硫丹的环境行为及水生态毒理效应研究进展
2015-06-05武焕阳丁诗华
武焕阳,丁诗华
1. 华南理工大学环境与能源学院,广州 510006 2. 西南大学动物科技学院 水产科学重庆市市级重点实验室,重庆 400715
硫丹的环境行为及水生态毒理效应研究进展
武焕阳1,2,丁诗华2,*
1. 华南理工大学环境与能源学院,广州 510006 2. 西南大学动物科技学院 水产科学重庆市市级重点实验室,重庆 400715
有机氯农药硫丹作为一种典型的持久性有机污染物(POPs)曾广泛应用于农业生产,我国曾大量使用。硫丹作为一种重要的污染物通过地表径流、淋、溶、干/湿沉降等方式进入水体,在直接影响大型水生植物和浮游藻类的同时,给鱼类等水生动物也带来了一定的毒性效应。由于其半衰期较长、迁移能力强、富集性高,在水体环境中已普遍检测出硫丹的存在,因此,对硫丹的水生生态安全性评价显得十分重要。硫丹对水生生物具有高毒性,它可影响生物正常受体配体作用、损伤生物膜、影响活性氧代谢并具有潜在的内分泌干扰作用。本文介绍了硫丹的环境行为效应,并综述了硫丹对水生生物的毒性及几种致毒机制,展望了该领域今后的研究重点和方向。
硫丹;POPs;水生生物;环境行为;毒理效应
硫丹(endosulfan)分子式:C9H6Cl6O3S,又称赛丹或安杀丹,纯品为白色晶体,易溶于氯仿、丙酮等有机溶剂。其在碱性介质中不稳定,可缓慢水解为硫丹二醇和二氧化硫,常见的α-硫丹和β-硫丹2种异构体混合物比例约为7:3[1]。作为一种危害性极高的有机氯农药,硫丹曾广泛用于棉花、烟草、茶叶和咖啡等农业生产中。据统计,全世界范围使用硫丹总量为30.8万t[2-3]。由于具有较强的迁移作用,硫丹在生产、使用和废弃过程中可通过污水、废水、地表径流或大气沉降等最终进入水环境中,且其在水体中的浓度水平危及水生生物和人类的健康,因此硫丹在水体中的分布及其对水生生物的毒理效应一直是人们关注的焦点[4]。然而从目前资料来看,关于硫丹在环境中的分布情况研究较多,而硫丹对水生生物的生理生化、内分泌毒性、遗传毒性及代谢机制等方面研究较少。本文综述了近年来硫丹的环境行为及对水生生物的毒性作用研究,并分析今后的研究思路,对以后的研究热点做了展望。
1 概 述
1.1 硫丹的环境行为及分布
环境中硫丹有2个来源:一是硫丹在农业生产中大量使用,使一部分硫丹挥发进入大气,另一部分是黏附在农作物上的硫丹在雨水冲刷、淋溶及地表径流的作用下,被转运至土壤和水体中;二是硫丹生产工厂废弃污染物排放使硫丹进入水体和土壤环境中。硫丹2种异构体均可被氧化、水解为硫丹硫酸盐(endosulfan sulphate)和硫丹二醇(endosulfan diol)[5]。
硫丹具有较强的环境迁移能力,据报道,硫丹作为一种有机氯农药广泛存在于大气环境中,并可随大气环流迁移到全球各个地区,高山地区、极地地区环境介质中均发现硫丹及硫丹硫酸盐存在。Pozo等[6]检测到智利北部大气硫丹浓度为4~101 pg·m-3,并发现硫丹主要生产使用地的北部地区大气硫丹含量高于南部地区。加拿大西部高山地区大气中也检测出硫丹存在,且α-硫丹浓度高于β-硫丹浓度[7]。我国大气中同样发现硫丹,通过对我国的37个城市及3个背景点的空气中有机氯进行分析,α-硫丹和β-硫丹的浓度范围分别为0~1 190 pg·d-1和0~422 pg·d-1[8],同时发现,含量较高采样点出现在棉花种植区,表明农业使用是我国空气中硫丹的重要来源。
水环境中同样有硫丹的存在,我国太湖中也检测出硫丹,表1列出了世界上部分典型水体中硫丹的含量。
美国高海拔(3 024~3 030 m)湖泊沉积物中也检测出硫丹硫酸盐存在[13]。研究发现加拿大北极圈群岛Devon岛DV09地平线以上湖底沉积物中有α-硫丹存在,最高浓度达0.04 ng·g-1(干重),且流量为6.2 ng·(m2y)-1,自1990年起,这些湖泊沉积物硫丹含量在逐渐增加[14]。

表1 硫丹在部分水环境中的浓度Table 1 The concentration of endosulfan in the water environment
1.2 硫丹在水生生物体内的蓄积
研究报道,水生生物体内也已普遍检测出硫丹。其中,我国华南沿海牡蛎(Crassostrea rivularis)体中硫丹含量为广东:2.13 ng·g-1(湿重),海南:1.23 ng·g-1(湿重),广西:0.76 ng·g-1(湿重)[15]。Kelly等[16]发现北极红点鲑(Salvelinus alpinus)、环斑海豹(Phoca hispida)和白鲸(Delphinapterus leucas)体内α-硫丹和β-硫丹含量分别为(0.12±0.09) ng·g-1(湿重)、(0.46±0.55) ng·g-1(湿重);(2.0±3.2) ng·g-1(湿重)、(1.7±2.1) ng·g-1(湿重)和(4.0±5.9) ng·g-1(湿重)、(6.5±2.8) ng·g-1(湿重)。而Stern等[24]发现加拿大北极群岛雄性白鲸体内硫丹硫酸盐含量从3.7 ng·g-1(脂重)到94 ng·g-1(脂重)不等。加拿大北极群岛Lancaster海峡和Jones海峡雄性白鲸体内(28~94 ng·g-1)发现更高浓度的硫丹硫酸盐,而Baffin岛Cumberland海峡和Frobisher湾的白鲸体内硫丹硫酸盐的含量为8.1~23 ng·g-1。据报道,印度超过60%的市售海水鱼可检测出硫丹,其浓度为5~22 ng·g-1(湿重)[17]。大量研究显示,硫丹及其降解产物主要在动物的肝脏、皮肤、脂肪及肌肉中分布[18]。可见,除了毒物代谢器官,脂肪及皮肤也是硫丹主要分布区域,这可能是因为硫丹的辛醇—水分配系数(logKow)显示其进入富含脂肪组织中的可能性较大,并可随着胚胎中脂肪转运进入卵细胞或者传递给下一代。
硫丹在水生生物体内的浓度水平能直接反映水体中硫丹的污染情况,进而可以评价其对生态系统的潜在危害。有研究表明,水体中β-硫丹较α-硫丹含量更高,这或许表明α-硫丹更容易被水生生物转化、富集[19]。α-硫丹、β-硫丹的logKow分别为4.94和4.78,因此沉积物对α-硫丹的吸附作用较β-硫丹强,α-硫丹生物富集能力略强于β-硫丹[20]。一般认为当有机化合物logKow>5时,该化合物具有生物富集性。浮游动物比浮游植物更易富集硫丹,而鱼类对硫丹富集能力明显大于浮游生物[21]。生物富集系数(BCF)经常用来评价污染物在水生生物体内的富集效果。研究表明,黄脂鲤鱼(Hyphessobrycon bifasciatus)对硫丹BCF高达11 000[22],淡水绿藻(Pseudokirchneriella subcapitatum)和淡水大型溞(Daphnia magna)对硫丹BCF分别为2 682和3 678[23],而野鲮(Labeo rohita)对硫丹BCF只有不到50[24],因此不同生物对硫丹富集能力存在较大差别。
2 硫丹的水生生物毒性效应
不同形态的硫丹在环境中的降解速率不同,生物毒性也不相同。β-硫丹较α-硫丹降解慢,α-硫丹半衰期为7~75 d,而β-硫丹半衰期为33~376 d,研究表明,硫丹硫酸盐是环境中硫丹的主要降解产物[25]。水中α-硫丹较β-硫丹更易降解为硫丹硫酸盐[26],在土壤环境中也有同样发现,并且硫丹降解速率受土壤水分、温度、含氧量、pH等环境因素影响,温度较低、水分较小、含氧量低、pH较低情况下硫丹的降解速率较慢[27]。硫丹降解产物毒性较小,如:硫丹硫酸盐对金鱼(Carassius auratus)和雅罗鱼(Leuciscus idus melanotus)48 h半数致死浓度(48 h LC50)接近100 μg·L-1,而α-硫丹 < 10 μg·L-1[28]。另外,硫丹与其他污染物的联合毒性效应更强。资料显示,394 μg·L-1毒死蜱与4.5 μg·L-1、7.9 μg·L-1、1 μg·L-1硫丹共同作用下,太平洋树蛙幼体(Pseudacris regilla)致死率显著高于硫丹单一染毒[29]。2.1 硫丹的急性致毒效应
几乎所有水生生物对硫丹都非常敏感。水生无脊椎动物是水生动物中较低等的动物类群,表2列出了硫丹对一些水生无脊椎动物的毒性值。

表2 硫丹对甲壳类动物毒性Table 2 Toxicity of endosulfan on shellfish
