Noninvasive Hemodynamic Monitoring for Heart Failure: A New Era of Heart Failure Management
2015-05-22GabrielHernandezMDVivianaNavasMDandSandraChaparroMD
Gabriel A. Hernandez, MD, Viviana Navas, MD and Sandra Chaparro, MD
1Division of Cardiology, Department of Medicine, University of Miami, Miami, FL, USA
2Cleveland Clinic Florida, Weston, FL, USA
Introduction
It is estimated that 5.7 million Americans have heart failure (HF), with an overall 1-year mortality rate as high as 29.6% [1, 2] at a high fi nancial cost [1].It is recognized that volume excess is the main reason for HF hospitalizations, but the evaluation and monitoring of fl uid status remains a challenge, with early signs of decompensation often being missed[3–5].
The pulmonary artery (PA) catheter (PAC) has become an essential tool to understand the physiologic and pathophysiologic changes in cardiovascular diseases [6, 7] and has revolutionized right-sided heart catheterization [8]. PACs remain an integral part of the cardiovascular evaluation but there is concern that they are overused, increase resource utilization, and might increase mortality [9, 10].Now with technological advances, new devices are available to monitor patients in a less intrusive way.
Heart Failure Hemodynamics
Patients with chronic HF have increased baseline filling pressures, which are required to maintain optimal cardiac output. The PA pressure will rise and its diastolic pressure will correlate with the mean wedge pressure, and the PA will adapt and dilate, causing an ameliorated increase in the right ventricular (RV) and right atrial pressures. Typically, the mean wedge pressure will be greater than the mean right atrial pressure in chronic HF. The depressed output will also cause an increase in the oxygen tissue extraction, decreasing the mixed venous oxygen saturation. In RV failure, the right ventricle becomes a conduit and the right atrial pressure may be equal to or exceed the mean wedge pressure [11].
Inpatient Hemodynamic Monitoring in the Current Era
Pulmonary Artery Catheter
The PAC became a reliable tool for use both in the cardiac catheterization laboratory and in the critical care unit. In the last decade, approximately one million PACs were used annually in the United States [12], and their overuse raised conf l icting opinions about their benef i t. It is important to emphasize that PAC use should not be considered a therapeutic intervention but instead should be considered a diagnostic tool and should not be considered as first-line intervention for evaluation of conditions where noninvasive tools can yield the same information. The outcome of patients undergoing PA catheterization will depend solely on the expertise of the treating physician, the accuracy of the results, and the treatment driven by the information obtained. Furthermore, inotropic therapy may be associated with myocardial exhaustion, arrhythmias, and adverse outcomes related not to the PAC but to the therapy [7].
Despite theoretical benef i ts of hemodynamic information obtained from PAC use, multiple trials failed to show improvement in outcomes[9, 12–14] in the intensive care setting. In contrast, retrospective trials suggested that in severe chronic HF there might be a role for PAC use when one is tailoring inotropic and vasoactive agents [15, 16].
Initially, randomized trials were diff i cult to perform as PAC use was deemed to be benef i cial and physicians considered it unethical to enroll patients in a nonintervention arm [9].
The SUPPORT study [9] included more than 5000 patients with a wide variety of diseases (less than 8% of the patients had HF). The investigators concluded that PAC use was associated with increased mortality and utilization of resources.
The ESCAPE trial [13] was designed to determine whether PAC use was safe and improved clinical outcomes in hospitalized patients with severe symptomatic and recurrent HF. This trial showed that therapy to reduce volume during hospitalization led to symptom abatement in patients with and without a PAC. Additionally, mortality and number of hospitalization days did not differ between groups.Some limitations included selection bias [13], a signif i cant number of patients received nesiritide, a drug that failed to show eff i cacy in later trials, and some patients did not receive inotrope or intravenous vasodilators [17].
The PAC-MAN trial [14] was a larger trial that enrolled patients who should be treated with a PAC(selection bias). This trial included only 11% of patients with decompensated HF and showed neither a clear benef i t nor harm in critically ill patients treated with a PAC.
