以甲氧基苯甲酸为配体的两个双核铜配合物的合成、晶体结构和理论计算
2015-06-01于良民夏树伟闫星辰倪春花
张 琦 于良民,2 夏树伟 李 霞*,,2 闫星辰 倪春花
(1中国海洋大学,海洋化学理论与工程技术教育部重点实验室,青岛266100)
(2中国海洋大学,海洋科学与技术青岛协同创新中心,青岛266100)
以甲氧基苯甲酸为配体的两个双核铜配合物的合成、晶体结构和理论计算
张 琦1于良民1,2夏树伟1李 霞*,1,2闫星辰1倪春花1
(1中国海洋大学,海洋化学理论与工程技术教育部重点实验室,青岛266100)
(2中国海洋大学,海洋科学与技术青岛协同创新中心,青岛266100)
以2-甲氧基苯甲酸(HL1)、2,3-二甲氧基苯甲酸(HL2)及甲醇为配体,合成了配合物Cu2(L1)4(CH3OH)2(1)和Cu2(L2)4(CH3OH)2(2),并通过红外、元素分析、X-射线粉末和单晶衍射等研究手段表征了其结构。配合物1属单斜晶系,空间群P21/n;配合物2属三斜晶系,空间群P1。2个配合物都具有双核铜结构,由2个铜离子、4个L配体分子和2个甲醇配体分子组成,其中配体L通过双齿配位模式与铜离子配合。研究了2个配合物的热稳定性,并通过Gaussian 09软件密度泛函理论B3LYP方法进行了理论研究。
甲氧基苯甲酸;双核铜配合物;晶体结构;量子化学计算
0 Introduction
In recent years,the design and synthesis of metal complexes have attracted widely interest because of their versatile networktopologies[1-3]and potential applicationsinelectrochemicalsensor,catalysis, magnetism,luminescence,antibacterial activity,biological activity,mental-base drugs[4-11]and so on.As anionic O-donor ligands,carboxylates have strong coordination ability to metal ions,which involve different coordination modes and their complexes exhibit diverse network structures[12-16].Copper is often selected to design and synthesize new complexes with novel structure because of its high probability in forming a variety of mononuclear or multinuclear complexes[17-18].The carboxylates can coordinate with copper cations by monodentate or bidentate bridging coordination mode.
In this paper,two dinuclear copper complexes with methoxybenzoic acids were synthesized and the singlecrystalstructureswereobtainedand characterized.DFT[19-21]studies of the complexes were performed,and frontier molecular orbital energies and components were calculated also.These results would provide theoretical support for further study of the chemical properties and applications.
1 Experimental
1.1 Materials and physical measurement
All reagents used in this work were of analytical grade.The X-ray diffraction data were collected on a Bruker Smart Apex CCD X-ray single-crystal diffractometer.X-ray powder diffraction was determined by a Bruker D8 powder diffractometer at room temperature.FT-IR spectra were recorded on an Avatar 360 FT-IR spectrometer in dry KBr pellets in the range of 4 000~400 cm-1.Elemental analyses were carried out with a model 2400 Perkin-Elmer analyzer.The quantum chemistry calculation was carried out by DFT B3LYP method with 6-31G(d,p)bases for C,H,O atoms and LANL2DZ for copper on Work Station by using Gaussian 09 program.Atom coordinates used in the calculation were derived from crystallographic data.
1.2 Syntheses of the complexes
2-methoxybenzoic acid(0.152 g,1.0 mmol)and KOH(0.056 g,1.0 mmol)were dissolved in 15 mL methanol,and then the solution was stirred for 15 min on a magnetic stirrer.After that Cu(CF3SO3)2·6H2O (0.235 g,0.5 mmol)was added and the solution was stirred for another 15 min.The obtained solution was filtered,and deep green block crystals of complex 1 were gained by slow evaporation of the filtrate at room temperature in the air for two weeks.The crystals were preserved in mother liquor for further detection (Yield:50.5%,based on Cu).Anal.Calcd.for C34H36Cu2O14(%):C 51.32,H 4.56;Found(%):C 51.15, H 4.52.IR(KBr,cm-1):3 488,1 606,1 396,1 247, 1 019,759,669.
