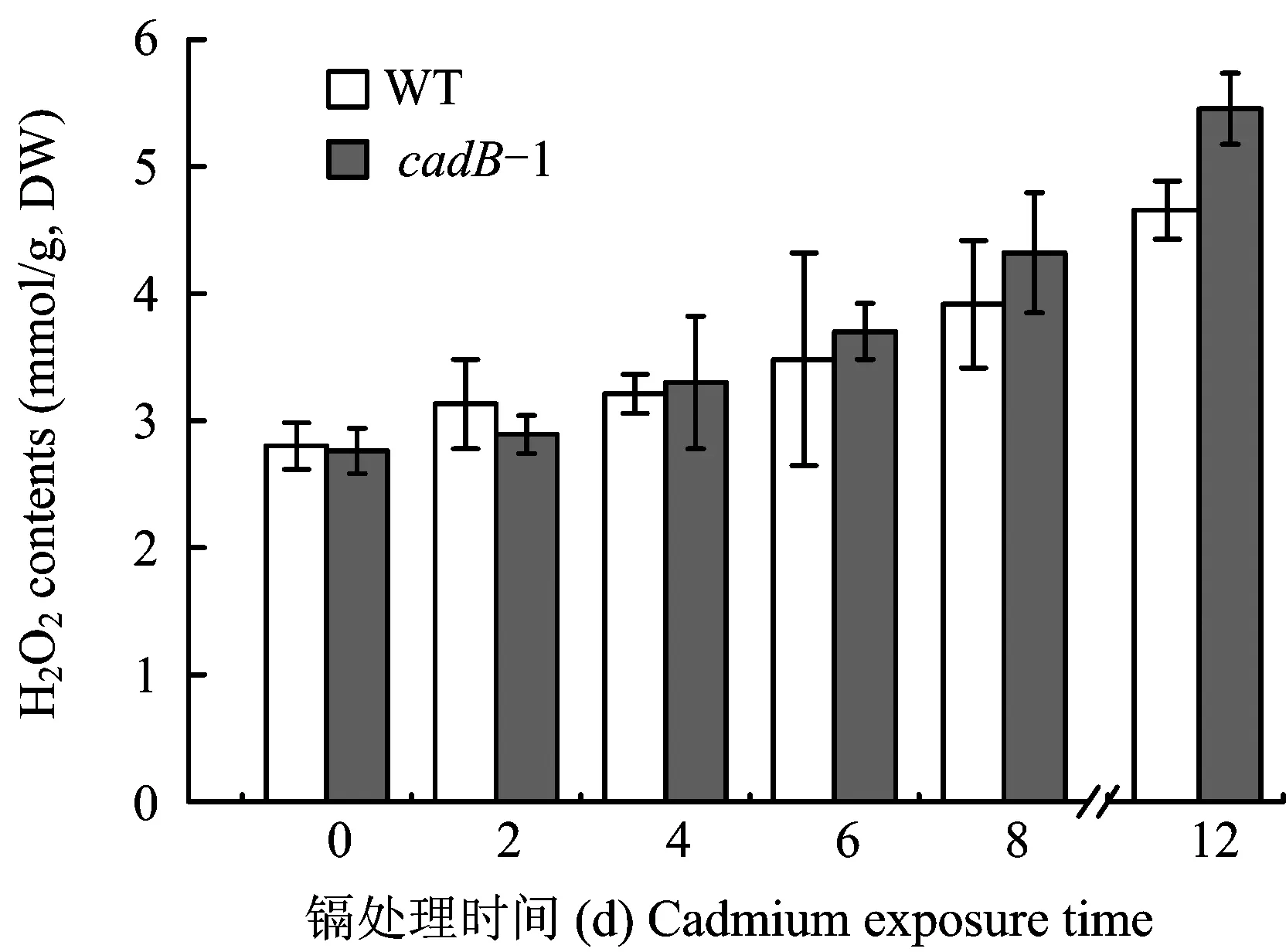
图1 野生型(WT)和Cd-敏感型(cadB-1)水稻秧苗叶片中H2O2含量Fig.1 H2O2 contents in leaves of WT and cadB-1 rice seedlings[注(Note):External Cd2+ concentration is 0.5 mmol/L 外源Cd2+浓度为0.5 mmol/L. Error bars represent standard error (n=3)误差线代表标准差(n=3)].