Lastly, a meta-analysis of 13 trials [12] that gathered 5051 patients demonstrated that PAC use did not improve outcomes in critically ill patients or increase mortality or the number of days in hospital.
PACs have been used in a wide and inappropriate patient population, and trials in cardiogenic shock patients, advanced HF patients, and pretransplant or ventricle-assisted device patients are needed to further elucidate appropriate use criteria.
ClearSight System
A new noninvasive cardiac output monitoring system, the ClearSight system (Edwards Lifesciences,Irvine, CA, USA) uses a previously described fi nger cuff method to measure arterial blood pressure continuously, the Nexf i n device (BMEYE, Amsterdam, Netherlands) in conjunction with the EV1000 clinical platform to assess beat-to-beat changes in blood pressure and heart rate, which are used to calculate stroke volume, cardiac output, and systemic vascular resistance [17].
This device has been compared with the PAC,pulse index continuous cardiac output [18], which uses information from arterial and central venous catheters [19], and other methods of cardiac output monitoring such as Doppler echocardiography with conf l icting results, being less accurate in low output states, where there is high peripheral vascular resistance and edema [20]. Nevertheless, this technology has not been evaluated in terms of mortality benef i t and usefulness in HF.
Despite the data on PAC use in the HF population, there is an increased need for remote hemodynamic monitoring in the outpatient setting to improve outcomes.
Ambulatory Hemodynamic Monitoring
To prevent hospital admission and thus decrease mortality and economic burden, increased attention has been paid to detecting early signs of HF decompensation and improving outpatient treatment by self-guidance (daily weight monitoring, diuretic sliding scales), close follow-ups, and/or remote monitoring systems (telemonitoring) [5].
For many years, the symptoms and signs on physical examination remained the best tool to evaluate congestion; unfortunately, symptoms occur late and within days before decompensation [21, 22],and even chest radiograph fi ndings correlate poorly with fl uid status measured by wedge pressures [23].
Noninvasive remote monitoring of the HF patient by frequent calls to evaluate changes in weight,vital signs, and symptoms has been studied and has shown conf l icting results [24, 25], raising questions as for how long patients should receive calls, when calls should be started, and which populations will benef i t from them. Nevertheless, the concept of remote monitoring opened a new pathway for further strategies.
Intrathoracic Impedance
When left-sided filling pressures increase, fl uid accumulates in the pulmonary interstitium, generating better electrical conductance; this causes electrical currents across the tissue to be faster, decreasing the impedance. With use of a generator (a pacemaker, an intracardiac def i brillator, or a cardiac resynchronization therapy device) and the RV lead,impedance can be obtained between them multiple times per day. This phenomenon has been studied,and intrathoracic impedance has been inversely correlated with wedge pressures [22]. Reductions in impedance begin 15 days before the onset (or worsening) of symptoms. In the nonrandomized Fluid Accumulation Status Trial (FAST) [26],the fl uid index derived from intrathoracic impedance measurement (OptiVol; Medtronic, Minneapolis, Minnesota, USA) revealed that impedance was more sensitive in predicting HF hospitalization than daily weight monitoring; this trial also showed that adherence to self-weight measurement decreased with time. A subsequent study, DOT-HF[27], incorporated an audible alert when possible fl uid accumulation was present, but failed to show a reduction in the composite end point of mortality and HF hospitalizations, leading in fact to more hospitalizations and more outpatient visits.
All these device-derived parameters when combined can improve the ability to identify patients at risk of HF events up to 30 days in advance [28].Patients required monthly visits to download the diagnostic data, increasing outpatient costs and resources. Ongoing trials are evaluating if remote monitoring with wireless transmission of thoracic impedance will reduce hospitalization rates for HF[29, 30]. Newer devices with multiple vectors are being investigated (i.e., use of the left ventricular lead in cardiac resynchronization therapy) to improve sensitivity and decrease the false positive rate for pulmonary congestion [31].