The preparation of complex 2 was performed in the same way as complex 1,and the dose of 2,3-dimethoxybenzioc acid was 0.182 g(1.0 mmol).Green block crystals were gained after about two weeks.The crystals were preserved in mother liquor for further detection(Yield:52.4%,based on Cu).Anal.Calcd. for C38H44Cu2O18(%):C 49.84,H 4.84;Found(%):C 49.67,H4.81.IR(KBr,cm-1):3451,1624,1397,1260, 1 061,1 000,780,757.
1.3 Crystallographic data collection and structure determination
A suitable crystal of complex 1 with dimensions of 0.45 mm×0.40 mm×0.37 mm was mounted on a Bruker Smart Apex CCD X-ray single-crystal diffractometer with a Mo Kα radiation(λ=0.071 073 nm)at 298(2)K by using a φ-ω scan mode.A total of 8 472 reflections were collected in the range of 2.66°≤θ≤25.02°(7≤h≤8,13≤k≤13,-25≤l≤17),and 3031 reflections were independent withRint=0.028 8,of which 2 366 reflections were observed with I>2σ(I).
A suitable crystal of complex 2 with dimensions of 0.43 mm×0.31 mm×0.16 mm was mounted on the diffractometer mentioned above.A total of 4 979 reflections were collected in the range of 2.53°≤θ≤25.02°(10≤h≤9,10≤k≤12,-14≤l≤13),and 3422 reflections were independent with Rint=0.019 5,of which 2 787 reflections were observed with I>2σ(I).
Empirical absorption corrections were appliedusing SADABS program[22].The structures of compoundsweresolvedbydirectmethodsusingthe SHELXS-97[23].Positional and thermal parameters of all non-hydrogen atoms were refined by full-matrix least-squaresmethodtoconvergenceusingthe SHELXTL software package[24].The methyl hydrogen atoms were positioned geometrically and refined using a riding model,with dC-H=0.096 nm and Uiso(H)=1.5 Ueq(C).All the other hydrogen atoms were fixed geometrically at calculated distances and allowed to ride on the parent non-hydrogen atoms.Crystal data and structure refinement of the complexes were listed in Table 1.
CCDC:1007777,1;1013765,2.
2 Results and discussion
2.1 Description of crystal structures
Complex1crystallizesinmonoclinic,space group P21/n,and complex 2 crystallizes in triclinic, space group P1.The crystal structures are shown in Fig.1 and Fig.2 ,and the selected bond lengths and bond angles are listed in Table 2.
Complex 1 has dinuclear structure,consisting of two Cucations,four L1ligands and two methanol Ligands molecules,in which the L1ligands are coordinated with copper cations by bidentate bridging coordination mode.The structure of complex 1 is centrosymmetric.The structure of complex 2 is similar as complex 1,and they are shown in Fig.1 (a)and Fig. 2(a).Each copper atom is coordinated with five oxygen atoms derived from four L1or L2ligands and one methanol,Cu1 and Cu1iare connected through the metallic bond.Namely,each Cuis six-coordinated, forming an octahedral configuration.Taking Cu1 of complex 1 as an example,the O5i-Cu1-O2i(88.69(10)°), O4-Cu1-O2i(89.82(10)°),O5i-Cu1-O1(91.31(10)°), O4-Cu1-O1(88.22(10)°)bond angles are all near 90°, indicating that the four atoms(O1,O2i,O4 and O5i) are approximately coplanar.In addition,the O5i-Cu1-O4(169.23(9)°),O2i-Cu1-O1(169.51(9)°),O7-Cu1-Cu1i(175.22(6)°)bond angles are deviated from 180°, indicating that the octahedron is slight distorted.TheCu1 of complex 2 forms a similarly distorted octahedral configuration as complex 1.

Table1 Crystallographic data and structure refinement for complexes 1 and 2

Fig.1 (a)Crystal structure of complex 1(Hydrogen atoms are omitted for clarity;Displacement ellipsoids are drawn at the 30%probability level),(b)1D chain structure of complex 1,(c)Packing diagram of complex 1
The Cu1-Cu1ibond length in complex 1 and complex 2 are 0.257 16(6)nm and 0.261 53(7)nm respectively,which are both shorter than 0.30 nm, indicating the existence of intense coupling effect[25-26]between Cu1 and Cu1i.In complex 1,the Cu1-O1 (0.197 23(19)nm),Cu1-O2i(0.196 72(19)nm),Cu1-O4(0.194 9(2)nm)and Cu1-O5i(0.194 6(2)nm)bond lengths are all shorter than that of Cu1-O7(0.213 87(19) nm),and in the complex 2,Cu1-O1(0.196 9(2)nm), Cu1-O2i(0.195 7(2)nm),Cu1-O5(0.196 7(2)nm), Cu1-O6i(0.196 6(2)nm)bond lengths are all shorter than that of Cu1-O9(0.219 7(2)nm),indicating that the oxygen atoms of L1and L2ligands have better coordinationabilitythantheoxygenatomsfrom methanol molecules.This might result from sterichindrance.In addition,the Cu1-O9 bond of complex 2 is longer than the Cu1-O7 bond of complex 1, suggesting that the steric hindrance effect of L2ligand is stronger than L1ligand.