研究表明,硫丹对藻类也有较高毒性,硫丹对近头状伪蹄型藻(Pseudokirchneriella subcapitatum)96 h EC50为428 μg·L-1[23]。此外,有研究显示,不同的环境条件也可能影响硫丹对甲壳动物的毒性。当暴露环境中有底泥存在时,硫丹对褐虾(Penaeus aztecus)96 h LC50从无底泥存在时的0.2 μg·L-1提高到6.9 μg·L-1[37];斑节对虾(Penaeus monodon)96 h LC50从无底泥存在时的1.6 μg·L-1降低到0.5 μg·L-1;96 h最低可观察效应浓度(LOEC)从无底泥存在时的1.038 μg·L-1降低到0.141 μg·L-1;96 h最低无可观察效应浓度(NOEC)从无底泥存在时的0.536 μg·L-1降低到 < 0.141 μg·L-1[38]。0.1 μg·L-1硫丹暴露96 h,美洲龙虾幼体(Homarus americanus)代谢范围较对照组显著降低25%[39]。
硫丹对鱼类同样具有较强毒性。研究显示,硫丹对大部分鱼类的96 h LC50为0.09~4.4 μg·L-1[40],且淡水鱼类相对海水鱼类具有更高的耐受性,见表3。
根据毒性分级,LC50< 1 000 μg·L-1为剧毒物质。绝大部分鱼类对硫丹极为敏感,96 h LC50均在10 μg·L-1以下,可见硫丹对鱼类毒性极强。硫丹对不同鱼类LC50有所差异,可能原因有2种:一是受试动物对硫丹的耐受程度不同,二是暴露试验的环境条件不同。另外,研究发现,高等鱼类较低等鱼类对硫丹的耐受能力更强一些,这可能是因为高等鱼类的代谢器官更为发达、解毒系统更为完善,使毒物对机体的毒性作用更小。
2.2 干扰正常受体—配体的相互作用
受体是许多组织细胞的生物大分子,与化学物质即配体相结合后形成受体—配体复合物,能产生一定的生物学效应。许多毒物尤其是某些神经毒物的毒性作用与其干扰正常受体—配体相互作用的能力有关。 目前有研究表明,硫丹可与γ-氨基丁酸(GABA)拮抗,从而抑制GABA受体聚集[45]。GABA是中枢神经系统抑制性神经递质,硫丹作为GABA非竞争性的拮抗物,可抑制GABA受体聚集,聚集程度的降低将导致神经元细胞去极化,使动物焦躁不安[46]。 胆碱能神经是以乙酰胆碱(ACh)为神经传递物质,在ACh完成传递任务后,若继续存在,则将不断刺激突触后膜,引起神经功能的紊乱,因此必须及时将之分解消除,这有赖于乙酰胆碱酯酶(AChE)对ACh的催化作用,AChE可将ACh分解为乙酸和胆碱,避免ACh积累对神经的过多刺激。有机磷农药已被证实可抑制动物胆碱酯酶(ChE)活性,使其失去分解ACh能力,导致ACh积聚,阻断神经传导,引起神经功能紊乱[51]。研究显示,3.3~5 μg·L-1硫丹暴露96 h,可显著抑制橙色莫桑比克罗非鱼(Oreochromis mossambicus)脑AChE活性[52]。同样发现,0.072~1.4 μg·L-1硫丹暴露,可显著抑制四眼青鳉(Jenynsia multidentata)肌肉AChE活性,并发现随着硫丹暴露质量浓度升高或时间延长,其活动能力明显下降,游泳能力受到显著影响[53]。2.4 μg·L-1硫丹暴露96 h,斑马鱼脑AChE活性显著降低,较对照组下降近40%,其活动能力同样显著降低[54]。
2.3 生物膜损伤作用
生物膜具有十分重要的生物功能,它可选择地进行物质交换,以维持细胞内部有一个相对稳定的理化特性,并维持细胞内自身稳定。Na+、K+-ATP酶和Ca2+、Mg2+-ATP酶又称依赖ATP膜结合蛋白酶,对建立跨膜的离子梯度、维持细胞膜电位与细胞生理活动、调节细胞渗透压、控制细胞容量和正常代谢以及为其他离子和营养物质的转运提供动力方面具有重要作用[55]。
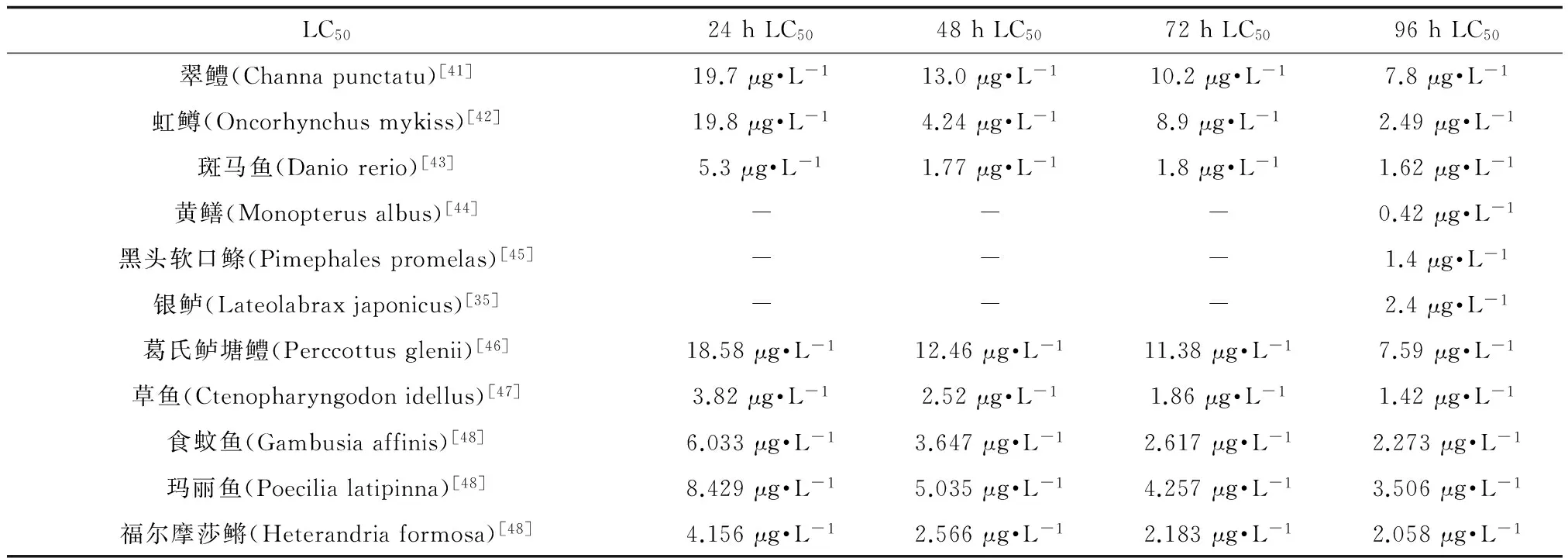
表3 硫丹对部分鱼类的LC50Table 3 LC50 of endosulfan on fish
研究表明,硫丹可影响鱼类ATP酶活性,从而影响细胞正常生物功能。2.2 μg·L-1硫丹暴露15 d,可激活宽额鳢(Channa gachua)Na+、K+-ATP酶和Mg2+-ATP酶活性;3.7 μg·L-1硫丹暴露30 d后,其肝脏、肾脏及肌肉ATP酶活性显著受到抑制[56]。暴露于0.010~0.264 μg·L-1硫丹下,罗氏沼虾仔虾(Macrobrachium rosenbergii)Na+、K+-ATPase活性显著升高[57]。翠鳢(Channa punctatus)鳃Na+、K+-ATP酶活性在1.2 μg·L-1硫丹暴露90 d后明显降低[58]。大西洋鲑(Salmo salar)硫丹(4~710 μg·kg-1)经口染毒14 d,鳃Na+、K+-ATP酶活性明显降低,35 d后恢复到正常水平;肠Na+、K+-ATP酶活性14 d和35 d均被显著抑制[59]。Velasco等[60]报道,0.16 μg·L-1和0.48 μg·L-1硫丹暴露14 d,可引起斑马鱼Na+、K+-ATP酶活性升高,并在28 d后恢复到正常水平,同时发现其鳃丝组织增生。
2.4 活性氧生成与氧化损伤机理
活性氧(ROS)是指在生物体内与氧代谢有关的含氧自由基和易形成自由基的过氧化物总称,如O2·-、·OH、H2O2、ROOH等。生物体自身生理活动可产生ROS,如分解氧以提供能量的电子传递链过程、吞噬细胞吞噬作用以及外源物质的分解过程,污染物也可诱导生物细胞内外源性ROS形成[61]。
体内的ROS具有一定的功能,如免疫和信号转导过程,但由于它有未成对电子,自由基和自由原子非常活泼,因此过多的ROS就会有破坏作用,导致正常细胞和组织的损坏。正常情况下细胞内的抗氧化酶类SOD、CAT、GSH-Px等可以清除ROS,而当ROS的产生与清除平衡被扰乱,细胞无法及时清除时,就会导致机体氧化损伤[62]。ROS过量生成可干扰多种信号转导通路,从而影响细胞凋亡,如MAPKs信号通路、ERK1/2通路、Nrf2-Keap1通路、JNK/SPAK通路等。研究表明,硫丹等机氯农药通过生成大量的ROS,可明显激活ERK1/2通路,激活的ERK通过磷酸化抗凋亡分子,同时激活转录因子,以刺激表达存活相关基因而产生抗凋亡作用[63]。
已有研究表明,硫丹可诱导斑马鱼[64]和草鱼(Ctenopharyngodon idellus)[65]肝脏Ⅰ相(APND;ERND)和Ⅱ相(GST)解毒酶活性升高,进而影响正常生理机能。硫丹暴露可诱导水芪草(Myriophyllum quitense)[66]、大型溞(Daphnia magna)[30]、虹鳟(Oncorhynchus mykiss)[67]、四眼青鳉(Jenynsia multidentata)[68]、斑马鱼[45-69]、草鱼[70-71]、奥尼罗非鱼(Oreochromis niloticus)[72]、中华大蟾蜍(Bufo bufo)[73]、鬼针草蟾(Bidens laevis)[74]、菲律宾蛤仔(Venerupis philippinarum)[75]等水生生物机体产生过量ROS,并产生氧化胁迫,表现为机体组织SOD、CAT、GSH-Px、GST等抗氧化酶活性的非正常变化,LPO升高,严重导致细胞NDA损伤、凋亡、组织病变甚至个体死亡。
2.5 内分泌干扰作用
研究表明硫丹对内分泌系统存在潜在的影响,对人类和生物具有较大的负面影响,其能够干扰生物体内源激素的合成、释放、转运、结合和代谢,从而影响机体的内环境稳定、生殖、发育及行为。体外毒性试验显示,硫丹可以激活雌激素受体α(ERα)的AF2功能,使孕酮受体(PR)水平升高和雌激素响应基层细胞增殖[76]。通过对ERα转染HeLa细胞系研究发现,硫丹与雌二醇竞争结合ERα,并可反馈激活ERα,诱导ERE依赖基因表达[77]。研究表明,硫丹暴露可下调胡子鲶(Clarias batrachus)卵巢泛素与Esco2蛋白表达,上调黑素皮质素受体-2蛋白表达[78];2.5 μg·L-1硫丹与33 μg·L-1氟他胺共同影响下,幼体胡子鲶睾丸发育相关转录因子(dmrt1、sox9a、wt1)、类固醇生成酶(11-hsd2、17-hsd12、P450c17)、类固醇激素合成急性调节蛋白、孤核受体(nr2c1、Ad4BP/SF-1)基因表达量显著降低[79]。硫丹是一种类雌激素,可模拟雌激素的生理作用促进子宫正常发育[80]。硫丹对鱼类也有类雌激素作用,硫丹暴露可诱导斑马鱼胚胎及幼体卵黄蛋白原(VTG)表达[81]。对大西洋鲑(Salmo salar)肝细胞卵透明带(ZP)和VTG基因表达研究[82]也有类似作用。正常情况下,只有性成熟的雌性动物卵子发生阶段在雌二醇的控制下才能产生ZP和VTG。雄鱼体内含有VTG后,雄性特征会逐步退化,雌性特征会逐步明显,雄鱼逐渐雌性化。
