The Evaluation of the Heart Failure Patient by Echocardiography: Time to go beyond the Ejection Fraction
2015-05-22JacquelineDawsonDoweJuanVilaroKarenHamiltonAnitaSzadyandJuanArandaJr
Jacqueline Dawson Dowe, Juan Vilaro, Karen Hamilton, Anita Szady and Juan M. Aranda, Jr
1Division of Cardiology, Western Kentucky Heart and Lung Associates, Bowling Green, KY, USA
2Division of Cardiology, Department of Medicine, University of Florida, Gainesville, FL, USA
Introduction
There are approximately 5.7 million people in the United States who have a diagnosis of heart failure.The echocardiogram is the single most performed study in these patients. Quantif i cation of the left ventricular (LV) chamber size and thickness, the evaluation for wall motion abnormalities, and the assessment of LV systolic function are important aspects of the echocardiogram and should be performed on every patient. However, the overall assessment of the heart function goes beyond measuring the ejection fraction and analysis of the left ventricle. This article reviews the role of the echocardiogram in the evaluation of the heart failure patient, without focusing on the left ventricle.
Right Ventricular Size and Function
The right ventricle is sometimes referred to as “the forgotten ventricle” as more attention is focused on the left ventricle. The right ventricle is a complex structure which cannot be completely visualized in any single echocardiographic imaging window,thereby making its assessment more diff i cult than that of the left ventricle. The right ventricle plays an essential role in the morbidity and mortality of patients presenting with signs and symptoms of cardiac disease and noncardiac disorders that affect the heart [1, 2]. Important information can be garnered by one paying careful attention to its size, structure,function, and associated hemodynamics [3].
The American Society of Echocardiography (ASE)has recommended that in all reported echocardiographic studies, the right heart should be examined with multiple acoustic windows, and the report should represent an assessment based on qualitative and quantitative parameters [4]. These parameters should include right ventricular (RV) size, right atrial(RA) size, and RV systolic function. In the assessment of RV systolic function, at least one of the following should be included: fractional area change (FAC),tricuspid annular plane systolic excursion (TAPSE),and RV index of myocardial performance, which is an index of global RV performance (RV index of myocardial performance is not discussed in this article).Estimation of the systolic pulmonary artery pressure(SPAP), which also includes estimation of the RA pressure on the basis of inferior vena cava (IVC) size and collapse, should also be documented. In many conditions, additional measurements such as pulmonary artery diastolic pressure (PADP) are indicated.The reference values for these recommended measurements are displayed in Table 1.
If three-dimensional (3D) echocardiography is available, use of it for measurements of RV volumes should be attempted as 3D imaging makes it easier to def i ne the endocardial border, thereby enhancing morphologic information. Normal values for indexed RV area and volume for men and women are included in Table 1. There are some limitations to 3D echocardiographic imaging. Three-dimensional (3D)echocardiographic values of RV volumes need to be established in larger groups of subjects as reference values are limited by lack of published data [5]. The routine clinical use of 3D echocardiography is limited by the need for excellent-quality transthoracic data sets for accurate analysis. The technique can be used only in patients with stable sinus rhythm, since several beats are need for the recording. In addition,intraobserver and interobserver variability is still a limitation. Cardiac magnetic resonance imaging is an alternative imaging modality to consider when one is evaluating the right ventricle as it provides a unique opportunity to image the right ventricle in motion and in three dimensions without some of the limitations of echocardiography [6].
Measuring Right Ventricular Dimension
RV dimension is best estimated at end diastole, from a focused apical four-chamber view [7]. An image demonstrating the maximum RV diameter without foreshortening it should be used. It can be acquiredby one ensuring that the crux and apex of the heart are in view as in Figure 1. The conventional apical four-chamber view focusing on the left ventricle results in considerable variability in how the right side of the heart is imaged. RV linear dimensions and areas may vary widely in the same patient with minor rotations in transducer position. An RV diameter greater than 41 mm at the base and greater then 38 mm at the mid level indicates RV dilatation.Similarly, a longitudinal dimension greater than 86 mm indicates RV enlargement. Qualitatively, the right ventricle should appear smaller than the left ventricle, usually no more than two thirds the size of the left ventricle in the standard apical view. RV liner measurements can also be measured from the parasternal long-axis and short-axis view as outlined in Figure 2.