Table2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
Hydrogen bonds exist in complex 1 and complex 2 leading to a one-dimensional structure,as shown in Fig.1 (b)and Fig.2 (b).In complex 1,there exist O7-H7…O1iiand O7-H7…O3iihydrogen bonds,and the symmetry code is-x,1-y,1-z.The distances between the hydrogen atom and oxygen atom are 0.220 7 and 0.255 6 nm,and the angles are 158.08°and 128.85° respectively.While in complex 2,there only exists O9-H9…O3iihydrogen bond(Symmetry code:2-x,1-y,1-z).The distance between the hydrogen atom and oxygen atom is 0.209 4 nm,and the angle is 169.68°. The differences are derived from the steric hindrance effect of methoxy group in meta-position.The steric hindrance effect of L2Ligand also leads to the deflectionofbenzenering,sothecomplex2 crystallizes in triclinic rather than monoclinic system.
There exist C-H…π interactions[27-28]in complex 1,and the distance between the hydrogen atom and the center of the phenyl ring is 0.346 7 nm,and the angle is 134.89°.A 3D network constructed by the CH…π interactions and hydrogen bond is shown in Fig.1 (c).In complex 2,there exist the C-H…O bond interaction.The distances between the hydrogen atom and oxygen atom are 0.270 3 and 0.261 4 nm,and the angles are 116.70°,136.31°respectively.A 3D network formed by the O-H…O and C-H…O hydrogen bond interactions is shown in Fig.2 (c).
2.2 IR spectroscopy
In the spectrum of complex 1,a strong wide absorption peak is observed at 3 488 cm-1,which can be attributed to the stretching vibration band of OH in CH3OH.The stretching vibration band of C=O in HL1at 1 693 cm-1disappears and appears at 1 606 cm-1after coordination,and the red shift of νC=Oindicates that the carbonyl group of L1ligand coordinates with Cucations.
In complex 2,a strong wide absorption peak is observed at 3 451 cm-1,which can be attributed to the stretching vibration band of OH in CH3OH.The stretching vibration band of C=O in HL2at 1 685 cm-1disappear and appears at 1 624 cm-1after coordination, and the red shift of νC=Oindicates that the carbonyl group of L2ligand coordinates with Cucations.The resultsareconsistentwiththecrystalstructure analysis.
2.3 X-ray powder diffraction
The simulated and experimental X-ray powder diffraction patterns of complex 1 and complex 2 are shown in Fig.3 .All peaks presented in the measured curves approximately match the simulated curves generated from single-crystal diffraction data,which confirms the phase purity of the complexes.

Fig.3 Comparison of simulated X-ray powder diffraction patterns(upper)and the measured values(lower)of complex 1(a)and complex 2(b)
2.4 Thermogravimetric analysis
Toinvestigatethermalstabilityofthetwo complexes,TG analyses were carried out with a Netzsch STA449 F3 instrument at a heating rate of 10℃·min-1from 30 to 800℃under nitrogen(Flow rate= 50 mL·min-1).
As shown in Fig.4 ,complex 1 displays three weight loss stages.The weight loss of 2.8%(Calcd. 8.1%)in the temperature range of 120~220℃corresponds to partial release of the coordinated methanol molecule.The quick weight loss of 34.2% (Calcd.43.3%)during the temperature range of 220~ 300℃couldbeattributedtothereleaseof coordinated methanol molecule and partial of L1ligand.The third step occurs between 300~800℃, which corresponds to the decomposition of residual ligands,and the final weight loss is 78.8%(Calcd. 84.0%).

