低温配位-沉淀法合成疏水ZnO及提高泡沫稳定性的应用
2015-06-01顾少楠李文军罗文利常志东周花蕾
顾少楠 李文军*, 罗文利 常志东 周花蕾
(1北京科技大学功能分子与晶态材料科学与应用北京市重点实验室,北京100083)
(2中国石油,提高石油采收率国家重点实验室,北京100083)
低温配位-沉淀法合成疏水ZnO及提高泡沫稳定性的应用
顾少楠1李文军*,1罗文利2常志东1周花蕾1
(1北京科技大学功能分子与晶态材料科学与应用北京市重点实验室,北京100083)
(2中国石油,提高石油采收率国家重点实验室,北京100083)
以Zn(NO3)2·6H2O为原料,25%氨水为配位剂,用NaOH做沉淀剂,十六烷基三甲溴化铵(CTAB)为改性剂制备了具有不同表面接触角的氧化锌微球。采用X射线衍射,扫描电镜和表面ζ电位技术对其进行了表征,并对合成过程中体系中的组分分布以及各组分对形貌的影响进行了分析。泡沫稳定性的实验结果表明:颗粒质量浓度为0.03%,接触角为52.9°的氧化锌颗粒具有对泡沫最强的稳定作用。同时对颗粒的疏水改性和提高泡沫性的机理进行了讨论。
氧化锌;配位沉淀;形貌可控;疏水效应;泡沫稳定性
0 Introduction
Aqueousfoams stabilized by micro-or nanoparticles have attracted a highly attention due to their extensive applications such as cosmetics,oil recovery, flotation and material manufacture[1-6].The strong and irreversibly attachment of particles at the air-water interface is responsible for the foam stability[7].As aresult,the destabilization mechanisms of foams such as bubble coalescence and Ostwald ripening can be prevented in this situation[8-9].Many previous studies indicated that the long-term stability of foams is a prerequisite for the successful application of foam flooding in enhanced oil recovery(EOR).Therefore theenvironmentfriendlyandlowcostparticles stabilizers with tunable surface property are needed.
Zinc oxide(ZnO)materials have wide application in several fields,such as luminescence materials,the laseractivemedium,sensors,photocatalystand antibacterial materials[10-13].However,very few studies, to the best of our knowledge,have investigated the application of surface modified ZnO particles in improving the stability of aqueous foams.ZnO is a non-toxic metal oxide with varies shapes and tunable surface properties[14-16],which have superiority in terms of the relationship between particles morphology or surfacepropertiesandfoamstability.Various techniques have been employed to synthesis of ZnO micro/nanostructuressuchassol-gelprocess[17], template-assisted growth[18],microwave methods[19], vaporphasedeposition[20]andhydro-solvothermal method[21-23].Most of these processes require high temperature or high pressure,and some of these fabrication methods are relatively expensive.This may greatlylimittheirthroughputaswellastheir efficiency.Therefore,it is still challenging to fabricate ZnO samples in uniform morphology using low energy consumption method.
In this work,we investigated a low temperature complex-precipitate(LTCP)method to obtain uniform ZnO micro-spheres.A system model of the reaction process was developed and the species distribution in the system was analyzed.Meanwhile,the samples weremodifiedbycationicsurfactant cetyltrimethylammonium bromide(CTAB)in order to obtain hydrophobic surfaces.Then,the influence of the surface contact angle of ZnO particles on foam stability was studied.Furthermore,the mechanism of foamstabilizedbysurfacemodifiedZnOwas discussed.
1 Experimental
1.1 Material
Cationicsurfactantcetyltrimethylammonium bromide(CTAB,Chemical Pure,M=364.45),Zn(NO3)2·6H2O(AR),NaOH(AR)and NH3·H2O(AR)were purchased from Sinopharm Chemical Reagent Co., Ltd.,China without further purification.The water was deionized water purified by ion exchange.Anionic surfactant sodium dodecyl sulfate(SDS,Chemical Pure, M=288)in 8.7 mmol·L-1was employed as the foam agent for all experiments.