Implantable Hemodynamic Monitor
Since all prior interventions to reduce HF hospitalization rates required face-to-face encounters to assess volume status, an accurate means to remotely assess cardiopulmonary filling pressures was developed in the last decade [32]. An advance in implantable wireless sensor technology allowed frequent and direct measurement of intracardiac filling pressures, which are monitored by health care providers and can help in the tailoring therapy to reduce filling pressures (Table 1).
Chronicle
The Chronicle (Medtronic, Minneapolis, Minnesota, USA) device is an implantable hemodynamic monitor (IHM) that continuously measures and stores hemodynamic information remotely. It consists of a generator (similar to an intracardiac def i -brillator or pacemaker) and a transvenous sensor lead in the RV outf l ow tract or septum [4, 38].
Reynolds et al. [39] estimated the PA diastolic pressures from the right ventricle, to avoid unnecessary pulmonary cannulation, with the objective to further calculate the wedge and left ventricular enddiastolic pressure. The Chronicle device continuously records the RV systolic, diastolic, and estimated PA diastolic pressures among other variables.
This device was studied in the Chronicle Offers Management to Patients with Advanced Signs
and Symptoms of Heart Failure (COMPASS-HF)study [4], a single-blinded, randomized trial of 274 patients with New York Heart Association (NYHA)class III/IV disease. A nonsignif i cant 21% reduction in the primary end point (HF-related events) was seen in the IHM group. The two safety end points –freedom form sensor failure and system-related complications – were met and showed a 36% relative risk reduction in HF hospitalization. Furthermore, COMPASS-HF demonstrated an intracardiac pressure upsurge, weeks before hospitalization,with a subsequent decline following treatment [38].
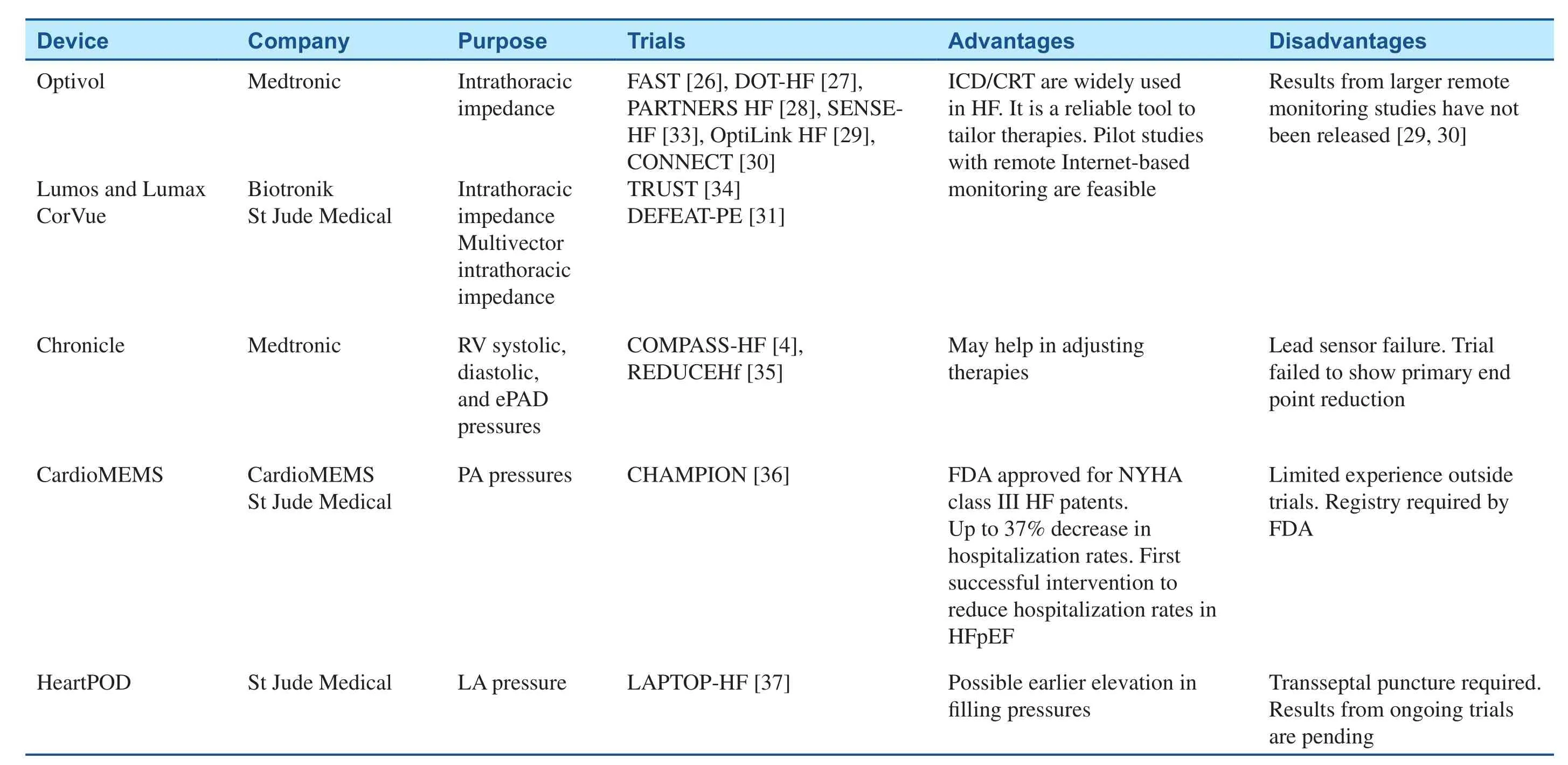
Table 1 Implantable Hemodynamic Monitors.
The Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients with Chronic Heart Failure (REDUCEhf) [35] was a singleblinded, randomized trial designed to recruit 850 NYHA class II/III HF patients but was stopped early,with 400 implants, because of a 4% IHM lead failure observed in previous trials. The primary safety end point was met but the trial was unable to test the primary clinical end point because of early termination.This trial, once again, supported the hypothesis that volume accumulation and increased filling pressures are mechanism preceding HF decompensation.