2.3 Cd effect on ratios of ASC/DHA, GSH/GSSG and NADPH/NADP+in rice seedlings
During exposure period, leaf contents of ASC decreased both in WT andcadB-1 (Fig.2-a), however the opposite effect was observed with DHA contents in leaves (Fig. 2-b). Significant differences were seen in ASC content between WT andcadB-1 rice seedling leaves at the 8th and 12th exposure day. Therefore, the ratio of ASC/DHA was reduced with prolonging exposure time (Fig.2-c). Furthermore, in WT andcadB-1 rice seedlings the ratio varied concomitantly with prolonging time at 0.5 mmol/L Cd2+. After 12 d exposure to 0.5 mmol/L Cd2+, the ratio of ASC/DHA at the 2nd, 4th, 6th, 8th and 12th exposure day compared to the control declined by 9.76%, 20.10%, 38.00%, 53.00% and 66.55%, respectively in the leaves of WT rice seedlings, while incadB-1 rice seedlings the percentage of decrease was 16.56%, 32.92%, 48.57%, 65.86% and 85.06%, respectively. Overall, the ratio of ASC:DHA declined more in leaves ofcadB-1 rice seedlings than in WT rice seedlings.
The GSH contents decrease and GSSG increase in leaves occurred not only in WT but also incadB-1 during the exposure (Fig. 2-d, e). Significant differences took place in GSH contents but not in GSSG between WT andcadB-1 rice seedling leaves during exposure period. Changes in GSH and GSSG led to changes in GSH/GSSG ratio (Fig. 2-f), significant differences could be found in the GSH/GSSG ratio between WT andcadB-1 rice seedling leaves at the 4th, 8th and 12th exposure day. During exposure period, the GSH/GSSG ratio in leaves ofcadB-1 rice seedlings declined by 35.80%, 61.09%, 70.65%, 82.31% and 85.91%, respectively, while ratios for leaves of the WT rice seedlings were increased by 26.37%, 37.52%, 59.15%, 63.00% and 68.09%, respectively compared to the control. GSH/GSSG ratios decreased more incadB-1 rice seedlings than in WT rice seedlings.
Oxidized nicotinamide adenine dinucleotide phosphate (NADP+) contents increased during exposure period (Fig. 2-h), while the NADPH contents meet the opposite to NADP+in leaves. NADPH:NADP+ratios were reduced (Fig. 2-i), similar to GSH/GSSG and ASC/DHA ratios. The leaves ofcadB-1 rice seedlings showed a decrease in the NADPH/NADP+ratios of 7.77%, 19.34%, 35.76%, 55.03%, and 67.94%, respectively. In the WT seedlings, the NADPH/NADP+ratios compared to the control were decreased by 3.81%, 18.30%, 28.76%, 45.09%, and 58.37%. The decrease in the NADPH/NADP+ratios was more pronounced incadB-1 rice seedlings than in WT rice seedlings.