甲状腺是动物重要的内分泌器官,其分泌的甲状腺激素T3、T4具有重要的生理功能:促进组织分化、生长与发育,作用于细胞核受体,刺激DNA转录过程,促进mRNA形成,加速蛋白质与各种酶生成,增强碳水化合物利用,促进脂肪酸及脂肪合成等。鱼类甲状腺素对代谢活动、生长、渗透压调节、生殖、体色、中枢神经活动和行为等方面都有影响[83]。某些有机氯农药可直接与甲状腺激素受体结合,激活受体或抑制受体,使激素不能发挥正常功能。研究显示,硫丹可影响鱼类的甲状腺激素水平。0.1 μg·L-1硫丹暴露35 d,尼罗罗非鱼(Oreochromis niloticus)血浆T4水平显著降低,T3水平变化不明显[84]。同样研究表明,硫丹可不同程度影响萨罗罗非鱼(Sarotherodon mossambicus)血清T3、T4水平[85]。有研究显示,硫丹是通过干扰肝脏Ⅰ型脱碘酶和Ⅲ型脱碘酶活性来影响甲状腺激素水平[86]。鱼类血浆T3的浓度与肾脏、肝脏中脱碘酶的活性密切相关[87]。此外,硫丹还可引起鱼类催乳激素、皮质醇、胰岛素水平变化,从而间接影响鱼类渗透压调节、应激反应及碳水化合物代谢等功能[85]。
3 总结与展望
本文总结了近年来硫丹的环境分布,并介绍了其对水生生物的毒性及致毒机制。由于硫丹与环境的相互作用复杂,已有的研究结果和认识还存在一定的局限性,因此有必要进一步加强硫丹对水生生物整个生命周期及在多种环境污染物共存条件下硫丹对水生生物的生理生化及生态学研究。另外,应进一步深入研究硫丹污染胁迫下,特别是低剂量长期暴露下,生物体内生理生化反应及分子机制,对于进一步揭示硫丹生物毒性的分子和细胞作用机制及其与机体健康的内在联系具有重要的意义。
[1] United Nations Environment Programme. Consideration of a Newly Proposed Chemical, Endosulfan, for Inclusion in Annexes A, B or C of the Convention [C]. Stockholm Convention on Persistent Organic Pollutants Review Committee Third Meeting, 2007
[2] Li Y F, Macdonald R W. Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: A review [J]. Science of the Total Environment, 2005, 342(1): 87-106
[3] Jia H, Li Y F, Wang D, et al. endosulfan in China 1: Gridded usage inventories [J]. Environmental Science and Pollution Research, 2009, 16(3): 295-301
[4] Miles C J, Pfeuffer R J. Pesticides in canals of South Florida [J]. Archives of Environmental Contamination and Toxicology, 1997, 32(4): 337-345
[5] Walse S S, Scott G I, Ferry J L, et al. Stereoselective degradation of aqueous endosulfan in modular estuarinemesocosms: Formation of endosulfan γ-hydroxycarboxylate [J]. Journal of Environmental Monitoring, 2003, 5: 373-379
[6] Pozo K, Harner T, Shoeib M, et al. Passive-sampler derived air concentrations of persistent organic pollutants on a north-south transect in Chile [J]. Environmental Science & Technology, 2004, 38(24): 6529-6537
[7] Daly G L, Lei Y D, Teixeira C, et al. Pesticides in western Canadian mountain air and soil [J]. Environmental Science & Technology, 2007, 41(17): 6020-6025
[8] Liu X, Zhang G, Li J, et al. Seasonal patterns and current sources of DDTs, chlordanes, hexachlorobenzene, and endosulfan in the atmosphere of 37 Chinese cities [J]. Environmental Science & Technology, 2009, 43(5): 1316-1321
[9] Gregor D J, Gummer W D. Evidence of atmospheric transport and deposition of organochlorine pesticides and polychlorinated biphenyls in Canadian Arctic snow [J]. Environmental Science & Technology, 1989, 23: 561-565
[10] Weber J, Halsall C J, Muir D C G, et al. Endosulfan and γ-HCH in the Arctic: An assessment of surface seawater concentrations and airsurface exchange [J]. Environmental Science & Technology, 2006, 40(24): 7570-7576
[11] Muir D C G, Alaee M, Teixeira C, et al. Newcontaminants in the Arctic and Subarctic Atmospheric and Aquatic Environments [R]. CEPA Resources Report-FY 2006-2007. Burlington ON: Environment Canada, Aquatic Ecosystem Protection Research Division, 2007
[12] Qiu X H, Zhu T, Wang F, et al. Air-water gas exchange of organochlorine pesticides in Taihu Lake China [J]. Environmental Science & Technology, 2008, 42(6): 1928-1932
[13] Usenko S, Landers D H, Appleby P G, et al. Current and historical deposition of PBDEs, pesticides, PCBs, and PAHs to Rocky Mountain national park [J]. Environmental Science & Technology, 2007, 41(21): 7235-7241
