Mechanical Circulatory Support for the Failing Heart: Which Device to Choose
2015-05-22MustafaAhmedMDandReneAlvarezJrMD
Mustafa Ahmed, MD and Rene Alvarez Jr., MD
1Division of Cardiovascular Medicine, University of Florida College of Medicine, Gainesville, FL, USA
2Division of Cardiology, Temple University, Philadelphia, PA, USA
Introduction
The spectrum of acute heart failure syndromes(AHFS) continues to broaden and frequently includes those with cardiogenic shock (CS). Despite advances in pharmacotherapy and revascularization techniques, these syndromes exert a tremendous mortality risk. Traditionally, support has been focused on the use of intra-aortic balloon pump(IABP) insertion along with vasoactive medication to improve hemodynamics in this population in an effort to improve outcomes. More recently, however, a number of percutaneous devices with greater hemodynamic support prof i les have been introduced, providing the clinician with more options to treat those with AHFS complicated by CS. In this new landscape, effectively and eff i ciently selecting the appropriate device for mechanical circulatory support (MCS) can be challenging, requiring the publication of updated consensus statements [1]. In this article, we aim to provide guidance on the appropriate use of these devices and how best to incorporate their implantation into a multi disciplinary care team approach.
Acute Heart Failure Syndromes and Medical Therapy
Historically, patients with AHFS have had a wide ranging risk of mortality, largely based on initial presentation. Data from the Acute Decompensated Heart Failure National Registry (ADHERE) registry show inpatient mortality as high as 21.9% in those who have elevated blood urea nitrogen and creatinine levels in the setting of hypotension [2]. Those who present with an AHFS complicated by frank CS have a dismal prognosis, with more contemporary registry data suggesting that two of every three such patients will fail to survive to discharge [3]. The need for, and the timing of, vasoactive medication administration can further ref i ne one’s risk of inhospital death. Data from ADHERE suggest that early vasoactive medication administration, within 6 h of presentation, reduces the risk of mortality,with the adjusted odds of death increasing by 6.8%with each 6-h incremental delay in treatment [4].Additionally, the type of vasoactive medication required, vasodilators versus inotropic support, has also been demonstrated to predict outcomes in a hospitalized group of patients, with those requiring inotropic support noted to have worse survival [5].The dose and number of inotropic agents required to maintain relative stability also correlates with outcomes, with a mortality rate of 80% in patients requiring three agents at a high dose [6].
Although these data paint a bleak picture for the management of AHFS, they do indicate that earlier recognition of shock syndromes and rapid initiation of therapies, as well as the magnitude of medical intervention, profoundly impact outcomes in this population. Therefore, the clinician is provided with an opportunity to quickly risk-stratify patients and identify those who may benef i t from escalation of care with the addition of MCS and transfer to an advanced heart failure center.
Acute Heart Failure Syndromes and Mechanical Circulatory Support
Although first described nearly 50 years ago [7],the IABP continues to be the mainstay of temporary MCS in clinical practice. The IABP system consists of a dual-lumen catheter and balloon connected to a pump. One lumen is connected directly to the pump and fi lls with helium with each inf l ation. The other lumen allows aortic pressure transduction and access for guidewire insertion. Balloon inf l ation occurs with the start of diastole, followed by rapid def l ation with the start of systole. Timing has traditionally been based on electrocardiogram or pressure triggers, with the former using the midpoint of the T wave for inf l ation and the start of the R wave for def l ation. Arrhythmias and tachycardia can perturb this method of counterpulsation timing. However, newer fi ber-optic catheters, which can improve timing algorithms, are now available in sites of 7F to 9F, with displacement capacities of 30–50 mL [8, 9].