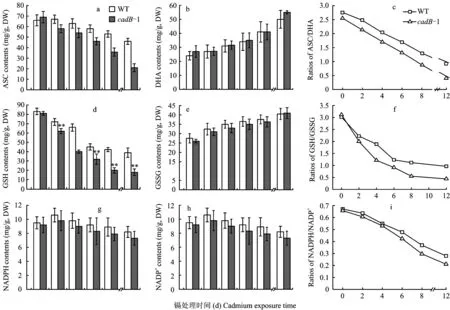
图2 野生型(WT)和Cd-敏感型(cadB-1)水稻秧苗叶片中ASC、DHA、GSH、GSSG、NADPH、NADP+值和ASC/DHA, GSH/GSSG and NADPH/NADP+比率Fig.2 The contesnt ASC, DHA, GSH, GSSG, NADPH, NADP+ and the ratio of ASC/DHA, GSH/GSSG and NADPH/NADP+ in leaves of WT and cadB-1 rice seedlings[注(Note): 外源Cd浓度为0.5 mmol/L External Cd concentration is 0.5 mmol/L; *P<0.05 and **P<0.01. 误差线代表标准差(n=3) Error bars represent standard error (n=3).]
2.4 Cd effect on APX, GR, DHAR and MDHAR activities in rice seedlings
APX activities increased and then decreased both in WT and incadB-1 rice seedlings during exposure (Fig.3-a). APX activity reached its highest level in WT and incadB-1 rice seedlings at the 6thexposure day, and the APX activities showed significant differences between WT andcadB-1 rice seedling. APX activities decreased more in the leaves ofcadB-1 seedlings than in WT seedlings during exposure.
GR activities increased first and then decreased in both WT andcadB-1 rice seedlings with the prolongation of exposure (Fig.3-b). GR activities were highest in both WT andcadB-1 rice seedlings at the 4th exposure day, GR activities were observed higher in WT than incadB-1 rice seedlings. to 0.5 mmol/L Cd2+, on the 4th day of exprosure, comparing to the control, GR activities increased by 64.85% incadB-1 seedlings and 101% in the WT rice seedlings. At the 8th day, significant differences in GR activities were observed betweencadB-1 and the WT rice seedling.