1.2 Synthesis of ZnO particles
10 mL NH3·H2O(13 mol·L-1)was slowly added into 0.15 mol·L-1Zn(NO3)2·6H2O with stirring by a constant temperature magnetic stirrer at 80℃.After that,100 mL 0.25 mol·L-1NaOH was added dropwise intothemixedsolution,followedbycontinually stirringfor3hatthesametemperature.The precipitate was filtered and was washed with deionized water for several times until the complete removal of residual impurities.The product was atmosphericdried at 60℃for 6~8 h.The final product was white powder.
1.3 Adsorption of CTAB on the surface of ZnO
The suspensions were prepared by dispersing ZnOsamplesintoCTABsolutionsinvaries concentrations.All the suspensions were vibrated at 25℃for 24 h.The detailed procedure was as follows. Firstly,a required amount of CTAB(10,50,170,220, 300,350,400 mg)were dissolved in 100 mL deionized water followed by stirring for a few minutes in order to produce homogeneous solutions.Secondly,30 mg ZnO samplesinseptuplicatewereaddedintoCTAB solutions,separately.Then,thepHvaluesof suspensions were adjusted to 9.5 with 0.1 mol·L-1NaOH solution.After vibrating,surface modified ZnO samples were collected by centrifugation before being washedwithdeionizedwaterandalcohol.Then samples were dried at 60℃for 4 h.
1.4 Characterization of ZnO particles
XRD pattern of obtained ZnO was recorded using powder X-ray diffraction at room temperature on a D/ MAX-RB Diffractometer(Rigaku,Japan)with Cu Kα source(λ=0.154 18 nm),operating at 40 kV and 30mA,scanning in the 2θ range from 10°to 90°.The morphology was observed via Quanta 450 emission scanning electron microscope(FEI,U.S.).All SEM samples were prepared by depositing a drop of diluted suspension in ethanol on conductive tape and imaged with carbon sputtering.ζ potentials of ZnO and ZnO/ CTAB samples were measured by Zetasizer Nano ZS90(Malvern,UK).
The contact angle of modified ZnO surface was measured using captive drop method[24]via OCA-20 (Dataphysics,Germany).SurfacemodifiedZnO samples were crushed into powders and compressed into circular disks at a pressure of 12 MPa.A drop of water was placed on the disk surface firstly and then the shape of the water was immediately photographed. The contact angle was directly measured using a protractor.
1.5 Foampreparationandfoamstability measurement
All the measurements were carried out under roomtemperature.Theaqueousdispersionswere prepared by mixing a required amount of surface modified ZnO samples(10,30,50,70 mg)in 100 mL SDS solutions.The dispersions were foamed using a Warring Blender(Cole-Parmer,US)for 30s.The foam stability was evaluated by the period used for 50 mL liquid draining out from foam(half-life,t1/2).After foaming,about 5 mL foam was transferred to a glass dish.The morphology of bubbles was observed by monocular straight cube microscope.
2 Results and discussion
2.1 Characterization of ZnO particles
Fig.1 shows the XRD pattern of the prepared ZnO sample.The characteristic peaks at 31.7°,34.4°, 36.2°,47.5°,56.5°,62.8°and 67.9°are identified to be(100),(002),(101),(102),(110),(103),and(112) lattice plane of Wurtzite ZnO,which indicates that the prepared ZnO possess a hexagonal crystal structure (PDF No.36-1451).The sharp and intense peaks show that the synthesized ZnO has good crystallinity. The relatively high intensity of the(101)peak suggeststheanisotropicgrowthandapreferred orientation of the crystallites[25].No impurity peaks are detected in the obtained-sample.