[14] Stern G A, Braekevelt E, Helm P A, et al. Modem and historical fluxes of halogenated organic contaminants to a lake in the Canadian arctic, as determined from annually laminated sediment cores [J]. Science of the Total Environment, 2005, 342(1-3): 223-2243
[15] 甘居利, 柯常亮, 陈洁文, 等. 华南沿海近江牡蛎体中硫丹残留特征研究[J]. 农业环境科学学报, 2014, 33(2): 271-275
Gan J L, Ke C L, Chen J W, et al. Residues ofendosulfan in ostrea oysters (Crassostrea rivularis) from near sea water of South China [J]. Journal of Agro-Environment Science, 2014, 33(2): 271-275 (in Chinese)
[16] Kelly B C, Ikonomou M G, Blair J D, et al. Food web-specific biomagnification of persistent organic pollutants [J]. Science, 2007, 317(5835): 236-239
[17] Muralidharan S, Dhananjayan V, Jayanthi P, et al. Organochlorine pesticides in commercial marine fishes of Coimbatore, India and their suitability for human consumption [J]. Environmental Research, 2009, 109(1): 15-21
[18] Johansen P, Muir D C G, Amund G, et al. Human exposure to contaminants in the traditional Greenland diet [J]. Science of the Total Environment, 2004, 331(1-3): 189-206
[19] Zhang Z L, Hong H S, Zhou J L, et al. Fate and assessment of persistent organic pollutants in water and sediment from Minjiang River estuary, Southeast China [J]. Chemosphere, 2003, 52(9): 1423-1430
[20] Shen L, Wania F, Ying D L, et al. Atmospheric distribution and long-range transport behaviour of organochlorine pesticides in North America [J]. Environmental Science & Technology, 2005, 39(2): 409-420
[21] Weber J, Halsall C J, Muir D, et al. endosulfan, a global pesticide: A review of its fate in the environment and occurrence in the Arctic [J]. Science of the Total Environment, 2010, 408(15): 2966-2984
[22] Jonsson C M, Toledo M C F. Bioaccumulation and elimination of endosulfan in the fish Yellow Tetra (Hyphessobrycon bifasciatus) [J]. Bulletin of Environmental Contamination and Toxicology, 1993, 50(4): 572-577
[23] DeLorenzo M E, Taylor L A, Lund S A, et al. Toxicity and bioconcentration potential of the agricultural pesticide endosulfan in phytoplankton and zooplankton [J]. Archives of Environmental Contamination and Toxicology, 2002, 42(2): 173-181
[24] Ramaneswari K, Rao L M. Bioconcentration of endosulfan and monocrotophos by Labeo Rohita and Channa Punciaia [J]. Bulletin of Environmental Contamination and Toxicology, 2000, 65(5): 618-622
[25] Laabs V, Amelung W, Pinto A A, et al. Pesticides in surface water, sediment, and rainfall of the northeastern Pantanal basin, Brazil [J]. Journal of Environmental Quality, 2002, 31(5): 1636-1648
[26] Leonard A W, Hyne R V, Lim R P, et al. Fate and toxicity of endosulfan in Namoi River water and bottom sediment [J]. Journal of Environmental Quality, 2001, 30(3): 750-759
[27] Ghadiri H, Rose C W. Degradation of endosulfan in a clay soil from cotton farms of western Queensland [J]. Journal of Environmental Management, 2001, 62(2): 155-169
[28] USEPA. ECOTOX User Guide: Ecotoxicology Database System, Version 4.0. United States Environmental Protection Agency; 2007c. [EB/OL]. [2011-10-16]. http://www.epa.gov/ecotox. htm
[29] Dimitrie D A, Sparling D W. Joint toxicity of chlorpyrifos and endosulfan to Pacific Treefrog (Pseudacris regilla) Tadpoles [J]. Archives of Environmental Contamination and Toxicology, 2014, 67: 444-452
[30] Barata C, Varo I, Navarro J C, et al. Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds [J]. Comparative Biochemistry and Physiology: Part C, 2005, 140(2): 175-186
[31] Bhavan P S, Zayapragassarazan Z, Geraldine P, et al. Acute toxicity tests of endosulfan and carbaryl for the freshwater prawn, Macrobrachium malcolmsonii (H.Milne Edwards) [J]. Environmental Science and Pollution Research, 1997, 16(5): 5-7