In this experiment, DHAR activities were lower incadB-1 than in WT seedlings with Cd2+exposure (Fig.3-c). DHAR activities in the WT increased and then decreased,cadB-1 varied concomitantly with WT. In thecadB-1 rice seedlings, DHAR activities reached maximum levels at the 4th exposure day, while those in WT seedlings reached maximum levels at the 8th day. Differences in DHAR activities betweencadB-1 and WT plants were significant at the 6th, 8th and 12th exposure day.
MDHAR activities increased and then decreased both in thecadB-1 and WT rice seedlings (Fig. 3-d). MDHAR activities were maximum at the 4th day incadB-1, and at the 6th day in WT seedlings. MDHAR activities decreased more in thecadB-1 than the WT rice seedlings. At the 12th exposure day, MDHAR activities decreased by 56.82% in the MT, and 47.79% in thecadB-1. MDHAR activity differences were significant betweencadB-1 and WT rice seedlings at the 6th exposure day.
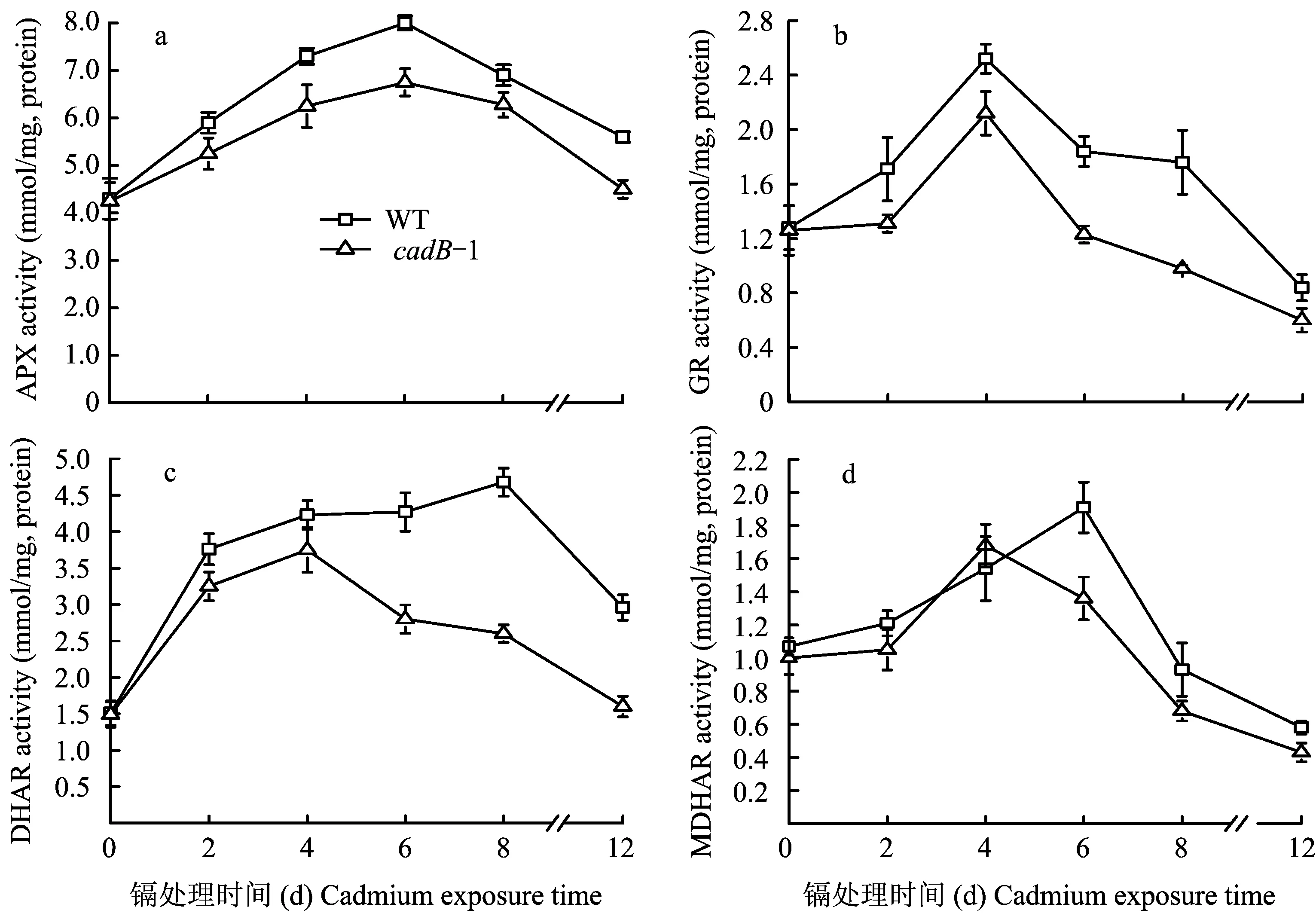
图3 野生型(WT)和Cd-敏感型(cadB-1)水稻秧苗根系中APX, GR, DHAR and MDHAR活性Fig.3 APX, GR, DHAR and MDHAR activities in roots of WT and cadH-5 rice seedlings[注(Note): 外源Cd浓度为0.5 mmol/L External Cd concentration is 0.5 mmol/L; *—P<0.05; **—P< 0.01. 误差线代表标准差 (n=3) Error bars represent standard error (n=3).]
3 Discussion
H2O2is induced inArabidopistreated with Cd2+[21], moreover, H2O2considered, as a signaling molecule in stress, is well documented[22], it defense and provide acclimation during various abiotic and biotic stresses. In general, we found the H2O2contents increased with prolongation of Cd2+exposure in bothcadB-1 and WT plants, and accumulated more incadB-1 than in WT (Fig.1).