IABP counterpulsation has a number of hemodynamic effects, including decreased afterload,diastolic blood pressure augmentation which improves coronary arterial perfusion, and a direct unloading of the left ventricle [10]. Despite these potential benef i ts, clinical trial data have been less than robust in regard to the hemodynamic impact of IABP placement in CS. The IABP Cardiogenic Shock Trial found no difference with IABP use in comparison with medical therapy in a small group of patients with acute myocardial infarction complicated by CS [11]. Serial hemodynamic measurements over a 96-h period demonstrated no signif i cant change in various parameters, including mean arterial pressure, cardiac output, left ventricular (LV) stroke work index, or cardiac power output. Although not used extensively in the clinical setting, cardiac power output, def i ned as mean arterial pressure × cardiac output/451 and reported in watts, has been demonstrated to hold prognostic value in CS patients as the strongest independent hemodynamic predictor of inpatient death [12]. These disappointing results were followed by the results of the IABP-SHOCK II trial, a prospective and randomized study of 600 patients, which demonstrated no mortality benef i t with IABP placement in a similar patient population [13]. In this analysis, there was no difference in serum lactate levels with the use of an IABP,suggesting no change in end-organ perfusion, confirming the hemodynamic results seen in its predecessor. Longer 12-month results also revealed no difference with IABP use [14].
These data suggest that the IABP may have limited utility in those individuals who have critical CS. There is, however, evidence that its use may be of benef i t in high-risk percutaneous coronary intervention as it pertains to the prevention of intraprocedural hypotension and improvement in long-term mortality in select patients [15, 16].
More robust percutaneous MCS is available in the form of the TandemHeart (CardiacAssist, Pittsburgh, PA, USA) and Impella (Abiomed, Danvers,MA, USA) devices, best thought of as percutaneous ventricular assist devices (pVADs) (Table 1). The TandemHeart (Figure 1) is a left atrial to femoral artery bypass system. Venous access is obtained via a 21F cannula, which is then placed transseptally in the left atrium. This cannula is attached toa centrifugal pump, controlled by an external console, which removes oxygenated blood from the left atrium and returns it to the arterial circulation via a 15F or 17F arterial return cannula which is capable of delivering a fl ow rate of up to 5 L/min.

Table 1 Percutaneous Mechanical Circulatory Support Options.

Figure 1 The TandemHeart System.
The TandemHeart system effectively reduces LV preload, wall stress, and myocardial oxygen demand [17, 18]. In a comparison of the IABP and the TandemHeart in 41 patients with CS following acute myocardial infarction, the TandemHeart system provided better hemodynamic improvements as assessed by changes in cardiac output, systemic vascular resistance, and cardiac power index[19]. Serum lactate levels were also signifi cantly improved, suggesting greater end-organ perfusion and an initial reversal of CS in the TandemHeart group as compared with support with an IABP. This was at the expense of increased bleeding and limb ischemia, owing to its larger prof i le, but did not provide a mortality advantage in this small study.A larger, albeit retrospective, single-center analysis further demonstrated the hemodynamic superiority of the TandemHeart in 117 patients with CS refractory to vasopressor and IABP support [20].Over an average duration of support of nearly 1 week, cardiac index, systolic blood pressure, and urine output all increased. More precise markers of end-organ perfusion, such as mixed venous oxygenation and lactate levels, were also signifi cantly improved. Although 6-month mortality remained high at 45.3%, nearly half of these patients were undergoing active cardiopulmonary resuscitation immediately before or during implantation of the TandemHeart, demonstrating the severity of illness in this population. This helps to establish the utility of more robust percutaneous MCS in those patients who do not respond to initial, more conservative measures. In particular, this can be an invaluable method of bridging patients to a more def i nitive treatment – namely, durable LV assist device(LVAD) implantation.