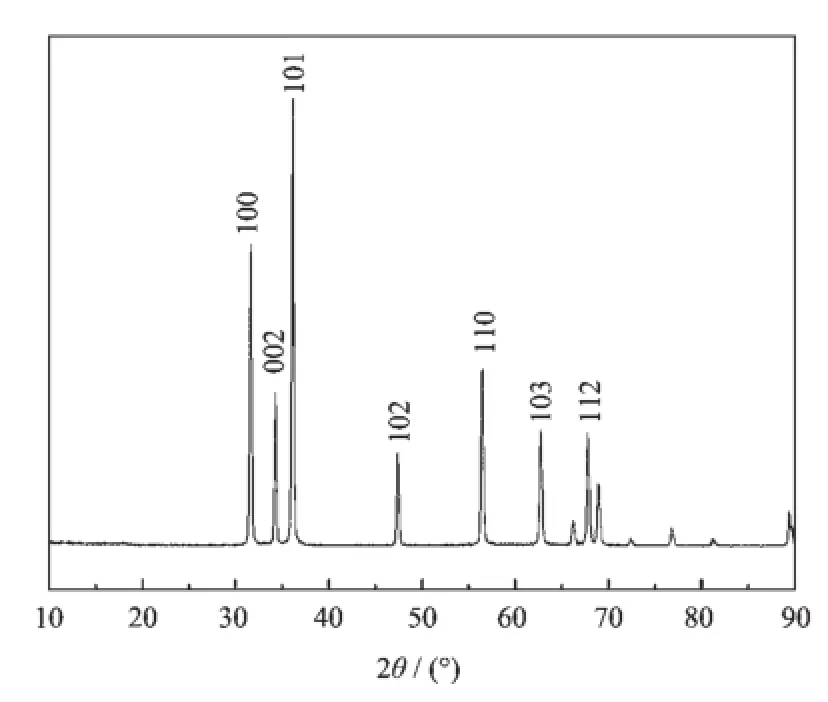
Fig.1 XRD pattern of the prepared ZnO
The SEM images of the prepared samples are shown in Fig.2 .It is clearly shown in Fig.2 A and Fig. 2B that the obtained ZnO is in micro-region with spherical morphologies.ZnO micro-spheres are almost in uniform size about 2 μm.According to previous reports[24,26-27],sphere particles with low aggregation make the greatest contribution to improving foam stability.In order to investigate the effect of ammonia and NaOH on the morphologies during the formation process of ZnO samples,two contrast experiments withoutammoniaorNaOHwereintroducedto compare with LTCP method mentioned in section 1.2. ZnO appears with different morphologies for the samples without addition of NaOH or ammonia as shown in Fig.2 C and Fig.2 D.When NaOH is absent from the process of reaction,ZnO micro-rods are observed(Fig.2 C).While,aggregated and nonuniform ZnO polyhedrons are obtained when there is no ammonia during the formation process of ZnO samples (Fig.2 D).

Fig.2 SEM images of the prepared ZnO samples
Zinc oxide particle size and morphology are directly influenced by the size of precursor Zn(OH)2. In alkaline aqueous solution,Zn(OH)2nucleus,produced by hydrolysis of Zn2+,would aggregate intensely into irregularmicro-polyhedrons(Fig.2 D).Inorderto control the morphology of ZnO,the intense aggregation of the precipitation must be prevented.Accordingly, ammonia is introduced.NH3molecules can coordinate with Zn2+to obtain steady Zn(NH3)x2+complexes.When OH-is gradually added into the system,the competition reaction between producing Zn(NH3)x2+and Zn(OH)2could effectively control the release rate of Zn2+ion from complexes,avoiding from aggregation of precipitation.Therefore,the presence of NH3molecules is helpful to control the morphology of ZnO samples.Fig. 3 and Fig.4 are species distribution in Zn2+-NH3-H2O system in our experimental conditions.Table 1 lists the corresponding equilibrium constants from MEDUSA database[28-29].