[32] Sumith J A, Parkpian P, Leadprathom N, et al. Dredging influenced sediment toxicity of endosulfan and lindane on black tiger shrimp (Penaeus monodon Fabricius) in Chantaburi River estuary in Thailand [J]. International Journal of Sediment Research, 2009, 24(4): 455-464
[33] Schimmel S C, Patrick J M, Wilson A J, et al. Acute Toxicity to and Bioconcentration of Endosulfan by Estuarine Animals [M]. USA: ASTM STP634, American Society for Testing and Material, Philadelphia, 1977: 241-252
[34] Wirth E F, Lund S A, Fulton M H, et al. Determination of acute mortality in adults and sublethal embryo responses of Palaemonetes pugio to endosulfan and methoprene exposure [J]. Aquatic Toxicology, 2001, 53(1): 9-18
[35] Johnson W W, Finley M T. Handbook of Acute Toxicity of Chemicals to Fish and Aquatic Invertebrates [M]. Washington DC: Resource Publication 137. U.S. Department of Interior, Fish and Wildlife Service, 1980: 6-56
[36] 陈尚朝, 陈敏东, 宋玉芝, 等. 2种有机杀虫剂对中华绒螯蟹毒性研究[J]. 环境科学与技术, 2014, 37(9): 5-9
Chen S C, Chen M D, Song Y Z, et al.Toxic effects of two organic pesticides on Eriocheir sinensis [J]. Environmental Science & Technology, 2014, 37(9): 5-9 (in Chinese)
[37] McLeese D W, Metcalfe C D. Toxicities of eight organochlorine compounds in sediment and seawater to Crangon septemspinosa [J]. Bulletin of Environmental Contamination and Toxicology, 1980, 25(6): 921-928
[38] Sumith J A, Parkpian P. Behaviour of Endosulfan and Lindane During Sediment Elutriate and Water Spike Toxicity Tests Under Saline Conditions [M]. Sri Lanka: Annals of the Sri Lanka Department of Agriculture, Vol. 10, 2008: 231-243
[39] Daoud D, Fairchild W L, Comeau M, et al. Impact of an acute sublethal exposure of endosulfan on early juvenile lobster (Homarus americanus) [J]. Aquatic Science and Technology, 2014, 2(2): 14-40
[40] 胡国成, 许木启, 戴家银, 等. 硫丹对水生生物毒理效应的研究进展[J]. 中国水产科学, 2007, 14(6): 1042-1047
Hu G C, Xu M Q, Dai J Y, et al. Progress in the study of toxicological effects of endosulfan on aquatic organisms [J]. Journal of Fishery Sciences of China, 2007, 14(6): 1042-1047 (in Chinese)
[41] Pandey S, Nagpure N S, Kumar R, et al. Genotoxicity evaluation of acute doses of endosulfan to freshwater teleost Channa punctatus (Bloch) by alkaline single-cell gel electrophoresis [J]. Ecotoxicology and Environmental Safety, 2006, 65(1): 56-61
[42] Capkin E, Altinok I, Karahan S, et al. Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout [J]. Chemosphere, 2006, 64(10): 1793-1800
[43] 胡国成, 甘炼, 吴天送, 等. 硫丹对斑马鱼的毒性效应[J]. 动物学杂志, 2008, 43(4): 1-6
Hu G C, Gan L, Wu T S, et al. Toxicological effects of endosulfan on Danio rerio [J]. Chinese Journal of Zoology, 2008, 43(4): 1-6 (in Chinese)
[44] Hii Y S, Lee M Y, Chuah T S, et al. Acute toxicity of organochlorine insecticide endosulfan and its effect on behaviour and some hematological parameters of Asian swamp eel (Monopterus albus, Zuiew) [J]. Pesticide Biochemistry and Physiology, 2007, 89(1): 46-53
[45] Dutta H M, Arends D A. Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish [J]. Environmental Research Letters, 2003, 91(3): 157-162
[46] 李思雯, 肖蓉, 程李芳, 等. 硫丹和高效氯氟氰菊酯对葛氏鲈塘鳢的急性毒性研究[J]. 安徽农业科学, 2014, 42(11): 3282-3283, 3286
Li S W, Xiao R, Cheng L F, et al. Study on the acute toxicity of endosulfan and lambda-cyhalothrin on Perccottus glenii [J]. Journal of Anhui Agricultural Sciences, 2014, 42(11): 3282-3283, 3286 (in Chinese)
[47] 武焕阳, 靳涛, 丁诗华, 等. 硫丹对草鱼鱼种的急性毒性效应[J]. 水产科学, 2012, 31(1): 37-40