The Impella is also a commercially available pVAD which provides axial fl ow from the left ventricle into the ascending aorta (Figure 2). There are three versions available, with the 2.5 and CP devices,12F and 15F, respectively, placed percutaneously via the femoral artery. The Impella 2.5 can provide a fl ow rate of up to 2.5 L/min, with the Impella CP capable of providing up to 4 L/min. A larger device(Impella 5.0) is also available, which requires a surgical cutdown and can alternatively be placed into the subclavian artery for more stable positioning and longer-term support [21]. As the name suggests,this device can provide a fl ow rate of up to 5 L/min.When it is placed from the femoral artery, insertion is similar to the placement of an IABP, over a wire into the aorta and across the aortic valve, with the distal end of the Impella having a pigtail loop which is taken into the left ventricle. Connected to this is the blood inlet area, followed by the outlet area and the motor housing. Via axial fl ow, blood is withdrawn from the left ventricle and propelled into the ascending aorta, thereby directly unloading the left ventricle. This provides decreased wall stress in addition to other benef i cial hemodynamic effects such as a decrease in pulmonary capillary wedge pressure and increased coronary perfusion pressure and fl ow, and a resultant decrease in myocardial oxygen demand [22, 23]. Although these data, and much of the available literature on the Impella system, are taken from the Impella 2.5 and its use in high-risk percutaneous coronary intervention, there are emerging data regarding the more powerful platforms. A small single-center study of 34 patients with profound CS in the setting of acute myocardial infarction suggested improved survival with the Impella 5.0 compared with the Impella 2.5, in both those in whom the larger device was initially implanted and those who received an upgrade [24].The Impella 5.0 has also been demonstrated to provide equivalent, if not superior, outcomes in critical CS when compared retrospectively with extracorporeal membrane oxygenation (ECMO) [25].In general, for the management of critical CS, the Impella CP and Impella 5.0 should be considered as the Impella 2.5 is unlikely to provide the needed hemodynamic support to improve outcomes.
Although there are promising data regarding the role of pVADs in critical CS as it pertains to hemodynamic and metabolic improvements, there has yet to be demonstrated a mortality benef i t with these devices. A meta-analysis of three prospective trials comparing pVADs with IABPs confirmed superiority of the former in regard to hemodynamic parameters, without a resultant improvement in 30-day mortality [26]. However, this was an analysis of 100 patients, and larger trials, with improved power, are likely necessary to adequately address the issue of mortality.
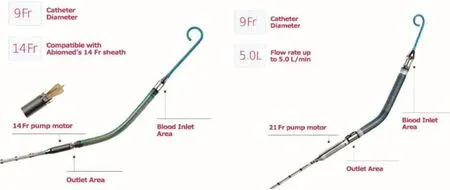
Figure 2 Impella Devices.
ECMO, and the large class of extracorporeal life support, provides full life support and devices can be placed at the bedside without the aid of fl uoroscopy. Venovenous ECMO provides gas exchange with circulatory support and is not of utility in CS.Venoarterial ECMO, however, offers full cardiopulmonary support and can be life-saving in critical CS. The venoarterial ECMO circuit is made up of a venous cannula and an arterial cannula connected to a centrifugal pump which has a membrane oxygenator. The venous cannula drains deoxygenated blood into the membrane oxygenator for gas exchange, and this is then propelled back to the patient through the arterial cannula. The cannulae are similar in size to those used with the Tandem-Heart, and venoarterial ECMO is able to provide a fl ow rate of up to 6 L/min. As there is no direct unloading of the left ventricle, and blood is returned into the arterial system resulting in an increase in afterload, this type of support can be combined with an IABP or Impella for optimal hemodynamic and metabolic affects [27]. Venoarterial ECMO has been demonstrated to be a viable, rapid, and transportable method of providing urgent MCS to patients as a method to bridge them to more durable support or transplantation [28].
The Special Case of Right Ventricular Heart Failure
Medical management of acute right ventricular failure focuses on reducing right ventricular afterload and improving the contractile function of the right side of the heart. MCS has largely been limited to surgical intervention with right ventricular assist devices, with an IABP providing indirect benef i t to the right ventricle by unloading the left ventricle. More recently, however, percutaneous support specific to the right side of the heart has been developed and approved for limited use. This can be provided in the form of continuous-f l ow right ventricular support devices – namely, the PROTEK Duo (CardiacAssist, Pittsburgh, PA, USA) and the Impella RP. The PROTEK Duo is a coaxial duallumen cannula, with a 29F outer cannula and a 16F inner cannula (Figure 3). The cannulae have inf l ow and outf l ow ports, and are connected to the TandemHeart centrifugal pump. This allows blood to be withdrawn from the right atrium and returned to the pulmonary artery. The Impella RP is a microaxial pump consisting of a 23F pump head and outf l ow cannula mounted on an 11F catheter (Figure 4). It can be implanted via the femoral vein and advanced across the pulmonic valve. The inf l ow portion of the catheter remains in the inferior vena cava, with the outf l ow in the pulmonary artery. Both devices have been demonstrated to improve hemodynamic status in patients with acute right ventricular failure[29, 30].