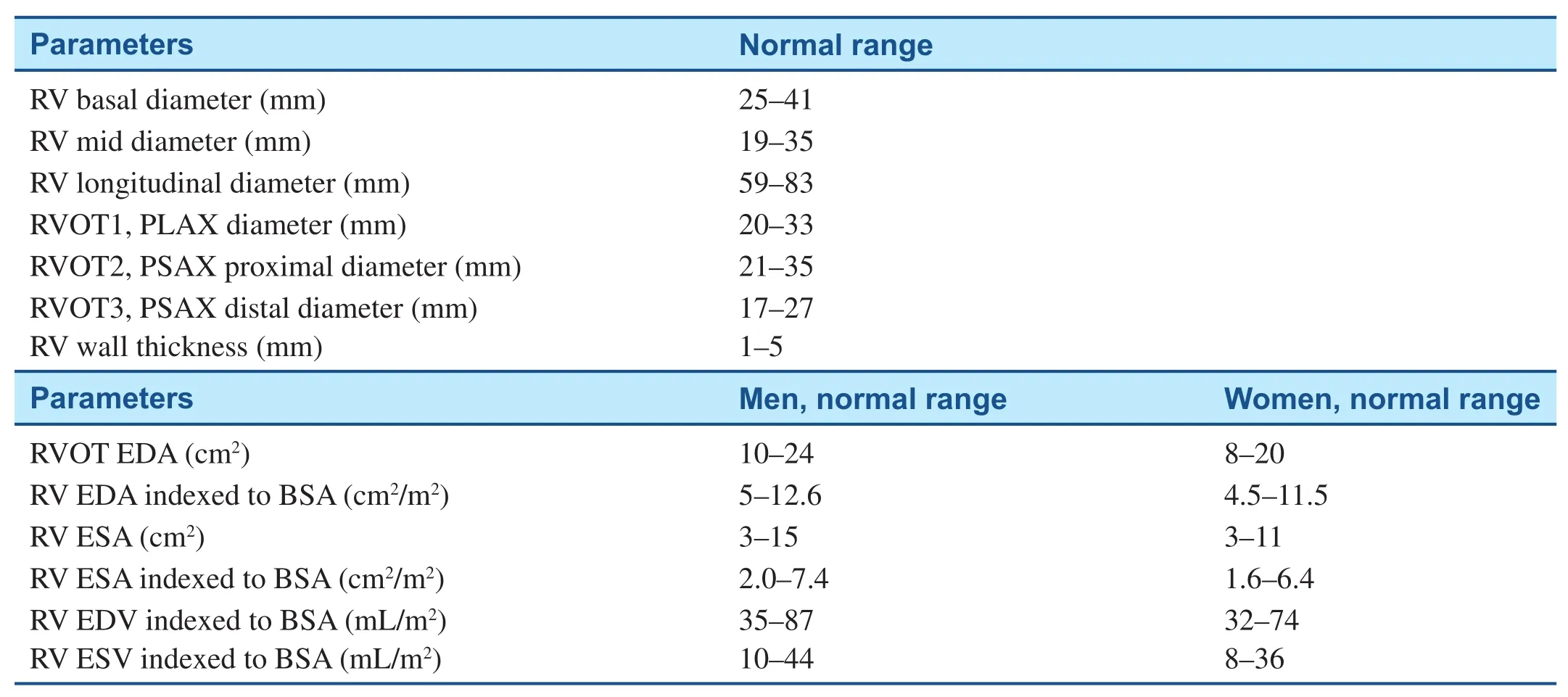
Table 1 Normal Measurements for Right Ventricular (RV) Chamber Size.