Wu H Y, Jin T, Ding S H, et al. Acute toxicity of endosulfan to juvenile grass carp Ctenopharyngodon idellus [J]. Fisheries Science, 2012, 31(1): 37-40 (in Chinese)
[48] Carriger J F, Hoang T C, Rand G M, et al. Acute toxicity and effects analysis of endosulfan sulfate to freshwater fish species [J]. Archives of Environmental Contamination and Toxicology, 2011, 60(2): 281-289
[49] Jia Z, Misra H P. Developmental exposure to pesticides zineb and/or endosulfan renders the nigrostriatal dopamine system more susceptible to these environmental chemicals later in life [J]. Neurotoxicology, 2007, 28(4): 727-735
[50] Scremin O U, Chialvo D R, Lavarello S, et al. The environmental pollutant endosulfan disrupts cerebral cortical function at low doses [J]. Neurotoxicology, 2011, 32(1): 31-37
[51] Garg U K, Pal A K, Jha G J, et al. Pathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides in broiler chicks [J]. International Immunopharmacology, 2004, 4(13): 709-722
[52] Kumar N, Prabhu P A J, Pal A K, et al. Anti-oxidative and immuno-hematological status of Tilapia (Oreochromis mossambicus) during acute toxicity test of endosulfan [J]. Pesticide Biochemistry and Physiology, 2011, 99(1): 45-52
[53] Ballesteros M L, Durando P E, Nores M L, et al. endosulfan induces changes in spontaneous swimming activity and acetylcholinesterase activity of Jenynsia multidentata (Anablepidae, Cyprinodontiformes) [J]. Environmental Pollution, 2009, 157(5): 1573-1580
[54] Pereira V M, Bortolotto J W, Kist L W, et al. endosulfan exposure inhibits brain AChE activity and impairs swimming performance in adult zebrafish (Danio rerio) [J]. Neurotoxicology, 2012, 33(3): 469-475
[55] Piovavroval N B. Effects of cadmium on ciliary and ATPase activity in the gills of freshwater mussel Anodonta cygnea [J]. Comparative Biochemistry and Physiology, 1992, 103(3): 27-30
[56] Sharma R M. Effect of endosulfan on adenosine triphosphatase (ATPase) activity in liver, kidney, and muscles of Channa gachua [J]. Bulletin of Environmental Contamination and Toxicology, 1988, 41(3): 317-323
[57] 熊昭娣, 戴习林, 谢剑, 等. 硫丹对罗氏沼虾溞状幼体及仔虾的急性毒性作用[J]. 广东农业科学, 2013, 11: 111-114
Xiong Z D, Dai X L, Xie J, et al.Acute toxicity of endosulfan to zoea and postlarve of Macrobrachium rosenbergii [J]. Guangdong Agricultural Sciences, 2013, 11: 111-114 (in Chinese)
[58] Sarma K, Pal A K, Sahu N P, et al. Dietary high protein and vitamin C mitigates endosulfan toxicity in the spotted murrel, Channa punctatus (Bloch, 1793) [J]. Science of the Total Environment, 2009, 407(12): 3668-3673
[59] Glover C N, Petri D, Tollefsen K E, et al. Assessing the sensitivity of Atlantic salmon (Salmo salar) to dietary endosulfan exposure using tissue biochemistry and histology [J]. Aquatic Toxicology, 2007, 84(3): 346-355
[60] Velasco S Y M, Handy R D, Katherine A, et al. endosulfan affects health variables in adult zebrafish (Danio rerio) and induces alterations in larvae development [J]. Comparative Biochemistry and Physiology, Part C, 2011, 153: 1-9
[61] Valavanidis A, Vlahogianni T, Dassenakis M, et al. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants [J]. Ecotoxicology and Environmental Safety, 2006, 64(2): 178-189
[62] Halliwell B, Gutteridge J M C. Free Radicals in Biology and Medicine [M]. Oxford: Oxford University Press, 1999: 543
[63] Ledirac N, Antherieu S, d'Uby A D, et al. Effects of organochlorine insecticides on MAP kinase pathways in human HaCat keratinocytes: Key role of reactive oxygen species [J]. Toxicological Sciences, 2005, 86(2): 444-452