As shown in Fig.3 ,with addition of ammonia into Zn2+-NH3-H2O system,Zn2+is decreased and Zn(OH)2will be the dominant component at pH value higher than 6.5 and lower than 8.5.At this stage,white flocculent sediment is observed.The morphology of as-obtained ZnO samples in this stage is shown in Fig.2 C.The growth rate of ZnO nuclei along the caxis is higher than the other axes,resulting in the formation of ZnO rod[30].When the concentration of ammonia is 4 times higher than that of Zn2+insolution,the white flocculentsedimentisslowly disappeared because of the following reaction:
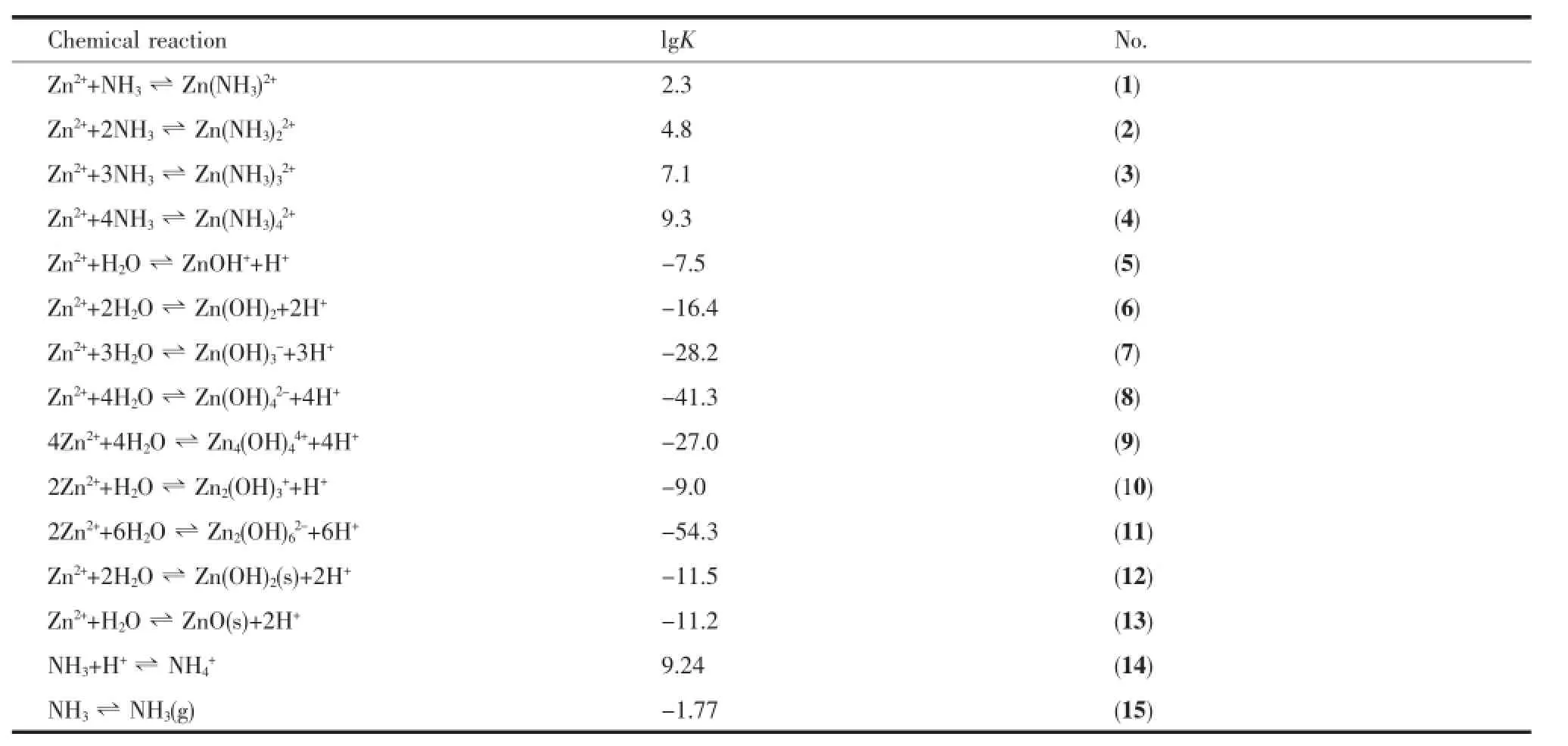
Table1 Solution chemical reactions in Zn2+-NH3-H2O system and corresponding equilibrium constant

Fig.3 Species distribution in Zn2+-NH3-H2O system as a function of pH value
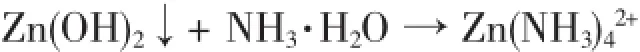
The system is transparent again and Zn(NH3)42+is the dominant component when pH value is between 8.5~10.5(Fig.3 ).Zn(NH3)42+could transfer to Zn(OH)2by introducing NaOH into the system(Fig.4 ).With the increase in OH-concentration,Zn2+is released from Zn(NH3)42+gradually and is combined with the OH-. Zn(OH)2becomes the dominant component in the system when the OH-concentration is about 25 mmol·L-1.All of the Zn2+are ransferred to Zn(OH)2when the OH-concentration reaches 118 mmol·L-1as shown in Fig.4 .
