一个基于苯并噻唑的铜离子荧光探针的合成、晶体结构和光谱性质
2015-06-01范方禄靖金球陈雪梅
范方禄 靖金球 陈雪梅
(1湖北理工学院材料与冶金学院,黄石435003)
(2湖北理工学院化学与化工学院,黄石435003)
一个基于苯并噻唑的铜离子荧光探针的合成、晶体结构和光谱性质
范方禄*,1靖金球1陈雪梅2
(1湖北理工学院材料与冶金学院,黄石435003)
(2湖北理工学院化学与化工学院,黄石435003)
合成和表征了一个苯并噻唑类的荧光探针N-(4-(苯并噻唑-2-基)苯基)-2-((2-羟乙基)(吡啶-2-甲基)氨基)乙酰胺(FL),用光谱法研究了它与各种金属离子的识别特性。结果表明:FL对Cu2+具有较高的选择性和灵敏度,并且对Cu2+的识别不受其它金属离子的干扰。FL与Cu2+形成配合物的结合比为1∶1,其荧光强度与Cu2+浓度(3.8~9.6 μmol·L-1)呈现较好的线性关系,而且它还可应用于自来水和湖水等水体样品中Cu2+的检测。
苯并噻唑;晶体结构;铜离子;荧光探针
Copper is an essential trace element at lower concentrationsinalllivingsystems.Despiteits important roles in organisms,the accumulation of excess amounts of copper ions or their misregulation can cause many severe diseases including Alzheimers and Wilsons diseases[1-4].Therefore,it is necessary to develop accurate and rapid detection methods to detect and quantify Cu2+in environmental and biological samples.Fluorescence detection is the most common method in the field of sensing technology because of its high sensitivity,selectivity and simplicity[5-6].Up to now, quite a few practical fluorescent probes for Cu2+havebeen achieved.The majority of them were based on fluorophoressuchasrhodamines,coumarins, benzoxadiazole,BODIPY,naphthalimides,cyanines, quinolines,fluoresceins,indoles,benzimidazoles, squaraines,anthracene[7-18].The acceptor of these probes included DPA(di-2-picolylamine),pyclen(3,6,9,15-tetraazabicyclo[9.3.1]pentadeca-1(15),11,13-triene)[12-13]. However,the practical applications of fluorescent probes for Cu2+with selectivity and sensitivity are still a challenge.Benzothiazole is often taken as a fluorophore for designing fluorescence probes to detect metal ions due to its good photophysic properties[19-20].2-(Pyridin-2-ylmethylamino)ethanol is a tridentate ligand,which can provide one O atom and two N atoms to coordinate with metal ions[21].In this paper,by using benzothiazole as the fluorophore and 2-(pyridin-2-ylmethylamino) ethanol as the acceptor,we designed and synthesized a fluorescent probe N-(4-(benzo[d]thiazol-2-yl)phenyl)-2-((2-hydroxyethyl)(pyridin-2-ylmethyl)amino)acetamide (FL)for detecting Cu2+.The crystal structureand fluorescent properties of FL were reported,and the practical application of FL for detecting Cu2+in different water samples was also investigated.
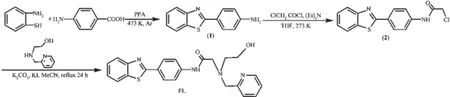
Scheme 1Synthesis of the probe FL
1 Experimental
Unless otherwisenoted,allreagentswere purchased from commercial companies and directly used without further purification.The melting point was determined with an XT4A micromelting point apparatus and was uncorrected.The IR spectrum were measured on a Perkin-Elmer Spectrum BX FT-IR instrument in tablets with potassium bromide.The1H NMR spectrum were recorded on a Mercury Plus-400 spectrometer in CDCl3.Electrospray ionization mass spectra(ESI-MS) were acquired on an Applied Biosystems API 2000 LC/ MS/MS system.Elemental analyses were carried out on a Perkin-Elmer 2400 instrument.UV-Vis spectra were recordedonaAnalytikjenaSpecord210 spectrophotometer.Fluorescencespectrawere performed on a FluoroMax-P spectrofluorimeter.