[64] Dong M, Zhu L S, Shao B, et al. The effects of endosulfan on cytochrome P450 enzymes and glutathione S-transferases in zebrafish (Danio rerio) livers [J]. Ecotoxicology and Environmental Safety, 2013, 92(1): 1-9
[65] 武焕阳, 靳涛, 许莉佳, 等. 硫丹对草鱼Ⅰ相Ⅱ相酶活性及DNA损伤的影响[J]. 生态与农村环境学报, 2012, 28(1): 54-60
Wu H Y, Jin T, Xu L J, et al. Effects of endosulfan on phase Ⅰ and Ⅱ enzyme activities and DNA damage of Ctenopharyngodon idellus [J]. Rural Eco-Environment, 2012, 28(1): 54-60 (in Chinese)
[66] Menone M L, Pesce S F, Díaz M P, et al. endosulfan induces oxidative stress and changes on detoxication enzymes in the aquatic macrophyte Myriophyllum quitense [J]. Phytochemistry, 2008, 69(5): 1150-1157
[67] Dorval J, Leblond V S, Hontela A, et al. Oxidative stress and loss of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) exposed in vitro to endosulfan, an organochlorine pesticide [J]. Aquatic Toxicology, 2003, 63(3): 229-241
[68] Ballesteros M L, Wunderlin D A, Bistoni M A, et al. Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan [J]. Ecotoxicology and Environmental Safety, 2009, 72(1): 199-205
[69] Shao B, Zhu L S, Dong M, et al. DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio) [J]. Ecotoxicology, 2012, 21(5): 1533-1540
[70] 武焕阳, 丁诗华, 唐毅, 等. 硫丹对草鱼外周血红细胞微核及核异常的影响[J]. 淡水渔业, 2011, 41(5): 28-34
Wu H Y, Ding S H, Tang Y, et al. Effects of endosuifan on micronuclei and nuclear anomalies in peripheral blood erythrocytes of Ctenopharyngodon idellus [J]. Freshwater Fisheries, 2011, 41(5): 28-34 (in Chinese)
[71] 武焕阳, OSCAR Ortegon, 许莉佳, 等. 硫丹对草鱼乙酰胆碱酯酶及抗氧化酶活性的影响[J]. 生态环境学报, 2011, 20(10): 1496-1502
Wu H Y, Oscar O, Xu L J, et al. Effects of endosulfan on activities of acetylcholinesterase and antioxidant enzyme of Ctenopharyngodon idellus [J]. Ecology and Environmental Sciences, 2011, 20(10): 1496-1502 (in Chinese)
[72] Tellez B M C, Santerre A, Casas S J, et al. Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan [J]. Fish & Shellfish Immunology, 2009, 27(2): 105-111
[73] Brunelli E, Bernabòa I, Berg C, et al. Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles [J]. Aquatic Toxicology, 2009, 91(2): 135-142
[74] Pérez D J, Menone M L, Camadro E L, et al. Genotoxicity evaluation of the insecticide endosulfan in the wetland macrophyte Bidens laevis L. [J]. Environmental Pollution, 2008, 153(3): 695-698
[75] 陶颜霞. 菲律宾蛤仔(Venerupis philippinarum)在硫丹胁迫下毒性效应与分子生物标志物的研究[D]. 青岛: 中国海洋大学, 2013: 26-28
Tao Y X. Study of toxicity effects and molecular biomarkers of clam Venerupis philippinarum exposed to endosulfan [D]. Qingdao: Ocean University of China, 2013: 26-28 (in Chinese)
[76] Hunter D S, Hodges L C, Vonier P M, et al. Estrogen receptor activation via activation function 2 predicts agonism of xenoestrogens in normal and neoplastic cells of the uterine myometrium [J]. Cancer Research, 1999, 59: 3090-3099
[77] Lemaire G, Mnif W, Mauvais P, et al. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines [J]. Life Sciences, 2006, 79(12): 1160-1169
[78] Laldinsangi C, Vijayaprasadarao K, Rajakumar A, et al. Two-dimensional proteomic analysis of gonads of air-breathing catfish, Clarias batrachus after the exposure of endosulfan and malathion [J]. Environmental Toxicology and Pharmacology, 2014, 37(3): 1006-1014
[79] Rajakumar A, Singh R, Chakrabarty S, et al. endosulfan and flutamide impair testicular development in the juvenile Asian catfish, Clarias batrachus [J]. Aquatic Toxicology, 2012, 110-111: 123-132
[80] Varayoud J, Monje L, Bernhardt T, et al. endosulfan modulates estrogen-dependent genes like a non-uterotrophic dose of 17β-estradiol [J]. Reproductive Toxicology, 2008, 26(2): 138-145