The Zn(NH3)42+ions play an important role for restraining the growth of ZnO nuclei along the c-axis. Asthecompetitionreactionbetweenproducing Zn(NH3)x2+and Zn(OH)2proceeds,Zn(NH3)42+ions gradually transfer to Zn(OH)2nucleus and finally dissociate to form ZnO.Initially the formed ZnO nuclei act as constituent units for the growth of final structure.According to the species distribution and experimental results,the above mentioned competition reaction drives the Zn2+to form uniform ZnO microspheres.At the same time,varying the components in Zn2+-NH3-H2O system could also control the morphology of ZnO samples.

Fig.4 Species distribution in Zn2+-NH3-H2O system as a function of COH-(T)
2.2 Surface modified of ZnO
2.2.1 Adsorption of CTAB on the surface of ZnO
The hydrolysis reaction of Zn2+on the surface of ZnO samples produces surface hydroxyl groups,which makesZnOmicro-spherescharged.Thecharge quantity of ZnO particles is described by ζ potential, which is varied with pH value as shown in Fig.5 .
When the pH value is increased from 6.0 to 9.0), ζ potential of ZnO particles decreases and changes to a negative value around pH value of 8.5.This is because that surface hydroxyl groups are presented as -O-and-OH2+in water solution with different pH values.When the pH value is lower than the value of isoelectric point,adsorption ofH+makessurface hydroxyl transfer to-OH2+and ζ potential of ZnO is positive.On the contrary,when the pH value of solution ishigherthanthe value of isoelectric point,OH-combines with H+from surface hydroxyl to generate H2O making surface hydroxyl represent as-O-with a negative value.In order to hydrophobically modify ZnO particles surface through electrostatic adsorption using positive-charged CTA+,pH value should be adjusted to 8.5~9.0 according to Fig.5 .

Fig.5 ζ potential of ZnO particles as a function of pH value
Since the ζ potential of ZnO particles directly reflects the adsorption of surfactant CTA+in Stern Plane,electrophoretic measurements are performed. Fig.6 represents the zeta potential of ZnO particles as a function of CTAB concentration.In the absence of surfactant,the pH value of the dispersion is adjusted to 9 and ζ potential of ZnO particles is about-6.7 mV.At the first stage of the curve,the ζ potential changes dramatically because the positive charged group CTA+adsorbs on the negative charged ZnO surfaceundertheattractiveinfluenceofstatic electricity.With the concentration of CTA+increasing, themagnitudeofζpotentialdecreasestothe isoelectric point and then increases rapidly.At the second stage,the ζ potential increases gradually and almost reaches the max value when the concentration of CTA+is 3.5~4.0 g·L-1.When the charge of ZnO surface is the same as CTA+,the surfactant molecules would condense onto the ZnO surface under the influence of hydrophobic interactions whose adsorption rate is smaller than that of static electricity attraction. The max value of ζ potential appears when the adsorption-desorption of CTA+on the surface of ZnO reaches equilibrium.