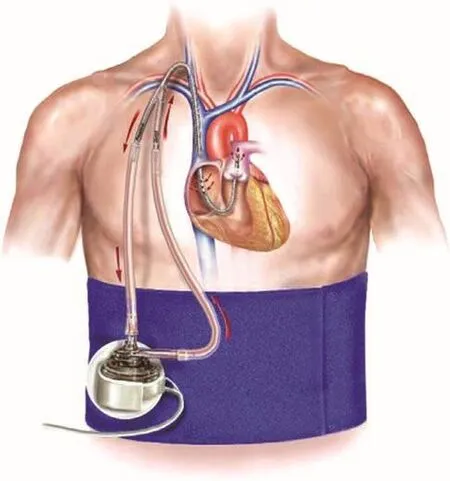
Figure 3 PROTEK Duo Right Ventricular Support Device with TandemHeart.

Figure 4 Impella RP Catheter.
Treating the Cardiogenic Shock Patient
Regardless of the method used to initially treat patients with CS, rapid identif i cation and early initiation of therapy is of paramount importance if one hopes to have any meaningful chance of survival. Patients with an acute coronary syndrome should first be taken to the cardiac catheterization laboratory. Inotropic support is a reasonable firstline choice for support; however, its use must be weighed against the spectrum of illness and degree of CS. As discussed earlier, as inotrope requirements increase, so too does mortality [6]. Therefore, inotropic support is likely to provide benef i t in more milder-shock syndromes. Its use in high doses, or in combination with other vasoactive medications, should trigger further evaluation with invasive hemodynamics and real-time consultation with a multidisciplinary team.
This realization of the spectrum of CS and the need to tailor therapy to the degree of hemodynamic support needed, with the goals of the patient in mind, has led to the concept of the “Shock Team.” Although it has been described in multiple formats and with different constituents, the premise is that real-time, early consultation between heart failure, interventional cardiology, critical care,and cardiac surgery specialists takes place to share decision making and devise an initial strategy for hemodynamic support. Depending on the degree of CS, this may include placement of a pVAD or ECMO if patients are felt to be acceptable candidates for more durable therapy such as an LVAD or transplantation.

Figure 5 Critical Cardiogenic Shock Algorithm.
We suggest that patients with an identif i ed shock syndrome be evaluated in the following, multidisciplinary team fashion (Figure 5). Following activation of the Shock Team, the patient should be taken to the cardiac catheterization laboratory for invasive hemodynamic measurements. In cases of mild-to-moderate CS, intotropic support and IABP placement is reasonable. More severe shock syndromes should be treated with a pVAD, again if, in the estimation of the team, the patient is an appropriate candidate for advanced therapies for heart failure. In the case of IABP placement and inotropic support, the patient and the team should remain in the cardiac catheterization laboratory. If no improvement in hemodynamics parameters is noted, such as pulmonary arterial saturation, the IABP should be exchanged for a pVAD. In the case of improvement in hemodynamic parameters, the patient’s lactate levels and pulmonary arterial saturation should be monitored every 6 h. If there is no sustained improvement, or if evidence of poor end-organ perfusion remains, therapy should be escalated either to a pVAD or ECMO, with the goal being to transition the patient to a durable LVAD within days.
This type of graded approach with a multidisciplinary team has been successful in improving outcomes with CS, with those who survived the period of initial temporary MCS having a survival rate of 80% at 1 year [31]. A modif i ed “team and network” approach has also been described, which incorporates early referral to advanced heart failure centers, and holds promise for improving outcomes in appropriately selected patients [32]. It remains unclear, however, what fi nancial burden this would place on the health care system, with initial analyses suggesting a cost benef i t for a pVAD over an IABP and ECMO; however, additional study is required[33, 34].