1.1 Synthesis of N-(4-(benzo[d]thiazol-2-yl)phenyl)-2-((2-hydroxyethyl)(pyridin-2-ylmethyl)amino) acetamide(FL)
The intermediate 2 was prepared according to reported procedures,which was further treated as follows to afford probe FL(Scheme 1)[22].2-[(Pyridin-2-ylmethyl)amino]ethanol(0.14 g,1 mmol)and the intermediate 2(0.31 g,1 mmol)were dissolved in CH3CN(100 mL),then K2CO3(0.14 g,1 mmol)and a catalytic amount of KI(0.02 g)were added to the resultant solution.The mixture was refluxed for 24 h until complete disappearance of the intermediate 2 (monitored by TLC).After the resulting solution was cooled to room temperature,the solvent was evaporated under vacuum,and the residue was purified via column chromatography(silica,Vpetroleumether:VAcOEt=5:1)to give a yellow solid.Yield:82%.m.p.129~131℃.IR(KBr): 3 350,3 075,2 935,1 645,1 560,1 545,1 460,1 342, 1 284,1 052 cm-1.1H NMR(400 MHz,CDCl3,):δ 2.56 (t,2H,J=7.2 Hz),3.48~3.74(m,5H),4.05(s,2H), 7.29~7.56(m,5H),7.78~8.06(m,6H),8.27(d,1H,J= 7.6 Hz),8.52(d,1H,J=6.8 Hz).ESI-MS:m/z 418.2 (M+).Anal.Calcd.for C23H22N4O2S(%):C,66.01;H, 5.30;N,13.39;Found:C,66.32;H,5.08;N,13.24.
1.2 X-ray crystallography
YellowcrystalsoftheprobeFLhaving approximate dimensions of 0.30 mm×0.20 mm×0.20 mm was mounted on a glass fibre in a random orientation at 298(2)K.The determination of unit cell and thedata collection were performed with Mo Kα radiation (λ=0.071 073 nm)on a Bruker Smart APEX-CCD diffactometer with a φ-ω scan mode.A total of 13 004 reflections were collected in the range of 0.99°<θ<26.00°at room temperature.The structures were solved bydirectmethodsandsemi-empiricalabsorption corrections were applied.The non-hydrogen atoms were refined anisotropically and the hydrogen atoms were determined by theoretical calculation.The final cycle of full matrix least-squares refinement was based on 4020 independent reflections[I>2σ(I)].All calculations were carriedoutusingSHELXS-97andSHELXL-97 programs[23-24].Crystallographic data of the probe FL are listed in Table 1.Selected bond lengths and bond angles are listed in Table 2,and hydrogen bond lengths and bond angles are given in Table 3.