Fig.4 TG curves of complex 1 and 2
In comparison with complex 1,complex 2 shows two weight loss stages.The weight loss of 1.6% (Calcd.7.0%)in the temperature range of 120~200℃might be caused by partial release of the coordinated methanol molecule.The second step occurs between 200~800℃,corresponding to release of residual methanol and all the ligands,and the final weight loss is 81.7%(Calcd.86.1%).
2.5 Quantum chemistry calculation
The natural atomic charges of the two complexes are listed in Table 3.The natural charges of Cuwere decreased to 0.77 or 0.78,suggesting that part of theelectrondensityhastransferredfromthe coordinated O atoms to the copper ions.The negative charges mostly concentrate on coordinated O atoms, which indicate O atoms are prone to coordinate with Cucations.The calculation results are in accordancewith the determination.
Some frontier molecular orbital energies and components of the two complexes are listed in Table 4,and views of frontier molecular orbital are shown in Fig.5 and Fig.6 .The total energy of complex 1 and complex 2 are-2 761.443 10 and-3 220.144 25 hartree,respectively.The HOMO-1,HOMO,LUMO, LUMO+1 orbital energies of the two complexes are -0.204 83,-0.166 89,-0.112 59,-0.013 31 a.u.and -0.201 09,-0.175 21,-0.123 61,-0.014 34 a.u..The energy gap between HOMO and LUMO of complex 1 and 2 are 0.054 3 and 0.051 6 a.u.respectively.
The orbital components of HOMO and LUMO are mainly distributed on central ion Cuand ligating O atom of L ligands,and show quite well symmetry as shown in Fig.5 and Fig.6 .The HOMO and LUMO components of Cucations in complex 1 are about 49.5%,42.6%,and in complex 2 are about 51.3%, 49.7%.Apart from the cations,the frontier molecular orbital evenly distributes on the ligands.The HOMO and LUMO components of coordinated O atoms from L1ligand are about 5.3%and 3.6%averagely,and that of L2ligand are about 5.2%and 4.6%averagely, while the orbital components of coordinated O atoms from methanol in the two complexes are both 0%. This indicates that although the methanol exists in the unit cell of the coordination compounds,it does not donate electrons to central ion Cuand just acts as an interstitial molecule.

Table4 Selected frontier molecular orbital energy (a.u.)and component(%)of complexes 1 and 2

Fig.5 View of frontier molecular orbital of complex 1
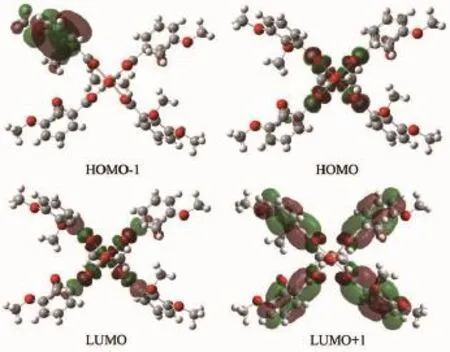
Fig.6 View of frontier molecular orbital of complex 2
The dipole moments of complex 1 and complex 2 are 0.011 4D,0.012 4D respectively,which due to the high symmetry of complexes.The calculation results identifycentrosymmetricstructuresofthetwo complexes.