The right ventricle dilates in response to chronic volume and/or pressure overload and with RV failure [8, 9]. Many heart failure patients will develop RV volume and pressure overload over a period of time. In general, the right ventricle adapts better to volume overload than to pressure overload.The right ventricle may tolerate volume overload for a long time without a signif i cant decrease in RV systolic function. In contrast to volume-overload states, moderate to severe acquired pulmonary arterial hypertension often leads to RV failure [10].Pressure overload of the right ventricle also may lead to RV ischemia, which may further aggravate ventricular dysfunction. Indexed RV end-diastolic diameter has been identif i ed as a predictor of survival in patients with heart failure [9].
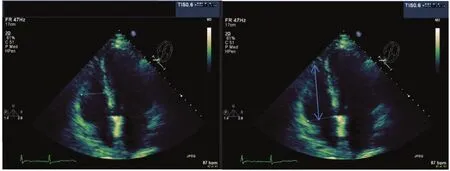
Figure 1 Measurement of Right Ventricular (RV) Diameter in the Apical Four-chamber View.
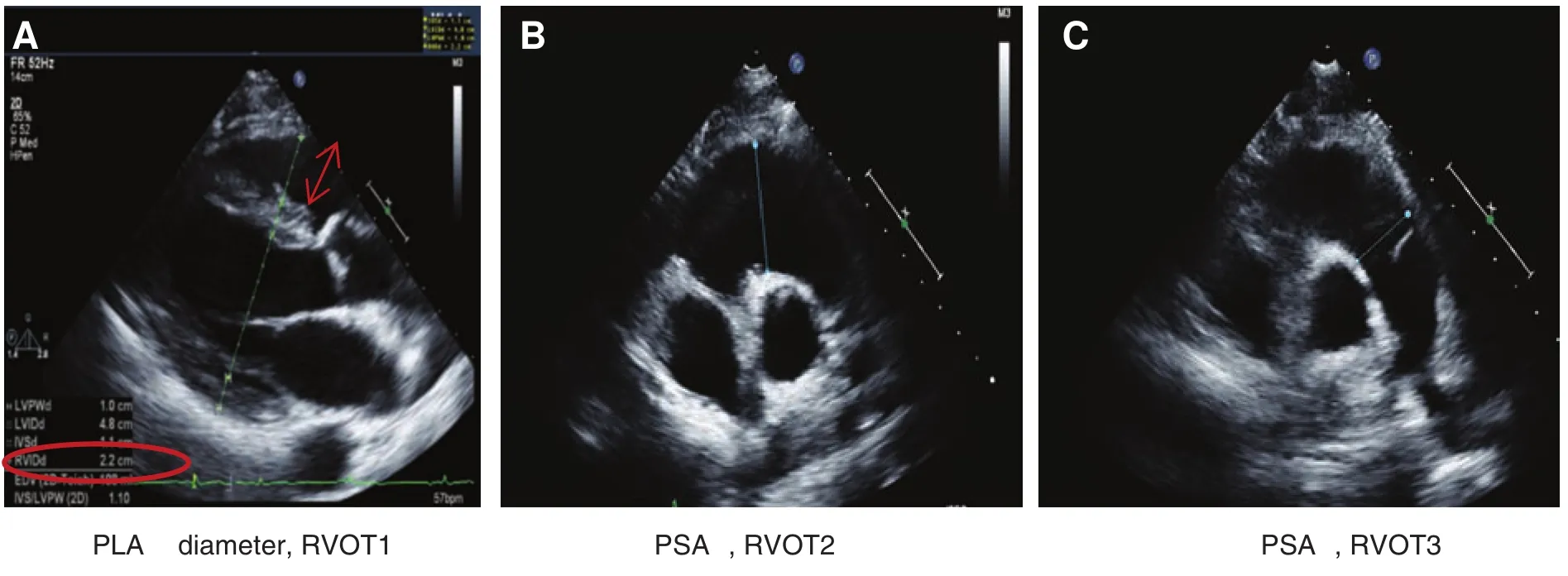
Figure 2 Measurement of Right Ventricular (RV) Diameter in the Parasternal Views.