2.2.2 Hydrophobicity of modified ZnO particles
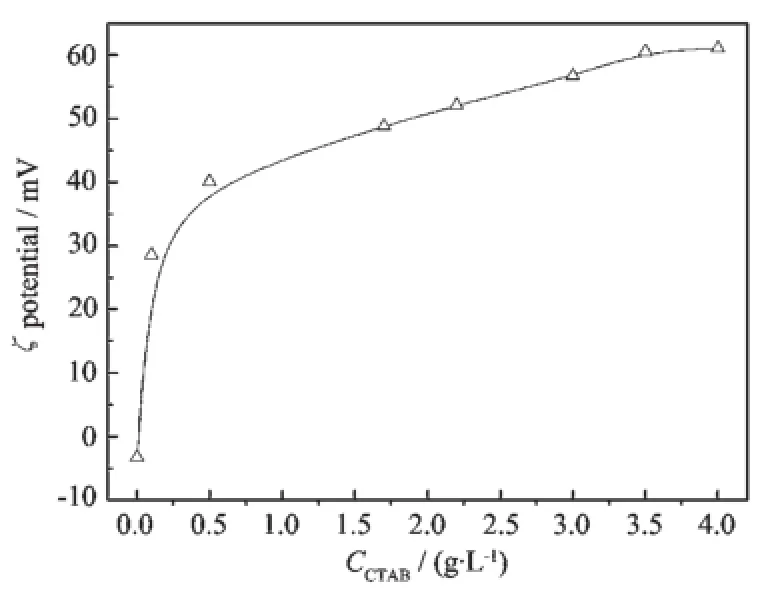
Fig.6 ζ potential of ZnO particles as a function of CTAB concentration
Aqueous foams could not be stabilized by entirely hydrophobic or entirely hydrophilic particles[31-32]. Generally,the hydrophobicity of particle surface is described by contact angle which is an important parameter in determining whether the particles could absorb on the air-water interface[7].The contact angles of surface modified ZnO particles are listed in table 2. The particles are almost hydrophilic in the absence of CTAB because the contact angle of ZnO surface is very small(19.6°).With the concentration of CTAB increasing,the surface contact angle first increases to a maximum(52.9°)and then decreases with further increaseinCTABconcentration.Whenthe concentration of CTAB is over 3.0 g·L-1,the CTAB-modified ZnO particles become hydrophilic again.The images of modified ZnO surface contact angle are shown in Fig.7 .
The adsorption feature of CTAB on ZnO particles may be further appreciated in virtue of the contact angles.When the concentration of CTAB is low,the adsorption of CTAB on the surface of ZnO results from the electrostatic interaction between CTA+and surface hydroxyl groups-O-.The hydrophobic tails towards to the solution and the contact angle of particles begins to increase(Fig.7 ).With the concentration of CTAB further increasing,the adsorption process is no longer driven by electrostatic interaction. The influence of hydrophobic interaction between hydrophobic tails is in effect when there is a reversal of ζ potential.When the concentration of CTAB reaches about 3.0 g·L-1,the hydrophobicity of particles attains the maximum value.After that,a secondsurfactant molecules layer forms with tails connected and head groups oriented towards to the solution.The contact angle decreases and the surface of ZnO particles becomes hydrophilic again.The possible process of surfactant absorbed on ZnO surface is shown in Fig.8 .

Table2 Contact angle of ZnO surface modified by CTAB in different concentrations

Fig.7 Images of different contact angles for ZnO particles

Fig.8 Possible process for surfactant absorption on ZnO surface
2.3 Properties of foam stabilized by CTAB-modified ZnO particles
2.3.1 Foam stability
TostudytheeffectofCTAB-modifiedZnO particles on the foam stability,liquid drainage half-life of SDS foams is investigated.
As foams generates,the liquid begins to drain out through the lamella layer under the action of gravity and the capillary forces followed by bubble coalescence[26].Therefore,liquid drainage half-life of foams is an important parameter in describing the foamstability.Drainagehalf-lifeofSDSfoams stabilized with CTAB-modified ZnO micro-spheres at differentparticle concentrations(wt%)and surface contact angles are shown in Fig.9 .The concentrations of CTAB-modified ZnO particles are 0.01,0.03,0.05 and 0.07wt%and the control experiment is performed with the only addition of corresponding amount of CTAB.The drainage half-life of foams in control experiment is almost not improved because CTAB holds opposite charge with SDS in aqueous solution and even reduce the foam stability.As shown in Fig.9 , the foam stability first increases with increasing of particle concentration until the particle concentration reaches0.03wt%,andthenthefoamstability decreasesasparticleconcentrationincreasesto 0.05wt%and 0.07wt%.Excessive concentration of particles could produce aggregates between bubbles, which is one of the adverse factors to form stable bubbles[5].At each particle concentration,the foam stability increases with increasing of ZnO surface contact angle and obtains the maximum value at the same contact angle(52.9°)according to Fig.9 .The particles are not able to adsorb on the air-water interface when the contact angle turns below the maximum value,as a result of the decease in foam stability(Fig.8 ,Fig.9 ).