CardioMEMS
A second IHM device also emerged during the last decade. The CardioMEMS HF sensor (Cardio-MEMS, Atlanta, Georgia, USA) is a 15-mm-long,3-mm-wide wireless PA pressure monitor, which consists of a coil and a pressure-sensitive capacitor encased within a hermetically sealed, fused-silica capsule completely covered in medical-grade silicone [40] and coupled to two wired nitinol loops that prevents distal migration. The insertion requires a right-sided heart catheterization to advance a guidewire. Subsequently, a 12F delivery catheter is advanced over the guidewire into the PA, where the sensor is separated from the tether wire and along with the delivery catheter removed [32]. The device is battery-free and powered by radiofrequency signals provided by an external antenna. The coil and capacitor form an electric circuit that resonates at a specific frequency, and changes in pressures around the sensor provokes change in the circuit’s resonant frequency that are then converted into a real-time pressure waveform. Prior studies demonstrated the safety prof i le [41] and accurate performance, the later when compared with a PAC and Doppler echocardiography [40].
The CardioMEMS HF Sensor Allows Monitoring of Pressures to Improve Outcomes in NYHA Functional Class III Heart Failure Patients ( CHAMPION)trial [36] was a prospective, multicenter, singleblinded, randomized trial of 550 patients. All of the patients underwent device implantation and were randomized to receive treatment versus standard care. This trial had a “hemodynamic-guided care strategy” [42] with an optivolemic target status(mean PA pressure of 10–25 mm Hg). If pressure values differed from the target, then a protocoldef i ned treatment with neurohormonal, diuretic, or vasodilator drugs was applied. The primary eff i cacy end point was the rate of HF hospitalizations during the 6 months after implantation, and the two primary safety end points were freedom from devicerelated or system-related complications with a total mean follow-up of 15 months.