3 Conclusions
Reference:
[1]Guo F,Wang F,Yang H,et al.Inorg.Chem.,2012,51(18): 9677-9682
[2]Xu J K,Sun X C,Ju C X,et al.J.Coord.Chem.,2013,66 (14):2541-2548
[3]Lin J D,Jia C C,Li Z H,et al.Inorg.Chem.Commun., 2009,12(6):558-562
[4]TANG Si-Ping(唐斯萍),XU Zhi-Feng(许志锋),FENG Yong-Lan(冯泳兰),et al.Chinese J.Inorg.Chem.(无机化学学报),2013,29(12):2683-2687
[5]Wang X L,Lin H Y,Liu G C,et al.J.Organomet.Chem., 2008,693(16):2767-2774
[6]Qu H,Qiu L,Leng X K,et al.Inorg.Chem.Commun.,2011, 14(9):1347-1351
[7]Qin L,Liu L W,Du X,et al.Transition Met.Chem.,2013, 38(1):85-91
[8]Ma L F,Han M L,Qin J H,et al.Inorg.Chem.,2012,51 (17):9431-9442
[9]LI Hai-Hua(李海华),ZHOU Xiao-Xia(周晓霞),YOU Zhong-Lu(由忠录).Chinese J.Inorg.Chem.(无机化学学报), 2013,29(3):649-653
[10]Mostafa M H K,Eglal R S,Eman H I,et al.Chinese J. Inorg.Chem.(无机化学学报),2013,29(9):1969-1978
[11]Huxford R C,Rocca J D,Lin W.Curr.Opin.Chem.Biol., 2010,14(2):262-268
[12]Haribabu P,Patil Y P,Reddy K H,et al.Transition Met. Chem.,2011,36(8):867-874
[13]WU Gang(吴刚),WANG Xiao-Feng(王小锋),XIAN Hua(鲜华),et al.Chinese J.Inorg.Chem.(无机化学学报),2010,26 (7):1315-1318
[14]Ke C H,Lin G R,Kuo B C,et al.Cryst.Growth Des.,2012, 12(7):3758-3765
[15]TANG Si-Ping(唐斯萍),ZHANG Shao-Hua(张少华),YANG Ying-Qun(杨颖群).Chinese J.Inorg.Chem.(无机化学学报),2013,29(11):2465-2469
[16]Yang W B,Lin X,Blake A J,et al.Inorg.Chem.,2009,48 (23):11067-11078
[17]Soltani B,Sadr M H,Engle J T,et al.Transition Met.Chem., 2012,37(8):687-694
[18]Lin H Y,Mu B,Wang X L,et al.J.Organomet.Chem., 2012,702:36-44
[19]SHI Zhi-Qiang(石志强),JI Ning-Ning(季宁宁),HE Guo-Fang(何国芳),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(6):1279-1285
[20]He H S,Zhong Y H,Si L P,et al.Inorg.Chim.Acta,2011, 378(1):30-35
[21]Jana M S,Pramanik A K,Sarkar D,et al.Polyhedron,2014, 81:66-73
[22]Sheldrick G M.SADABS,Program for Empirical Absorption Correction of Area Detector Data,University of Göttingen, Germany,1996.
[23]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[24]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[25]Zhao L L,Luo X Z,Xu L,et al.Inorg.Chem.Commun., 2010,13(4):554-557
[26]Yang Q,Zhang X F,Zhao J P,et al.Cryst.Growth Des., 2011,11(7):2839-2845
[27]Kamiński R,Kowalski J,Mames I,et al.Eur.J.Inorg. Chem.,2011,2011(4):479-488
[28]Santana A M,Ferreira J G,Moro A C,et al.Inorg.Chem. Commun.,2011,14(1):83-86
Syntheses,Crystal Structures and Theoretical Calculation of Two Dinuclear CopperComplexes with Methoxybenzoic Acids Ligands
ZHANG Qi1YU Liang-Min1,2XIA Shu-Wei1LI Xia*,1,2YAN Xing-Chen1NI Chun-Hua1
(1Key Laboratory of Marine Chemistry Theory and Technology,Ministry of Education, Ocean University of China,Qingdao,Shandong 266100,China)
(2Qingdao colobrative innovation center of marine science and technology, Ocean University of China,Qingdao,Shandong 266100,China)
Two complexes,Cu2(L1)4(CH3OH)2(1)and Cu2(L2)4(CH3OH)2(2)(HL1=2-methoxybenzoic acid,HL2=2,3-dimethoxybenzoic acid)were synthesized and characterized by IR spectorscopy,elemental analysis,X-ray powder and X-ray single-crystal diffraction.Complex 1 crystallizes in monoclinic,space group P21/n;Complex 2 crystallizes in triclinic,space group P1.Both of the complexes have dinuclear structure,consisting of two Cucations,four L ligands and two methanol ligands molecules,in which the L ligands are coordinated with copper cations by bidentate bridging coordination mode.The thermal stability of the complexes was investigated,and theoretical study of the complexes was carried out by Density Functional Theory(DFT)B3LYP method using Gaussian 09 program.CCDC:1007777,1;1013765,2.
methoxybenzoic acid;dinuclear coppercomplex;crystal structure;quantum chemistry calculation
O614.121
A
1001-4861(2015)03-0585-09
10.11862/CJIC.2015.086
2014-10-13。收修改稿日期:2014-12-23。
国家自然科学基金(No.51003099、51102219),中国海洋大学青年教师专项基金(No.201013017、201113024),国家科技支撑计划(No.2012BAB15B02)资助项目。
*通讯联系人。E-mail:xiali@ouc.edu.cn