2.3.2 Morphology of bubbles stabilized by CTAB modified-ZnO
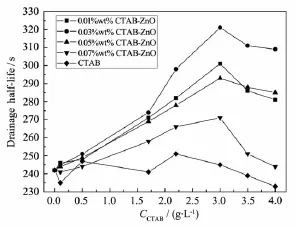
Fig.9 Drainage half-life of SDS foams stabilized with CTAB and CTAB-modified ZnO at different particle concentrations(wt%)
Optical microscope images of the SDS foams stabilized by CTAB modified-ZnO particles are shown in Fig.1 0.
SDS bubbles are in different size when CTAB modified-ZnO particles are absent from foam system shown in Fig.1 0A and B.After addition of 0.01wt% and 0.03wt%particles,the bubbles become uniform with better dispersity as shown in Fig.1 0C and D.The CTAB modified-ZnO particles are able to attach onto air-water interface of bubbles,as a result the film of bubbles obviously thickens.Adsorption of surface modified ZnO particles produces a shell on the bubble surface,which armors bubbles and enhances the stabilityoffoams.Fromtheanalysisabove, incompletely hydrophobic ZnO modified by CTAB can attach to air-water interface and achieve stable foams.

Fig.1 0Optical microscope images of the SDS foams stabilized by CTAB modified-ZnO particles
3 Conclusions
Zinc oxide micro-spheres with better dispersity and about 2 μm were synthesized by LTCP method. The effect of NH3and OH-in Zn2+-NH3-H2O system on the morphology of ZnO samples was discussed.The ZnOsamplesareinmicro-rodsandirregular polyhedrons with no addition of NaOH or ammonia. ZnOparticleswerehydrophobicalymodifiedby CTAB.The hydrophobicity of ZnO particles first increases with increasing of CTAB concentration,and thendecreaseswithfurtherincreaseinCTAB concentration.TheincompletelyhydrophobicZnO particles can attach onto the air-water interface to enhance the foam stability.The most stable foams are formed at particle concentration of 0.03wt%with the particle contact angle of 52.9°.
Acknowledgments:This work was financially supported by the National Natural Science Foundation of China(No. 21276022)and Petro China Company Limited Project(2011B-1303)and CNPC Innovation Foundation(2012D-5006-0208)
[1]Liu Q,Zhang S Y,Sun D J,et al.Colloids Surf.A,2010, 355(1/2/3):151-157
[2]Ata S,Yates P D.Colloids Surf.A,2006,277(1/2/3):1-7
[3]Dong X Q,Xu J,Cao C B,et al.Colloids Surf.A,2010,353 (2/3):181-188
[4]Gonzenbach U T,Studart A R,Tervoort E,et al.Langmuir, 2006,22(26):10983-10988
[5]RU Mian(茹冕),CHANG Zhi-Dong(常志东),GU Shao-Nan (顾少楠),et al.Chin.J.Chem.Eng.(中国化学工程学报), 2012,63(6):1943-1950
[6]Liu Q,Zhang S Y,Sun D J,et al.Colloids Surf.A,2009, 388(1/2/3):40-46
[7]Binks B P.Curr.Opin.Colloid Interf.Sci.,2002,7(1/2):21-41
[8]Binks B P,Kirkland M,Rodrigues J A.Soft Matter,2008,4: 2373-2382
[9]Gonzenbach U T,Studart A R,Gauckler L J,et al.Angew. Chem.Int.Ed.,2006,45(21):3526-3530
[10]Talam S,Srivinasa R K,Nagarjuna G.IRSN Nanotechnol., 2012,2012:1-6
[11]Rajendran R,Balakumar C,Hasabo A M A,et al.Int.J. Eng.Sci.Technol.,2010,2(1):202-208
[12]Rai P,Yeon T Y.Sens.Actuators B:Chem.,2012,173:58 -65
[13]Shinde V V,Dalavi D S,Mali S S,et al.Appl.Surf.Sci., 2014,307:495-502