CCDC:960513.
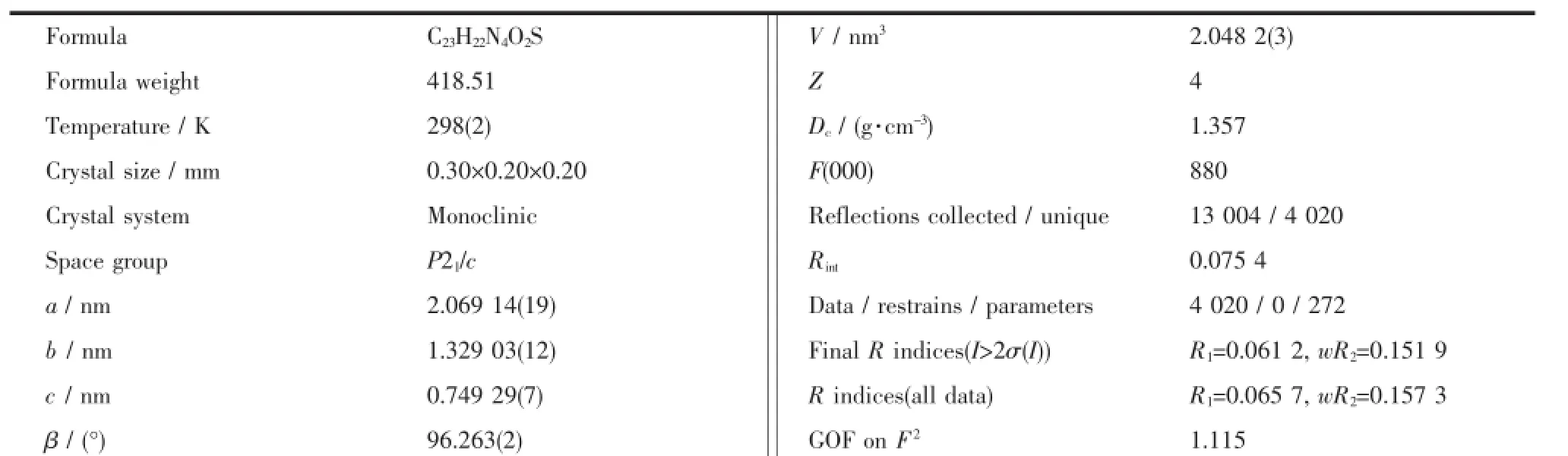
Table1 Crystal data and structure refinements of the probe FL
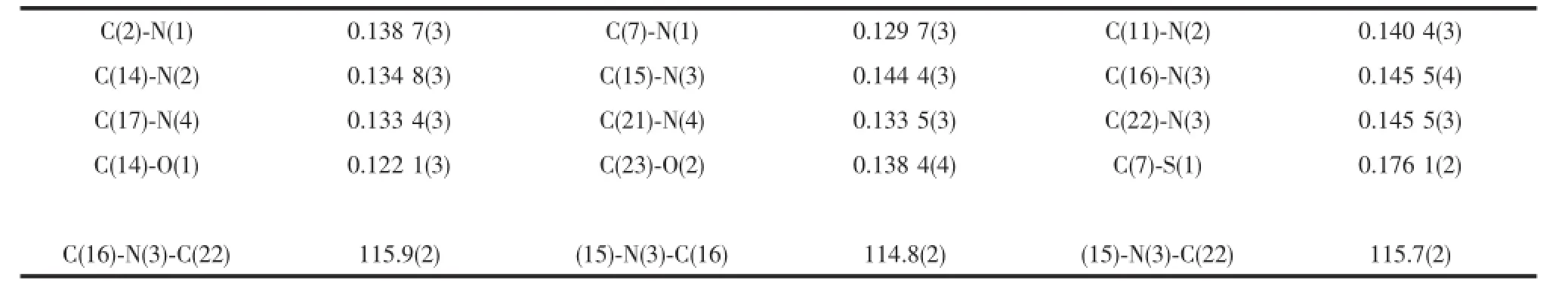
Table2 Selected bond lengths(nm)and bond angles(°)of the probe FL

Table3 Hydrogen bond lengths(nm)and bond angles(°)of the probe FL
1.3 The detection of Cu2+in water samples
The recovery study of the detection of Cu2+in various water samples was carried on according to the literature methods[25-26].Three selected water samples in the experiment were tap water,lake water and mineral water,and they were obtained from our laboratory tap water supply,Cihu Lake in Huangshi city(Hubei province,China)and Wahaha mineral water(Hangzhou WahahaGroup,China),respectively.Nofurther purification was adopted before the water samples were applied.Various water samples were used instead of the distilled water in the fluorescence measurements.
2 Results and discussion
2.1 Crystal structure of the probe FL
Yellow crystals of the probe FL were obtained by slow evaporation of a dichloromethane solution at room temperature.The structure of FL was characterized by single crystal X-ray diffraction,as shown in Fig.1 .The benzothiazole ring system and adjacent benzene ring constitute the fluorescent moiety of the probe FL,they are nearly coplanar,and the dihedral angle between them is 10.25(2)°,while that between the pyridine ring and each of them are 44.00°and 41.72°,respectively. The C-N bond distances range from 0.129 7(3)to 0.145 5(4)nm,and the C-N(benzothiazolyl)bonds are shorter than the C-N(amino)bonds(Table 2).The bond length of C(11)-N(2)is longer than that of C(14)-N(2), due to the fact that C(14)is sp2hybridized,whereas C(11)is part of the aromatic ring.The C(23)-O(2)bond length(0.138 4(4)nm)is also found to be longer than the C(14)-O(1)bond length(0.122 1(3)nm)for the similar reason.The angle of C(16)-N(3)-C(22)is 115.9(2)°,which leads to V-shape of the chelating moiety.As shown in Table 3 and Fig.2 ,the molecules are stablized by N-H…N and O-H…O hydrogen bonds,leading to the formation of a three dimension network.