All primary end points were met; there were no pressure-sensor failures, and 2.6% of patients experienced procedure-, device-, or system-related complications, which included mostly bleeding events;no episodes of pulmonary infarction or embolism associated with the sensor occurred during the trial.
The rate of HF hospitalizations was reduced by 28% in the treatment group during the first 6 months,and by 37% over the entire randomized follow-up.Patients in the treatment group had a greater reduction in PA pressure, increase in days alive outside the hospital, and quality of life improvement. A subgroup analysis of patients with a preserved ejection fraction (n= 119) showed a signif i cant primary end point reduction by 46% at 6 months in the treatment group compared with the control group [43].
Despite the notable results from CHAMPION,the sponsor and national principal investigators had frequent contact with the sites and made therapeutic recommendations for the treatment group, however, the absolute number of those communications was low and had no impact on outcomes when they were analyzed between groups [44].
In May 2014, the FDA approved this device, with the indication of wirelessly measuring and monitoring PA pressure and heart rate in NYHA class III HF patients who have been hospitalized for HF in the previous year with the goal of reducing HF hospitalization rates.
Further studies in a real-life scenario are encouraged, as the fl awless nature of the trials might be in disparity with off i ce-based physicians with fewer personnel capable of following up on measurements and intervening appropriately.
HeartPOD
A newer left atrial pressure device monitor, Heart-POD (St Jude Medical, Sylmar, CA, USA) [45,46], consists of an implantable sensor lead coupled with a subcutaneous antenna coil and an external module. The lead has a 3×7 mm hermetically sealed sensor module in the tip, with distal anchors that attach the sensor in the interatrial septum; the implantation requires a transseptal puncture with either transesophageal or intracardiac echocardiographic guidance, which will also serve to exclude the presence of a thrombus. This device is powered and interrogated wirelessly and will measure the left atrial pressure and intracardiac electrogram.
With the hypothesis that direct left atrial pressure measurements might have some advantages compared with estimated measurements, possibly noticing pressure elevations earlier, the ongoing Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy (LAPTOP-HF) study [37] will enroll up to 730 patients with NYHA class III HF and evaluate the HeartPOD (as a stand-alone system) or the Promote CRT-D LAP (if cardiac resynchronization therapy is indicated and the physician chooses to use a single device). The unique strategy in this study is that the device will alert the patient as to which medications to take and when to take them.The primary safety end point is freedom from major cardiovascular or neurological events, and the primary effectiveness end point is the reduction in HF hospitalization rates (broadening to decompensated HF and complications of treatment such as hypotension and acute renal failure).
Conclusion and Take-Home Message
Hemodynamic monitoring is an important tool in the treatment of HF. With the improvement of device technologies, safe and remote monitoring systems have shown promising results, decreasing the rate of hospital readmission in NYHA class III HF outpatients. Ongoing trials are evaluating newer technologies and software capable of interacting with the patient and facilitating compliance with therapies. This opens a new window of possibilities to prevent HF hospitalizations, with devices that can let patients evaluate their fl uid status and manage their own diuretic scale and let the provider titrate guideline-directed therapies.
Conflict of Interest
The authors declare no conf l ict of interest.
Funding
SC has received grants and personal fees from St Jude and Medtronic.
1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29–322.
2. Setoguchi S, Stevenson LW,Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007;154(2):260–6.
3. Murray CM, Agha SA, Rathi S,Germany RE. The evaluation and monitoring of volume status in congestive heart failure. Congest Heart Fail 2008;14(3):135–40.
4. Bourge RC, Abraham WT,Adamson PB, Aaron MF, Aranda JM, Jr., Magalski A, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 2008;51(11):1073–9.
5. Abraham WT. Disease management:remote monitoring in heart failure patients with implantable def i brillators, resynchronization devices, and haemodynamic monitors. Europace 2013;15 Suppl 1:i40–6.
6. Nossaman BD, Scruggs BA,Nossaman VE, Murthy SN, Kadowitz PJ. History of right heart catheterization: 100 years of experimentation and methodology development. Cardiol Rev 2010;18(2):94–101.
7. Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation 2009;119(1):147–52.
8. Swan HJ, Ganz W, Forrester J,Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a fl ow-directed balloon-tipped catheter. N Engl J Med 1970;283(9):447–51.
9. Connors AF, Jr., Speroff T, Dawson NV, Thomas C, Harrell FE, Jr.,Wagner D, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients.SUPPORT Investigators. J Am Med Assoc 1996;276(11):889–97.
10. Sharkey SW. Beyond the wedge:clinical physiology and the Swan-Ganz catheter. Am J Med 1987;83(1):111–22.
11. Lopez-Sendon J, Coma-Canella I,Gamallo C. Sensitivity and specificity of hemodynamic criteria in the diagnosis of acute right ventricular infarction. Circulation 1981;64(3):515–25.
12. Shah MR, Hasselblad V, Stevenson LW, Binanay C, O’Connor CM,Sopko G, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. J Am Med Assoc 2005;294(13):1664–70.
13. Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness:the ESCAPE trial. J Am Med Assoc 2005;294(13):1625–33.
14. Harvey S, Harrison DA, Singer M,Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 2005;366(9484):472–7.
15. Stevenson LW, Tillisch JH. Maintenance of cardiac output with normal filling pressures in patients with dilated heart failure. Circulation 1986;74(6):1303–8.
16. Steimle AE, Stevenson LW,Chelimsky-Fallick C, Fonarow GC, Hamilton MA, Moriguchi JD,et al. Sustained hemodynamic eff i -cacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation 1997;96(4):1165–72.
17. Yancy CW, Jessup M, Bozkurt B,Butler J, Casey DE, Jr., Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol 2013;62(16):e147–239.
18. Ameloot K, Van De Vijver K,Van Regenmortel N, De Laet I,Schoonheydt K, Dits H, et al. Validation study of Nexf i n® continuous non-invasive blood pressure monitoring in critically ill adult patients.Minerva Anestesiol 2014;80(12):1294–301.
19. Litton E, Morgan M. The PiCCO monitor: a review. Anaesth Intens Care 2012;40(3):393–409.
20. Ameloot K, Palmers PJ, Malbrain ML. The accuracy of noninvasive cardiac output and pressure measurements with fi nger cuff: a concise review. Curr Opin Crit Care 2015;21(3):232–9.
21. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep 2009;6(4):287–92.
22. Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, et al.Intrathoracic impedance monitoring in patients with heart failure:correlation with fl uid status and feasibility of early warning preceding hospitalization. Circulation 2005;112(6):841–8.
23. Chakko S, Woska D, Martinez H, de Marchena E, Futterman L,Kessler KM, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conf l icting results may lead to inappropriate care. Am J Med 1991;90(3):353–9.
24. Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A metaanalysis of remote monitoring of heart failure patients. J Am Coll Cardiol 2009;54(18):1683–94.
25. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z,et al. Telemonitoring in patients with heart failure. N Engl J Med 2010;363(24):2301–9.
26. Abraham WT, Compton S, Haas G, Foreman B, Canby RC, Fishel R, et al. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST). Congest Heart Fail 2011;17(2):51–5.
27. Van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011;124(16):1719–26.
28. Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol 2010;55(17):1803–10.
29. Brachmann J, Bohm M, Rybak K, Klein G, Butter C, Klemm H, et al. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the OptiLink HF study (Optimization of Heart Failure Management using OptiVol Fluid Status Monitoring and CareLink). Eur J Heart Fail 2011;13(7):796–804.
30. Zabel M, Vollmann D, Luthje L,Seegers J, Sohns C, Zenker D,et al. Randomized Clinical evaluatiON of wireless fl uid monitoriNg and rEmote ICD managemenT using OptiVol alert-based predefined management to reduce cardiac decompensation and health care utilization: the CONNECTOptiVol study. Contemp Clin Trials 2013;34(1):109–16.