[14]Meng X M,Liu J,Jiang Y,et al.Chem.Phys.Lett.,2003, 382(3/4):434-438
[15]Pillai S C,Kelly J M,McCormack D E,et al.Mater.Sci. Technol.,2004,20(8):964-968
[16]Tang E J,Cheng G X,Ma X L,et al.Appl.Surf.Sci.,2006, 252(14):5227-5232
[17]Pan J H,Dou H,Xiong Z,et al.J.Mater.Chem.,2010,20 (22):4512-4528
[18]Moafi H F,Shojaie A F,Zanjanchi M A.Thin Solid Films, 2011,519(11):3641-3646
[19]Hu X H,Gong J M,Zhang L Z,et al.Adv.Mater.,2008,20: 4845-4850
[20]Tian Y,Lu H B,Wu Y,et al.Mater.Sci.Technol.,2010,5 (3):1248-1252
[21]Wang Y X,Sun J,Fan X Y,et al.Ceram.Int.,2011,37(8): 3431-3436
[22]Kiomarsipour N,Razavi R S.Superlattices Microstruct., 2012,52(4):704-710
[23]Yu J G,Yu X X.Environ.Sci.Technol.,2008,42(13):4902 -4907
[24]Kostakis T,Ettelaie R,Murray B S.Langmuir,2006,22(3): 1273-1280
[25]Muruganandham M,Chen I S,Wu J J.J.Hazard.Mater., 2009,172(2/3):700-706
[26]Binks B P,Horozov T S.Angew.Chem.Int.Ed.,2005,117 (44):2-5
[27]Zhang S Y,Sun D J,Dong X Q.Colloids Surf.A,2008,324 (1/2/3):1-8
[28]GU Shao-Nan(顾少楠),SUN He-Yun(孙和云),FAN Ying-Ju (范迎菊),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29(6):1185-1191
[29]Puigdomenech I.MEDUSA.Sweden:Royal Institute of Technology,1999,Stockholm
[30]Liang S,Zhu L F,Gai G S,et al.Ultrason.Sonochem., 2014,21(4):1335-1342
[31]Dickinson E,Ettelaie R,Kostakis T,et al.Langmuir,2004, 20(20):8517-8525
[32]Zhang S Y,Lan Q,Liu Q.Colloids Surf.A,2008,317(1/2/3): 406-413
Hydrophobic ZnO:Low Temperature Complex-Precipitate Synthesis and Application in Improving Foam Stability
GU Shao-Nan1LI Wen-Jun*,1LUO Wen-Li2CHANG Zhi-Dong1ZHOU Hua-Lei1
(1Beijing Key Laboratory for Science and Application of Functional Molecular and Crystalline Materials,University of Science and Technology Beijing,Beijing 100083,China)
(2Research Institute of Petroleum Exploration and Development,Petro China,Beijing 100083,China)
Using Zn(NO3)2·6H2O as raw material,25%ammonia as complexing agent and NaOH as precipitant, ZnO micro-spheres were synthesized by low temperature complex-precipitate(LTCP)method.The species distribution in the system was also analyzed.XRD and SEM were employed to characterize ZnO samples.The SEM images demonstrate that the uniform micro-spheres are about 2 μm.The morphology of the samples appears as micro-rods and as irregular polyhedrons without addition of NaOH or ammonia.ZnO micro-spheres were hydrophobically modified by varies amounts of cetyltrimethylammonium bromide(CTAB)in order to obtain different contact angles.Meanwhile,the process for surfactant molecules absorption on ZnO micro-spheres surface was discussed.The results of foam stability reveal that 0.03wt%ZnO micro-sphere with surface contact angle of 52.9°makes the greatest contribution to improving the stability of foams.
zinc oxide;complex-precipitate;morphology tunable;hydrophobic effect;foam stability
O614.24+1
A
1001-4861(2015)03-0594-09
10.11862/CJIC.2015.069
2014-10-17。收修改稿日期:2014-12-16。
国家自然科学基金(No.21276022)资助项目。
*通讯联系人。E-mail:wjli@sas.ustb.edu.cn