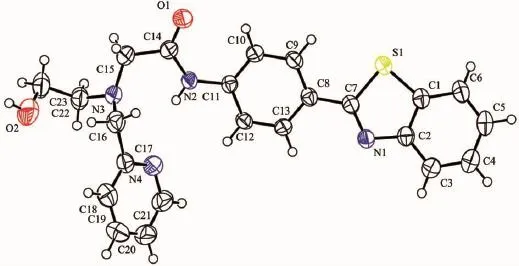
Fig.1 ORTEP drawing of the probe FL with displacement ellipsoids shown at the 30%probability level
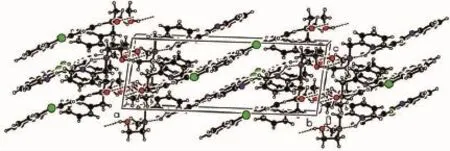
Fig.2 Packing of the probe FL in unit cell
2.2 Response of FL to Cu2+
The maximum absorption wavelength of FL was 320 nm,and its maximum excitation(λex)and emission (λem)wavelengths were 325 and 380 nm,respectively. The interactions of FL(10 μmol·L-1)toward 1 equivalent of various metal ions(K+,Ca2+,Na+,Mg2+, Mn2+,Fe2+,Fe3+,Co2+,Ni2+,Cu2+,Zn2+,Ag+,Pb2+,Cd2+, Hg2+)was studied in EtOH/Tris-HCl buffer(1∶1,V/V, pH=7.4).As shown in Fig.3 ,Cu2+significantly quenched the fluorescence of FL,and Co2+responded with weak decrease in the fluorescent intensity,while other metal ions showed nearly negligible effect on the fluorescence behavior of FL.The result indicated that FL displayed a good selectivity to Cu2+.Competition experiments for Cu2+(10 μmol·L-1)mixed with other metal ions at a 100-fold excess concentration were also performed(Fig. 4).Its found that the Cu-induced fluorescence response of FL(10 μmol·L-1)was nearly unperturbed in thepresence of other background metal ions,indicating that FL could have potential practical applicability in Cu2+detection.The UV spectroscopy showed that the absorption of FL revealed that a strong absorption band at 320 nm,and the maximum absorption wavelength of Cu-complex was almost unchanged upon addition of the same amount of Cu2+,but its absorption intensity decreased(Fig.5 ).The fluorescence spectrum of FL was invariable in a wide pH range of 2~12.Upon addition of Cu2+,the stable fluorescent intensity of FL was weakened with increasing pH from 2 to 8,So FL could be suitable for Cu2+detection in pH variations close to physiological conditions(Fig.6 ).