31. Heist EK, Herre JM, Binkley PF,Van Bakel AB, Porterf i eld JG,Porterf i eld LM, et al. Analysis of different device-based intrathoracic impedance vectors for detection of heart failure events(from the Detect Fluid Early from Intrathoracic Impedance Monitoring study). Am J Cardiol 2014;114(8):1249–56.
32. Castro PF, Concepcion R, Bourge RC, Martinez A, Alcaino M, Deck C, et al. A wireless pressure sensor for monitoring pulmonary artery pressure in advanced heart failure:initial experience. J Heart Lung Transplant 2007;26(1):85–8.
33. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, et al.Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations:the SENSE-HF trial. Eur Heart J 2011;32(18):2266–73.
34. Varma N, Epstein AE, Irimpen A,Schweikert R, Love C. Eff i cacy and safety of automatic remote monitoring for implantable cardioverterdef i brillator follow-up: the Lumos-T Safely Reduces Routine Off i ce Device Follow-up (TRUST) trial.Circulation 2010;122(4):325–32.
35. Adamson PB, Gold MR, Bennett T,Bourge RC, Stevenson LW, Trupp R, et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of The Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCEhf) trial. Congest Heart Fail 2011;17(5):248–54.
36. Abraham WT, Adamson PB,Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure:a randomised controlled trial. Lancet 2011;377(9766):658–66.
37. Maurer MS, Adamson PB,Costanzo MR, Eigler N, Gilbert J, Gold MR, et al. Rationale and design of the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy study (LAPTOP-HF).J Card Fail 2015;21(6):479–88.
38. Adamson PB, Conti JB, Smith AL,Abraham WT, Aaron MF, Aranda JM, Jr., et al. Reducing events in patients with chronic heart failure(REDUCEhf) study design: continuous hemodynamic monitoring with an implantable def i brillator.Clin Cardiol 2007;30(11):567–75.
39. Reynolds DW, Bartelt N, Taepke R, Bennett TD. Measurement of pulmonary artery diastolic pressure from the right ventricle. J Am Coll Cardiol 1995;25(5):1176–82.
40. Verdejo HE, Castro PF, Concepcion R, Ferrada MA, Alfaro MA, Alcaino ME, et al. Comparison of a radiofrequency-based wireless pressure sensor to swan-ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol 2007;50(25):2375–82.
41. Abraham WT, Adamson PB, Hasan A, Bourge RC, Pamboukian SV,Aaron MF, et al. Safety and accuracy of a wireless pulmonary artery pressure monitoring system in patients with heart failure. Am Heart J 2011;161(3):558–66.
42. Adamson PB, Abraham WT, Aaron M, Aranda JM, Jr., Bourge RC,Smith A, et al. CHAMPION trial rationale and design: the long-term safety and clinical eff i cacy of a wireless pulmonary artery pressure monitoring system. J Card Fail 2011;17(1):3–10.
43. Adamson PB, Abraham WT,Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction.Circ Heart Fail 2014;7(6):935–44.
44. Loh JP, Barbash IM, Waksman R.Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion heart failure monitoring system. J Am Coll Cardiol 2013;61(15):1571–6.
45. Ritzema J, Melton IC, Richards AM, Crozier IG, Frampton C,Doughty RN, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation 2007;116(25):2952–9.
46. Troughton RW, Ritzema J, Eigler NL, Melton IC, Krum H, Adamson PB, et al. Direct left atrial pressure monitoring in severe heart failure: long-term sensor performance. J Cardiovasc Transl Res 2011;4(1):3–13.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Mechanical Circulatory Support for the Failing Heart: Which Device to Choose
- Continuous Flow Left Ventricular Assist Device Therapy: A Focused Review on Optimal Patient Selection and Long-Term Follow-up Using Echocardiography
- Cardiac Resynchronization Therapy in 2015:Lessons Learned
- Pulmonary Arterial Hypertension and the Failing Ventricle: Getting It Right
- The Evaluation of the Heart Failure Patient by Echocardiography: Time to go beyond the Ejection Fraction
- Epidemiological Study of Heart Failure in China