Quantitative Assessment of Right Ventricular Systolic Function
Per the ASE guidelines, there should be at least one attempt to quantitatively assess the RV systolic function in all echo report. The following are some of the quantitative methods suggested.
Tricuspid Annular Plane Systolic Excursion
TAPSE is easily obtainable and is a measure of RV longitudinal function. RV muscle fi ber orientation results in contraction that occurs predominantly along the longitudinal plane. The measurement is taken from the lateral tricuspid annulus in twodimensional (2D) M-mode and requires a good standard apical four-chamber window (Figure 3).The cursor is placed at the junction of the tricuspid valve plane and the free wall of the right ventricle.The data are averaged over fi ve beats. The normal value for TAPSE is 17 mm or greater [11] and has shown good correlation with other measures used to estimate RV global systolic function, such as the radionuclide-derived RV ejection fraction, the 2D RV FAC, and the biplane Simpson 2D RV ejection fraction. There are three practical limitations that should be considered with regard to TAPSE.Some reference values are based on values obtained from normal individuals without a history of heart disease. The values are not indexed to body surface area or height, and therefore patients at either extreme may be misclassif i ed as having values outside the reference ranges. Finally, it assumes that the displacement of this lateral annular segment in the apical four-chamber view is representative of the function of the entire right ventricle, an assumption that is not valid in some disease states or when there are regional RV wall motion abnormalities.
Two-Dimensional Fractional Area Change
An alternative method for assessing RV function is to use the FAC. The FAC is a surrogate of the RV ejection fraction.
The RV FAC is calculated as follows (Figure 4): (RV diastolic area - RV systolic area)/RV diastolic area.