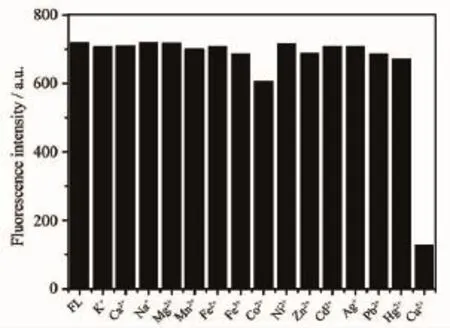
Fig.3 Effect of metal ions(10 μmol·L-1)on the fluorescent property of probe FL(10 μmol· L-1)in EtOH/Tris-HCl buffer(1∶1,V/V,pH= 7.4)with an excitation at 325 nm

Fig.4 Effect of Cu2+on FL(10 μmol·L-1)in the presence of different metal ions(1 mmol·L-1)
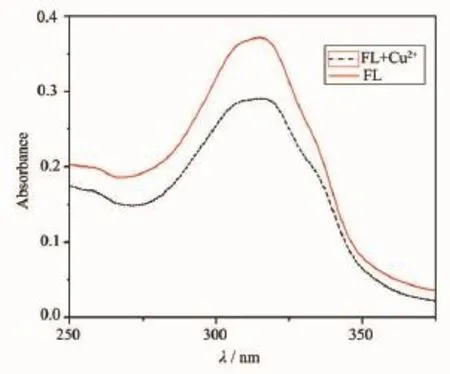
Fig.5 Change in UV spectra of FL upon interaction with Cu2+

Fig.6 Fluorescence intensity of FL(10 μmol·L-1)and FL+Cu2+over a pH range from 2 to 12 at room temperature
To investigate the sensitivity of FL toward Cu2+,the fluorescence titration experiments were carried out at room temperature.Fig.7 illustrated the changes of fluorescence spectra of FL upon the addition of increasing amount of Cu2+.In the absence of Cu2+,the maximum fluorescence intensity was observed,and the fluorescence intensity decreased slowly as the Cu2+concentration was increased.With 2 equivalent of Cu2+, about 80%quenching of initial fluorescence of FL was observed and the fluorescence intensity was at a minimum,at this moment the fluorescence color of FL changed from bright blue to pale blue under the irradiation at 365 nm(Inset of Fig.7 ).It was found that a linear regression curve(linear correlation coefficient R2=0.989 9)of the Cu2+-FL complex fitted the relationship between the fluorescence of FL and the concentration of Cu2+(3.8~9.6 μmol·L-1)(Fig.8 ).The detection limit of FL to Cu2+was determined to be 1.9 μmol·L-1according to the calculation method reported in the literature[27].Moreover,the binding stoichiometry between FL and Cu2+was examined by the Jobs plot experiment,In Fig.9 ,the fluorescence intensity at 380nm was plotted as a function of the mole fraction of Cu2+,and the total molar concentration of FL and Cu2+is 10 μmol·L-1.A maximum point at a mole fraction of 0.5 was observed when the change of fluorescence emission approached a maximum.This clearly demonstrated that FL formed a 1:1 complex with Cu2+.On the basis of a 1: 1 stoichiometry,the association constant of FL for Cu2+was determined to be 1.02×105L·mol-1according to the Benesi-Hildebrand equation[28].