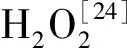
ASC behaves as an electron donor for APX scavenging of H2O2, and would be oxidated to DHA. On the other hand, DHA could be regenerated to ASC by DHAR, using GSH as an electron donor. The balance of ASC and DHA is crucial for the enzymatic systems that scavenge H2O2. In the present study, we found that prolonged Cd2+exposure time produced a decrease in the ASC content while increased in the DHA content both incadB-1 and WT. These changes resulted in decreases in the ASC:HA ratio. The decrease in ASC content might be due to an inhibition of the DHAR activity, or more excessive use of ASC in metal detoxification, or because the activity of GSH, an electron donor, is lower. The ASC content and the ASC:DHA ratio were lower in the mutant than in the wild type. This could be the result of lower DHAR activities incadB-1 than in the wild type (Fig.3). From this, we inferred that plants resistant to Cd2+toxicity maintain a high ASC content and require a high ratio of ASC/DHA.
H2O2scavenging by APX is the first step in the ASC-GSH cycle[26]. As demonstrated in the choroplasts of pea (Pisumsativum) and spinach (Spinaceaoleracea), GR, DHAR, and MDHAR also participate in this cycle[26]. In this study, we found at the short time of Cd2+exposure can induce APX activity, and may indicate the H2O2content is increasing; after long time of Cd2+exposure the APX activities decreased, which not suggesting the H2O2content is decreasing but showing Cd2+inhibition of APX activities. With the prolongation of exposure, APX activity reductions were more pronounced in the mutant than in the wild type, showing that Cd2+strongly inhibits APX activity in the mutant. GR activities vary concomitantly with APX activities; short time Cd2+exposure can induce GR activities, which catalyze GSSG to synthesize GSH by consuming NADPH as an electron donor. DHAR and MDHAR take part in the regeneration of ASC. At first can induce DHAR and MDHAR activity, possibly as the result of increased APX activities, hence DHAR and MDHAR caught regenerate enough ASC as an electron donor for APX scavenging for H2O2.With long time of Cd2+, DHAR and MDHAR activities are inhibited, and DHAR and MDHAR activities decrease more in the mutant than in the wild type, showing that Cd2+inhibited both DHAR and MDHAR activities in the mutant acutely. At the short time, ROS is effectively scavenged; at the long time the capability is overridden and they cannot scavenge ROS effectively. From this we inferred the plant is irreversibly damaged and inhibited by ROS at the long time to Cd2+exposure.
4 Conclusion
The higher ASC, GSH and NADPH levels and the higher ratios of ASC/DHA, GSH/GSSG and NADPH/NADP+, as well as the higher antioxidative enzymatic activities in plants will be more effective to resist Cd2+toxicity. Compare to WT, the mutantcadB-1 has lower level of antioxidants as well as lower activity of antioxidant enzymes;A little more Cd accumulated incadB-1 means a little more reactive oxygen species production (ROS). Videlicet,cadB-1 is deficient of the defense power against increased level of ROS which leads to a lower growth potential and sensitive to Cd.
Acknowledgements:
The authors are very grateful to Dr Wang Jiang-Xin (Shenzhen University) read the manuscript carefully, and proposed many revisions. This work supported by Heze University fund to PhD (XY13BS01).
[1] Liu Y G, Wang X, Zeng G Metal. Cadmium-induced oxidative stress and response of the ascorbate-glutathione cycle inBechmerianivea(L.) Gaud[J]. Chemosphere, 2007, 69(1): 99-107.
[2] Anjum A N, Gill S S, Gill Retal. Metal/metalloid stress tolerance in plants: role of ascorbate, its redox couple, and associated enzymes[J]. Protoplasma, 2014, 251(6): 1265-1283.
[3] Li Z, Su D, Lei Betal. Transcriptional profile of genes involved in ascorbate glutathione cycle in senescing leaves for an early senescence leaf (esl) rice mutant[J]. Journal of Plant Physiology, 2014, 176(25): 1-15.
[4] Chaparzadeh N D, Amico M L, Khavari-Nejad R Aetal. Antioxidative responses ofCalendulaofficinalisunder salinity conditions[J]. Plant Physiology and Biochemistry, 2004, 42(9): 695-701.