The RV diastolic and systolic areas are traced in the apical four-chamber view. To ensure as accurate a measurement as possible, the entire right ventricle should be in the fi eld of view during both systole and diastole, including the apex and the lateral wall.Trabeculations should be excluded when one is tracing the RV area. An RV FAC of 35% or greater is considered normal [7]. Measurement of the RV FAC has been studied in certain groups; for example, a typical LV assist device (LVAD) candidate has an RV FAC between 20% and 30%. Patients with an RV FAC less than 20% are at risk of RV failure with the initiation of LVAD therapy [12].
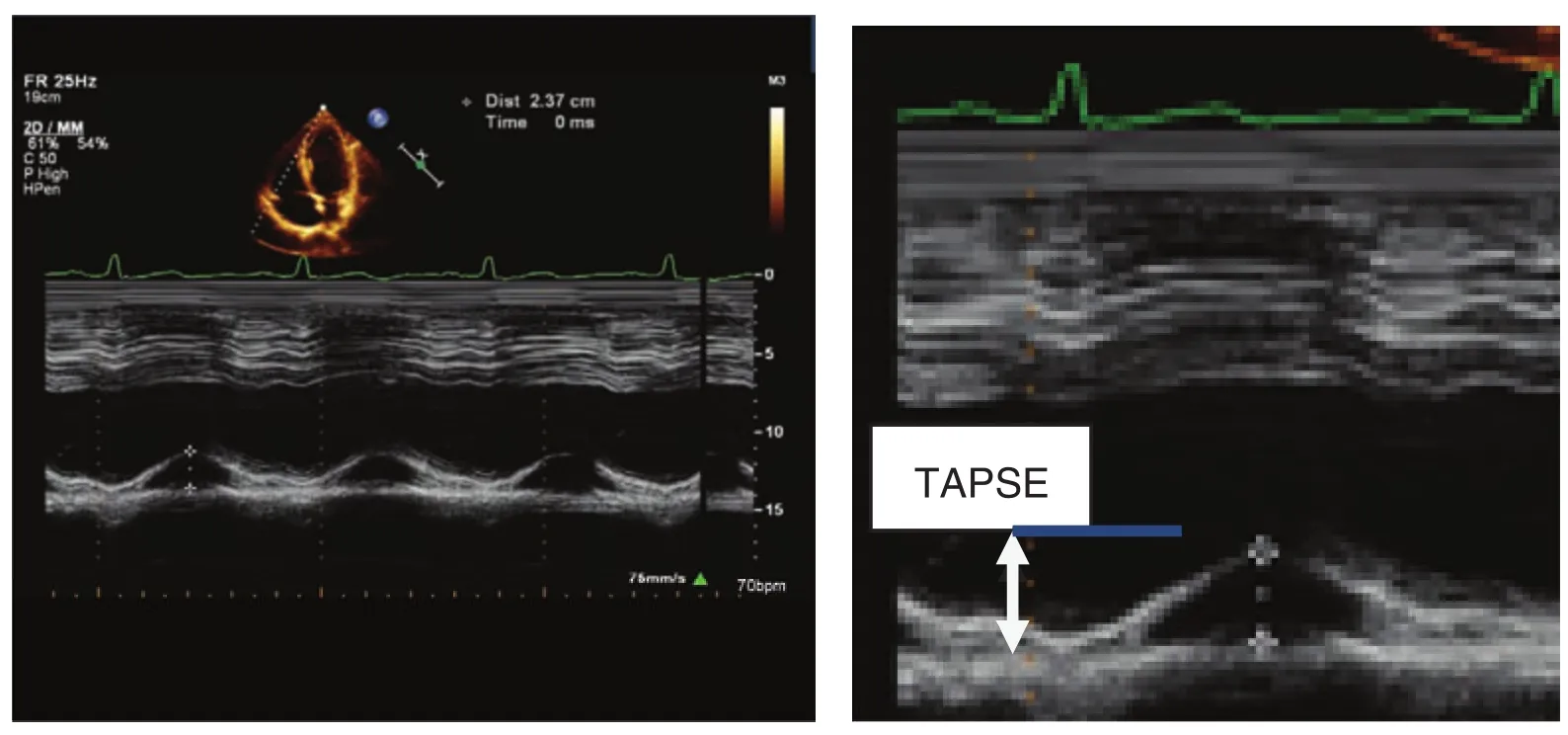
Figure 3 Tricuspid Annular Plane Systolic Excursion (TAPSE) M-mode Recording in a Patient with Preserved Right Ventricular Function.
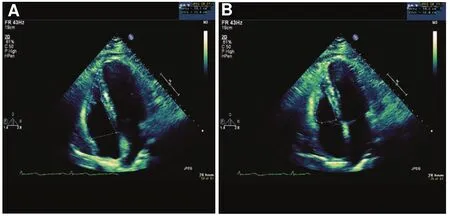
Figure 4 Fractional Area Change (FAC).
Peak Tricuspid Annular Systolic Velocity
A quick and reliable method of measuring RV systolic function is by measuring the peak tricuspid annular systolic velocity (S′). This is achieved by tissue Doppler imaging (Figure 5). Tissue Doppler imaging is superior to blood fl ow Doppler imaging as it directly ref l ects myocardial function and is less subjected to preload changes. Similarly to TAPSE,it does not take into account RV outf l ow tract contraction and septal contribution to RV ejection.S′less than 10 cm/s indicates RV systolic dysfunction.S′ has been shown to correlate well with other measures of global RV systolic function, regardless of pulmonary artery pressure [13].
Estimation of Pulmonary Artery Systolic Pressure
The estimation of pulmonary artery systolic pressure (PASP) via echocardiography is important in the treatment of any patient with heart failure. This entails one measuring the peak tricuspid regurgitant jet velocity using the simplif i ed Bernoulli equation and combining the measurement with the estimated RA pressure measurement. One can estimate the RA pressure by determining the diameter of the IVC and its inspiratory collapsibility (Figure 6).The PASP is equivalent to the RV systolic pressure in the absence of a gradient across the pulmonic valve or RV outf l ow tract.