Fig.7 Changes on the fluorescence spectra of FL (10 μmol·L-1)in the presence of increasing Cu2+concentrations(0~20 μmol·L-1)with an excitation at 325 nm
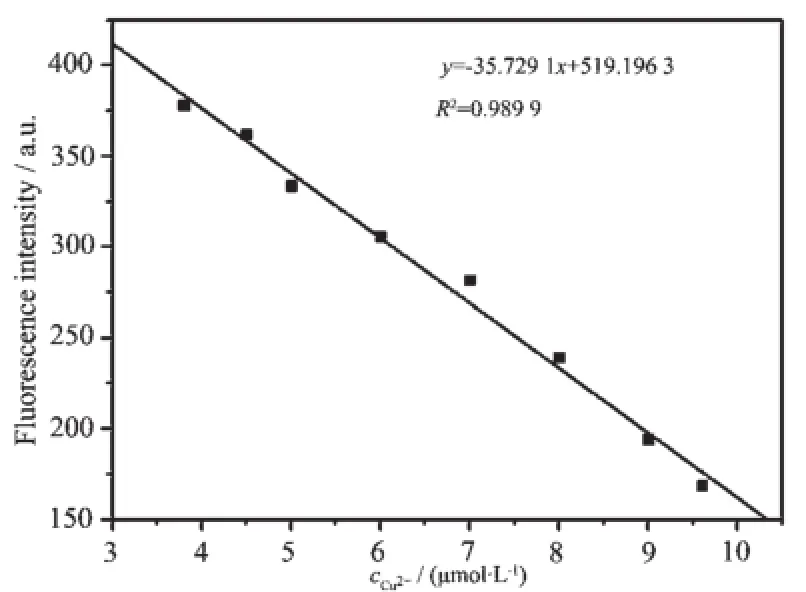
Fig.8 Fluorescence intensity of FL as a function of concentration of Cu2+(3.8~9.6 μmol·L-1)
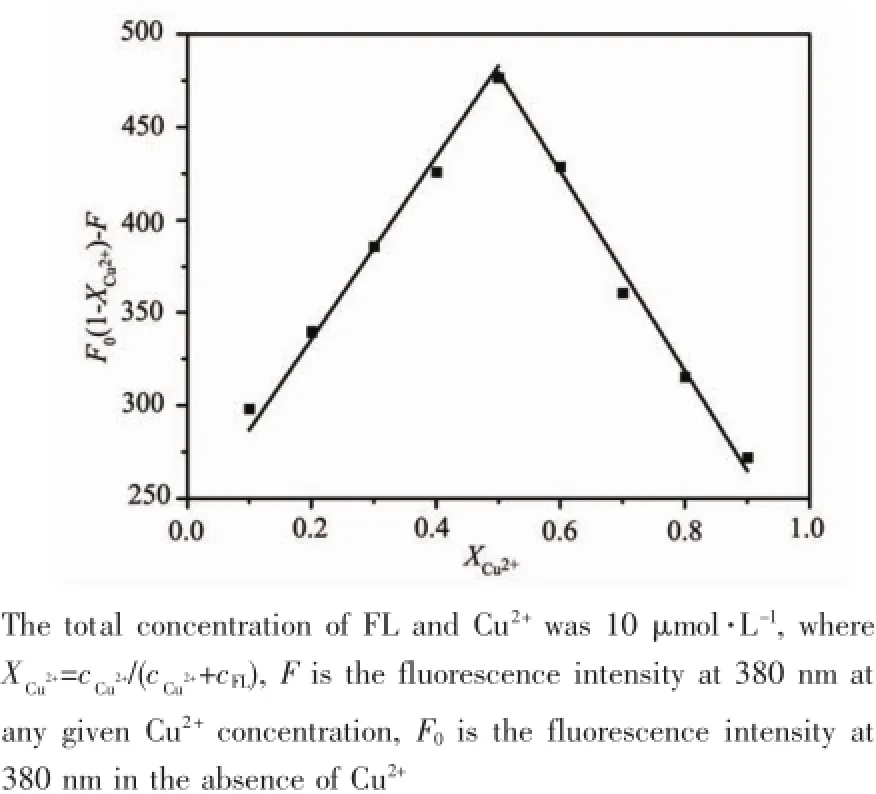
Fig.9 Jobs plot of interaction between FL and Cu2+
Based on above experiments and some reports,we propose a possible binding model of FL with Cu2+,as shown in Scheme 2[21,29].In the chelating moiety of FL, two nitrogen atoms,one hydroxyl oxygen atom and one oxygen atom of amide might bind with Cu2+.The capture of Cu2+caused the electron or energy transfer between the chelating unit and the benzothiazole fluorophore, thus resulting in the fluorescence quenching of the benzothiazolefluorophore.Inaddition,the paramagnetic effect from spin-orbit coupling of Cu2+could also induce fluorescence quenching[30].
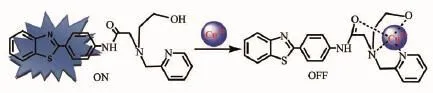
Scheme 2Proposed binding model of FL with Cu2+
2.3 Applications in real samples
The practical applications of FL were evaluated by determining the Cu2+contents in tap water,lake water and mineral water samples.All the samples without or with the addition of Cu2+at different concentration levels of 0~9.00 μmol·L-1were analyzed by FL for Cu2+(Table 4).The observed results showed that the recovery studies of Cu2+based on FL were in good agreement with RSD values ranging from 1.81% to4.29%.Thehigh-quality recovery experiments indicated the possible feasibility of FL for the detection of Cu2+from real water samples without any interference from other environmentally relevant competitive metal ions.