[5] Kuzniak E. Maria S. Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected byBotrytiscinerea[J]. Plant Science, 2001,160(4): 723-731.
[6] Kuo M C, Kao C H. Antioxidant enzyme activities are upregulated in response to cadmium in sensitive, but not in tolerant rice (OryzasativaL.) seedlings[J]. Botany Bulletin of Academy Sinica, 2004, 45: 91-299.
[7] Srivastava S, Tripathi R D, Dwivedi U N. Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress inCuscutareflexa-An angiospermic parasite[J]. Journal of Plant Physiology, 2004, 161(6): 665-674.
[8] Monrás J P, Collao B, Molina-Quiroz R C, Pradenas G Aetal. Microarray analysis of theEscherichiacoliresponse to CdTe-GSH Quantum Dots: understanding the bacterial toxicity of semiconductor nanoparticles[J]. BMC Genomics. 2014,15(1): 1099.
[9] 林冬, 朱诚, 孙宗修. 镉敏感水稻突变体在镉胁迫下活性氧代谢的变化[J]. 环境科学, 2006, 27(3): 561-566. Lin D, Zhu C, Sun Z X. Alterations of oxidative metabolism respond to cadmium stress in Cd-sensitive mutant rice seedlings[J]. Environmental Sciences 2006, 27(3): 561-566.
[10] He J Y, Zhu C, Ren Y Fetal. Root morphology and cadmium uptake kinetics of the cadmium-sensitive rice mutant[J]. Biologia Plantarum, 2007, 51(4): 791-794.
[11] Shen G M, Zhu C, Du Q Z, Shangguan L N. Ascorbate- Glutathione cycle alteration in a cadmium sensitive mutant from rice[J]. Rice Science, 2012, 19(3): 185-192
[12] Shen G M, Zhu C, Shangguan L N, Du Q Z. The Cd-tolerant rice mutantcadH-5 is a high Cd accumulator and shows enhanced antioxidant activity[J]. Journal of Plant Nutrition and Soil Science, 2012, 175(2): 309-318.
[13] Shah K, Dubey R S. A 18 kDa cadmium inducible protein Complex: its isolation and characterisation from rice (OryzasativaL.) seedlings[J]. Journal of Plant Physiology, 1998, 152(4): 448-454.
[14] Jana S, Choudhuri M A. Glycolate metabolism of three submersed aquatic angiosperms during aging[J]. Aquatic Botany, 1982, 12: 345-354.
[15] Law M Y, Charles S A, Halliwell B. Glutathione and ascorbic acid in spinach (Spinaciaoleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat[J]. Biochemistry Journal, 1983, 210: 899-903.
[16] Anderson J V, Chevone B I, Hess J L. Seasonal variation in the antioxidant system of eastern white pine needles evidence for thermal dependence[J]. Plant Physiology, 1992, 98(2): 501-508.
[17] Nisselbaum J S, Green S. A simple ultramicro method for determination of pyridine nucleotides in tissues[J]. Analytical Biochemistry, 1969, 27(2): 212-217.
[18] Nakano Y, ASCda K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical[J]. Plant and Cell Physiology, 1987, 28(1): 131-140.
[19] Dalton D A, Russell S A, Hanus F Jetal. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules[J]. Proceedings of the National Academy of Sciences, 1986, 83(11): 3811-3815.
[20] Arrigoni O, Dipierro S, Borraccino G. Ascorbate free radical recuctase: A key enzyme of ascorbic acid system[J]. FEBS Letters, 1981, 125: 242-244.
[21] Cho U, Seo N. Oxidative stress inArabidopsisthalianaexposed to cadmium is due to hydrogen peroxide accumulation[J]. Plant Science, 2005, 168(1): 113-120.
[22] Chen C, Twito S, Miller G. New cross talk between ROS, ABA and auxin controlling seed maturation and germination unraveled in APX6 deficient Arabidopsis seeds[J]. Plant Signal & Behavior, 2014, 9(12): e976489.