Conclusion and Take-Home Message
CS remains a problem with signif i cant impact on the health care system, and although outcomes remain poor, early identif i cation and aggressive therapy may provide benef i t. The key to such an approach is ongoing evaluation by a multidisciplinary team,and rapid escalation of care when appropriate. The heterogeneity of patients with CS and the wide spectrum of the severity of illness encountered with this clinical syndrome are likely to confound trial design and preclude def i nitive data to guide therapies. Therefore, care should be tailored to the fi ndings in specific patients, with an understanding of patient and family goals, while next utilizing individual and facility expertise. Early referral to an advanced heart failure center should be considered in all patients with a high-risk CS syndrome, where more robust MCS can be delivered. Cost considerations will need to be evaluated further to help def i ne the optimal delivery method for these strategies.
Conflict of Interest
The authors declare no conf l ict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or notfor-profit sectors.
1. Rihal CS, Naidu SS, Givertz MM,Szeto WY, Burke JA, Kapur NK,et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Association,the Cardiological Society of India,and Sociedad Latino Americana de Cardiologia Intervencion; aff i rmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 2015;65:e7–26.
2. Fonarow GC, Adams KF, Jr.,Abraham WT, Yancy CW, Boscardin WJ. Risk stratif i cation for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.J Am Med Assoc 2005;293:572–80.
3. Tomcikova D, Felsoci M, Spinar J,Miklik R, Mikusova T, Vitovec J,et al. Risk of in-hospital mortality identif i ed according to the typology of patients with acute heart failure: classif i cation tree analysis on data from the Acute Heart Failure Database-Main registry. J Crit Care 2013;28:250–8.
4. Peacock WF, Emerman C,Costanzo MR, Diercks DB,Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail 2009;15:256–64.
5. Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL,LeJemtel TH, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol 2005;46:57–64.
6. Samuels LE, Kaufman MS, Thomas MP, Holmes EC, Brockman SK,Wechsler AS. Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: experience with the Abiomed BVS system. J Card Surg 1999;14:288–93.
7. Kantrowitz A, Tjonneland S,Freed PS, Phillips SJ, Butner AN,Sherman JL, Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. J Am Med Assoc 1968;203:113–8.
8. Schreuder JJ, Castiglioni A,Donelli A, Maisano F, Jansen JR, Hanania R, et al. Automatic intraaortic balloon pump timing using an intrabeat dicrotic notch prediction algorithm. Ann Thorac Surg 2005;79:1017–1022; discussion 1022.
9. Mulholland J, Yarham G, Clements A, Morris C, Loja D. Mechanical left ventricular support using a 50 cc 8 Fr fi bre-optic intra-aortic balloon technology: a case report.Perfusion 2013;28:109–13.
10. Papaioannou TG, Stefanadis C.Basic principles of the intraaortic balloon pump and mechanisms affecting its performance. ASAIO J 2005;51:296–300.
11. Prondzinsky R, Unverzagt S, Russ M, Lemm H, Swyter M, Wegener N, et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial.Shock 2012;37:378–84.
12. Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004;44:340–8.
13. Thiele H, Zeymer U, Neumann FJ,Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96.
14. Thiele H, Zeymer U, Neumann FJ,Ferenc M, Olbrich HG, Hausleiter J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II):fi nal 12 month results of a randomised, open-label trial. Lancet 2013;382:1638–45.
15. Perera D, Stables R, Clayton T, De Silva K, Lumley M, Clack L, et al.Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1):a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation 2013;127:207–12.
16. Perera D, Stables R, Thomas M,Booth J, Pitt M, Blackman D, et al.Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. J Am Med Assoc 2010;304:867–74.
17. Basra SS, Loyalka P, Kar B. Current status of percutaneous ventricular assist devices for cardiogenic shock. Curr Opin Cardiol 2011;26:548–54.