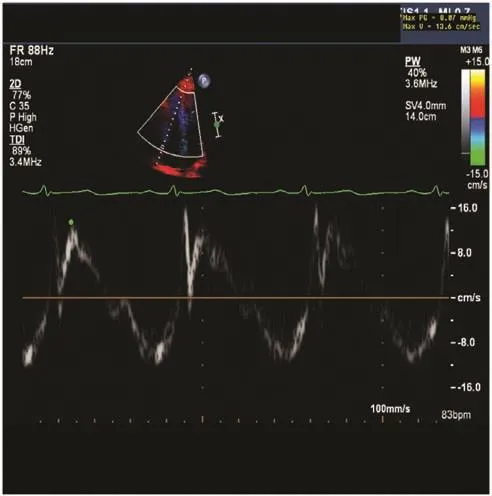
Figure 5 Peak Tricuspid Annular Systolic Velocity, S′.
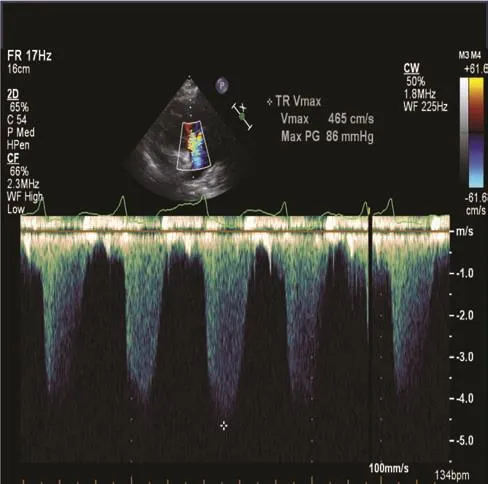
Figure 6 Doppler Echocardiographic Determination of Pulmonary Artery Systolic Pressure (PASP).
Spectral continuous-wave Doppler signal of tricuspid regurgitation corresponding to the right ventricular (RV)–right atrial (RA) pressure gradient. PASP was calculated as RV systolic pressure =4V2+ RA pressure, whereVis the peak velocity (in meters per second) of the tricuspid valve regurgitant jet, and RA pressure is estimated from the inferior vena cava diameter and respiratory changes. In this example, PASP is estimated as 86 + central venous pressure, or 101 mmHg, if RA pressure is assumed to be 15 mmHg.
The ASE recommends that the PASP should be estimated and reported in all subjects with reliable tricuspid regurgitant jets [7]. It is advised to obtain tricuspid regurgitation signals from several windows as velocity measurements are angle dependent. The signal with the highest velocity should be used in the calculation. If the signal is weak, it may be enhanced with agitated saline or blood-saline contrast. Careful attention of where the peak signal is measured should be confirmed by the echocardiographer to avoid overestimation of the spectral envelope, which would overestimate the PASP. There is the potential for error in the measurement of the estimated PASP in patients with severe tricuspid regurgitation. The tricuspid regurgitation spectral Doppler envelope may be cut off early (called the “V wave cut-off sign”) because of an early equalization of RV and RA pressures, which would underestimate the PASP calculated with the simplif i ed Bernoulli equation.
In practice, when one is assessing pulmonary hemodynamics via echocardiography, PASP is more commonly measured and reported than are the mean pulmonary artery pressure and PADP. Normal resting PASP corresponds to a peak tricuspid regurgitation gradient of 2.8 m/s or less to 2.9 m/s or a peak systolic pressure of 35 or 36 mmHg, assuming a RA pressure of 3 (0–5) mmHg [14]. It is recommended that further evaluation of patients with dyspnea without a known cause should be performed if the estimated RV systolic pressure is greater than 40 mmHg [15].