[81] Chow W S, Chan W K L, Chan K M, et al. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A [J]. Journal of Applied Toxicology, 2013, 33(7): 670-678
[82] Krøvel A V, Søfteland L, Torstensen B E, et al. endosulfan in vitro toxicity in Atlantic salmon hepatocytes obtained from fish fed either fish oil or vegetable oil [J]. Comparative Biochemistry and Physiology, Part C, 2010, 151(2): 175-186
[83] 林浩然. 鱼类生理学(第二版)[M]. 广州: 广东高等教育出版社, 2007: 241
[84] Coimbra A M, Figueiredo F A, Reis H M A, et al. Nile tilapia (Oreochromis niloticus), liver morphology, CYP1A activity and thyroid hormones after endosulfan dietary exposure [J]. Pesticide Biochemistry and Physiology, 2007, 89(3): 230-236
[85] Thangavel P, Sumathiral K, Maheswari S, et al. Hormone profile of an edible, freshwater teleost, Sarotherodon mossambicus (Peters) under endosulfan toxicity [J]. Pesticide Biochemistry and Physiology, 2010, 97(3): 229-234
[86] Coimbra A M, Reis H M A, Darras V A, et al. Circulating thyroid hormone levels and iodothyronine deiodinase activities in Nile Tilapia (Oreochromis niloticus) following dietary exposure to endosulfan and aroclor 1254 [J]. Comparative Biochemistry and Physiology, Part C, 2005, 141(1): 8-14
[87] Mol K A, Vandergeyten S, Darras V M, et al. Characterization of iodothyronine outer ring and inner ring deiodinase activities in the blue tilapia, Oreochromis aureus [J]. Endocrinology, 1997, 138(5): 1787-1793
◆
Research Progress in the Environmental Behavior and Water Ecotoxicological Effects of Endosulfan
Wu Huanyang1,2, Ding Shihua2,*
1. College of Environment and Energy, South China University of Technology, Guangzhou 510006, China 2. College of Animal Science and Technology, Key Laboratory of Aquatic Science of Chongqing, Southwest University, Chongqing 400715, China
15 October 2014 accepted 6 February 2015
As a typical persistent organic pollutants, endosulfan, the organochlorine pesticide, has been widely used in agricultural production in China. Endosulfan could go into the water envronment through the surface runoff, leaching and wet/dry deposition, which will have a direct impact on aquatic macroohytes and planktonic algae, and produce a certain amount of toxic effects on fish and other aquatic animals as well. Because of its longer half-life period, better migration abilities and higher enrichment, endosulfan could be detectable widely in the water body, herein the safety evaluation of endosulfan in the aquatic ecosystem is very important. Endosulfan is so highly-toxic to aquatic organisms that it could have influences on normally biological receptor-ligand function, membrane damage, active oxygen metabolism and have a potential role of endocrine disruption. The environmental behavior effects and several toxic mechanisms of endosulfan on aquatic organisms will be reviewed, and the future prospects in this filed will be also discussed.
endosulfan; POPs; aquatic organisms; environmental behaviors; toxicology effects
国家自然科学基金项目(30670226);重庆市科委农业科技成果转化资金项目(cstc2013jcsf-nycgzhA80008)
武焕阳(1986-),男,博士,研究方向为环境生态毒理学,E-mail: wuhuanyang@163.com;
*通讯作者(Corresponding author), E-mail: shhding@yahoo.com.cn
10.7524/AJE.1673-5897.20141015002
2014-10-15 录用日期:2015-02-06
1673-5897(2015)2-113-10
X171.5
A
丁诗华(1966—),男,遗传学博士,教授,从事水产动物生理及研究环境生态学研究,发表学术论文50余篇。
武焕阳, 丁诗华. 硫丹的环境行为及水生态毒理效应研究进展[J]. 生态毒理学报, 2015, 10(2): 113-122
Wu H Y, Ding S H. Research progress in the environmental behavior and water ecotoxicological effects of endosulfan [J]. Asian Journal of Ecotoxicology, 2015, 10(2): 113-122 (in Chinese)