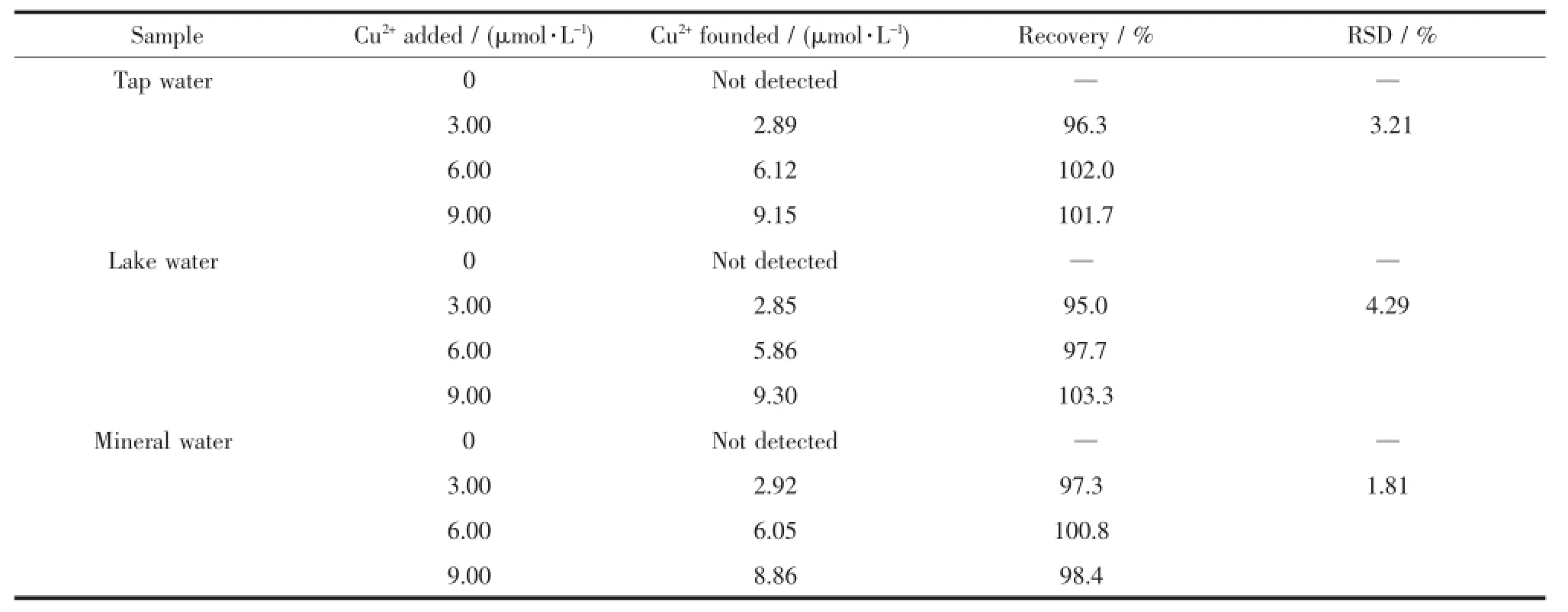
Table4 Recovery study of the detection of Cu2+in various water samples
3 Conclusions
In conclusion,we prepared a benzothiazole-based fluorescent probe FL for Cu2+,which was characterized by single crystal X-ray diffraction.The fluorescent study was showed that probe FL was sensitive and selective to Cu2+and formed a 1∶1 complex with Cu2+. Moreover,FL was successfully applied to determine Cu2+content in real water samples.