18. Kapur NK, Paruchuri V, Urbano-Morales JA, Mackey EE, Daly GH, Qiao X, et al. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013;128:328–36.
19. Thiele H, Sick P, Boudriot E,Diederich KW, Hambrecht R,Niebauer J, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–83.
20. Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock.J Am Coll Cardiol 2011;57:688–96.
21. Pozzi M, Quessard A, Nguyen A,Mastroianni C, Niculescu M, Pavie A, et al. Using the Impella 5.0 with a right axillary artery approach as bridge to long-term mechanical circulatory assistance. Int J Artif Organs 2013;36:605–11.
22. Raess DH, Weber DM. Impella 2.5. J Cardiovasc Transl Res 2009;2:168–72.
23. Remmelink M, Sjauw KD,Henriques JP, de Winter RJ, Koch KT, van der Schaaf RJ, et al.Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv 2007;70:532–7.
24. Engstrom AE, Cocchieri R,Driessen AH, Sjauw KD, Vis MM,Baan J, et al. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: the Academic Medical Center intensive care unit experience. Crit Care Med 2011;39:2072–9.
25. Lamarche Y, Cheung A,Ignaszewski A, Higgins J, Kaan A, Griesdale DE, et al. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J Thorac Cardiovasc Surg 2011;142:60–5.
26. Cheng JM, den Uil CA, Hoeks SE,van der Ent M, Jewbali LS, van Domburg RT, et al. Percutaneous left ventricular assist devices vs.intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J 2009;30:2102–8.
27. Koeckert MS, Jorde UP, Naka Y,Moses JW, Takayama H. Impella LP 2.5 for left ventricular unloading during venoarterial extracorporeal membrane oxygenation support. J Card Surg 2011;26:666–8.
28. Guenther S, Theiss HD, Fischer M, Sattler S, Peterss S, Born F,et al. Percutaneous extracorporeal life support for patients in therapy refractory cardiogenic shock: initial results of an interdisciplinary team. Interact Cardiovasc Thorac Surg 2014;18:283–91.
29. Cheung AW, White CW, Davis MK,Freed DH. Short-term mechanical circulatory support for recovery from acute right ventricular failure:clinical outcomes. J Heart Lung Transplant 2014;33:794–9.
30. Kapur NK, Paruchuri V,Jagannathan A, Steinberg D,Chakrabarti AK, Pinto D, et al.Mechanical circulatory support for right ventricular failure. JACC Heart Fail 2013;1:127–34.
31. Takayama H, Truby L, Koekort M, Uriel N, Colombo P, Mancini DM, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant 2013;32:106–11.
32. El-Banayosy A, Cobaugh D,Zittermann A, Kitzner L, Arusoglu L, Morshuis M, et al. A multidisciplinary network to save the lives of severe, persistent cardiogenic shock patients. Ann Thorac Surg 2005;80:543–7.
33. Maini B, Gregory D, Scotti DJ,Buyantseva L. Percutaneous cardiac assist devices compared with surgical hemodynamic support alternatives: cost-effectiveness in the emergent setting. Catheter Cardiovasc Interv 2014;83:E183–92.
34. Roos JB, Doshi SN, Konorza T,Palacios I, Schreiber T, Borisenko OV, et al. The cost-effectiveness of a new percutaneous ventricular assist device for high-risk PCI patients: mid-stage evaluation from the European perspective. J Med Econ 2013;16:381–90.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Continuous Flow Left Ventricular Assist Device Therapy: A Focused Review on Optimal Patient Selection and Long-Term Follow-up Using Echocardiography
- Cardiac Resynchronization Therapy in 2015:Lessons Learned
- Pulmonary Arterial Hypertension and the Failing Ventricle: Getting It Right
- The Evaluation of the Heart Failure Patient by Echocardiography: Time to go beyond the Ejection Fraction
- Noninvasive Hemodynamic Monitoring for Heart Failure: A New Era of Heart Failure Management
- Epidemiological Study of Heart Failure in China