[23] Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis[J]. Annual Review of Plant Biology, 2002, 53(1): 159-182.
[24] Dalton D A, Russell S A, Hanus F Jetal. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules[J]. Proceedings of the National Academy of Sciences, 1986, 83(11): 3811-3815.
[25] Ortega-VillASCnte C, Rellán-álvarez R, Del Campo F Fetal. Cellular damage induced by cadmium and mercury inMedicagosativa[J]. Journal of Experimental Botany, 2005, 56(418): 2239-2251.
[26] Jimenez A, Hernandez J A, Del Rio L A, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves[J]. Plant Physiology, 1997, 114(1): 275-284.
Cd2+induced changes of ascorbate-glutathione cycle in Cd sensitive rice mutantcadB-1 leaves
SHEN Guo-ming
(KeyLaboratoryofPlantBiology/DepartmentofLifeSciences,HezeUniversity,Heze,Shandong274015,China)
【Objectives】 Cd2+is easily absorbed from the soil by plants and accumulation in plants which health threat to humans through human food chain. To investigate the mechanism of cadmium (Cd2+) induced oxidative stress and inhibit growth in a Cd sensitive rice mutant (cadB-1), a hydroponic experiment was conducted. 【Methods】 Ajaponicarice (Oryzasativa) variety Zhonghua 11 and the mutant rice seedlings obtained from the same rice variety as that formerly constructed with T-DNA/Ds insertion mediated byAgrobacterium. The transgenetic rice generations have stable hereditability and were used in this experiment. The seeds were surface sterilized in 0.5% sodium hypochlorite for 20 min, rinsed, and germinated in the dark on moistened filter paper at 30℃ for 2 d, and then moved to a plastic screen floating on distilled water at 28℃ for 4 d. Then uniformly germinated seedlings were transferred to black polyethylene barrels which contained 6 L of rice culture solution. Seedlings were grown in a growth chamber with a photo flux density of 500 μmol/(m2·s), relative humidity of approximately 65% and day/night temperatures of 32℃/27℃ (14 h/10 h). During the growth period, the solution was renewed every 5 d. At the seven leaf stage, rice seedlings include wild-type (WT) andcadB-1 exposed to 0.5 mmol/L CdCl2for 0 (as control), 2, 4, 6, 8,or 12 d.【Results】 1) Cd and hydrogen peroxide (H2O2) accumulation were higher incadB-1 than in wild one; 2) The ratios of reduced glutathione (GSH) and oxidized glutathione (GSSG), ascorbate (ASC) and dehydroascorbate (DHA), or reduced nicotinamide adenine dinucleotide phosphate (NADPH) and oxidized nicotinamide adenine dinucleotide phosphate (NADP+) were lower incadB-1 than in WT; 3) Ascorbate peroxidase (APX, EC 1.11.1.11), glutathione reductase (GR, EC 1.6.4.2), dehydroascorbate reductase (DHAR, EC 1.8.5.1) and monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) activities were lower incadB-1 than in WT in leaves during CdCl2exposure periods.【Conclusion】cadB-1 has lower level of antioxidants as well as lower activity of antioxidant enzymes. In addition,cadB-1 accumulates more Cd means that it can produce more reactive oxygen species (ROS). Videlicet,cadB-1 is deficient of the defense power against increased level of ROS which leads to a lower growth potential and sensitive to Cd.Key words: ascorbate-glutathione cycle; cadmium sensitive mutant; growth inhibit; hydrogen peroxide; rice
2014-08-18 接受日期: 2015-01-22
菏泽学院博士基金项目(XY13BS01)资助。
沈国明(1975—), 男, 浙江绍兴人, 博士,讲师,主要从事植物逆境分子生理和农产品安全生产研究。 E-mail: gmshen@tzc.edu.cn
S511.01
A
1008-505X(2015)02-0346-08