[1]Que E L,Domaille D W,Chang C J.Chem.Rev.,2008,108: 1517-1549
[2]Millhauser G L.Acc.Chem.Res.,2004,37:79-85
[3]Gaggelli E,Kozlowski H,Valensin G.Chem.Rev.,2006,106: 1995-2044
[4]Camakaris J,Voskoboinik I,Mercer J F.Biochem.Biophys. Res.Commun.,1999,261:225-232
[5]Nolan E M,Lippard S J.Acc.Chem.Res.,2009,42:193-203
[6]Bozdemir O A,Guliyev R,Buyukcakir O,et al.J.Am.Chem. Soc.,2010,132:8029-8036
[7]Xu Z,Yoon J,Spring D R.Chem.Commun.,2010,46:2563-2565
[8]You Q H,Lee A W M,Chan W H,et al.Chem.Commun., 2014,50:6207-6210
[9]Zhang L,Sun J,Liu S,et al.Inorg.Chem.Commun.,2013,35: 311-314
[10]Tian M,Hu M,Fan J,et al.Bioorg.Med.Chem.Lett., 2013,23:2916-2919
[11]Wang J,Xie Y,Wang Z,et al.Sens.Actuators B:Chem., 2014,194:149-155
[12]Wang X B,Ma X Y,Yang Z,et al.Chem.Commun.,2013,49: 11263-11265
[13]Wang H H,Xue L,Fang Z J,et al.New J.Chem.,2010,34: 1239-1242
[14]Kou S,Lee H N,Noort D,et al.Angew.Chem.Int.Ed.,2008, 47:872-876
[15]Li P,Duan X,Chen Z,et al.Chem.Commun.,2011,47:7755-7757
[16]Tang L J,Cai M J,Huang Z L,et al.Sens.Actuators B: Chem.,2013,185:188-194
[17]Hewage H S,Anslyn E V.J.Am.Chem.Soc.,2009,131: 13099-13106
[18]Lee Y J,Seo D,Kwon J Y,et al.Tetrahedron,2006,62:12340 -12344
[19]Santra M,Roy B,Ahn K H.Org.Lett.,2011,13:3422-3425
[20]Sun W,Li W H,Li J,et al.Tetrahedron Lett.,2012,53:2332 -2335
[21]Chen J W,Wang X Y,Zhu Y G,et al.Inrog.Chem.,2005,44: 3422-3430
[22]Lin G W,Wang Y,Jin Q M,et al.Inorg.Chim.Acta,2012, 382:35-42
[23]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[24]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[25]Liu Y,Fei Q,Shan H,et al.Analyst,2014,139:1868-1875
[26]Tayade K,Bondhopadhyay B,Basu A,et al.Talanta, 2014,122:16-22
[27]Shortreed M,Kopelman R,Kuhn M,et al.Anal.Chem., 1996,68:1414-1418
[28]Benesi H A,Hildebrand J H.J.Am.Chem.Soc.,1949,71: 2703-2707
[29]LiuYM,FeiQ,ShanHY,etal.Analyst,2014,139:1868-1875
[30]Qu L,Yin C,Huo F,et al.Sens.Actuators B:Chem.,2013, 183:636-640
Synthesis,Crystal Structure and Spectroscopic Property of a Benzothiazole-Based Fluorescent Probe for Cu2+
FAN Fang-Lu*,1JING Jin-Qiu1CHEN Xue-Mei2
(1School of Materials and metallurgy,Hubei Polytechnic University,Huangshi,Hubei 435003,China)
(2College of Chemistry and Chemical Engineering,Hubei Polytechnic University,Huangshi,Hubei 435003,China)
A benzothiazole-based fluorescent probe N-(4-(benzo[d]thiazol-2-yl)phenyl)-2-((2-hydroxyethyl)(pyridin-2-ylmethyl)amino)acetamide(FL)has been synthesized and characterized,and its recognition properties towards various metal ions have been studied by spectrometry.The results showed that FL was highly sensitive and selective to Cu2+,and other metal ions did not interfere with its recognition for Cu2+.The stoichiometry of the complex formation of FL with Cu2+was determined to be 1:1,and the fluorescence intensity of FL varied almost linearly versus the concentration of Cu2+(3.8~9.6 μmol·L-1).FL can also be applied to detect Cu2+in different water samples such as tap and lake water.CCDC:960513.
benzothiazole;crystal structure;Cu2+;fluorescent probe
O614.121
A
1001-4861(2015)03-0548-07
10.11862/CJIC.2015.026
2014-09-19。收修改稿日期:2014-10-21。
国家自然科学基金(No.51173060)资助。
*通讯联系人。E-mail:flfan2014@163.com