Estimating the Right Atrial Pressure
The subcostal view allows imaging and measurement of the IVC and assessment of the inspiratory collapsibility. According to the ASE guidelines [7],the diameter of the IVC should be measured just proximal to the entrance of hepatic veins at the end of expiration. The entrance of the hepatic vein usually lies approximately 0.5–3.0 cm proximal to the ostium of the right atrium. For simplicity and uniformity of reporting, the ASE also recommends that specific values of RA pressure, rather than ranges,should be used in the determination of SPAP. If the diameter of the IVC is less than 2.1 cm and collapses by more than 50% with a sniff, the RA pressure is then estimated to be normal and should be recorded as 3 mmHg (range 0–5 mmHg). If the diameter of the IVC is greater than or equal to 2.1 cm and collapses by more than 50% with a sniff, this suggests a high RA pressure of 15 mmHg (range 10–20 mm Hg). In scenarios where the IVC diameter and collapse do not fi t this paradigm, an intermediate value of 8 mmHg (range 5–10 mmHg) may be used or, preferably, other indices of RA pressure should be integrated to downgrade or upgrade the pressure to the normal, lower or higher value of RA pressure. If the IVC is greater than 2.1 cm in diameter and does not collapse at all with sniff, an RA pressure of 20 mmHg can be assigned. In normal young athletes, the IVC may be dilated in the presence of normal RA pressure. In addition, the IVC is commonly dilated and may not collapse in patients on ventilators. Caution should be exercised in these cases and others in an effort to not overestimate the RA pressure. Although a distended IVC usually denotes elevated RA pressures, in patients with otherwise normal examination results, reassessment of the IVC size and collapsibility in the left lateral position may be useful to avoid overestimation of the RA pressure.
Estimation of Pulmonary Artery Diastolic Pressure
PADP can be estimated from the end-diastolic pulmonary regurgitation velocity and the estimated RA pressure. This is done by use of the simplif i ed Bernoulli equation to quantify the pulmonary artery to RV gradient, PADP = 4 × (end-diastolic pulmonary regurgitant velocity)2+ RA pressure. The PADP increases disproportionately in pulmonary hypertension, causing a higher pressure gradient and, subsequently, an increased end diastolic regurgitant velocity. Ristow et al. found [16] that an increase in the pulmonary regurgitation end-diastolic pressure gradient of more than 5 mmHg was associated with RV systolic dysfunction, diastolic dysfunction, higher New York Heart Association functional class, lower metabolic equivalents achieved on treadmill testing,and elevated brain natriuretic peptide concentration.
Mean Pulmonary Artery Pressure
One can estimate the mean pulmonary artery pressure by measuring the pulmonary artery acceleration time (AT) using the Mahan formula,by using the peak early PR velocity, or one can derive it from the systolic and diastolic pulmonary pressures. It is recommended to use more than one method when one is estimating the mean pulmonary artery pressure:
Method 1, Mahan formula: mean pulmonary artery pressure = 79 – (0.45 × AT). In patients with ATs less than 120 ms, the following formula for the mean pulmonary artery pressure should be used instead [17]: mean pulmonary artery pressure = 90– (0.62× AT). AT is measured by pulsed wave (PW)Doppler echocardiography of the pulmonary artery in systole.
Method 2, peak early PR velocity [18]: 4 × (early PR velocity)2+ estimated RA pressure (Figure 7B).
Method 3, systolic and diastolic pulmonary pressures. Once systolic and diastolic pressures are known, the mean pulmonary artery pressure may be estimated by the standard formula: mean pulmonary artery pressure = 1/3(SPAP) + 2/3(PADP).
Diastolic Function
It is important to assess LV diastolic function in all echocardiographic studies as diastolic dysfunction develops quite early in many cardiac diseases and causes an elevation of LV filling pressures. Echocardiographic measurements of diastolic function can yield important prognostic information [19–28].
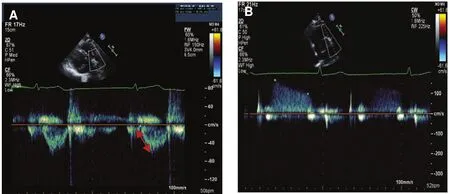
Figure 7 Measurement of Mean Pulmonary Artery Pressure by Means of Acceleration Time (AT) and Early PR Velocity.
Among all patients with heart failure, diastolic heart failure or heart failure with preserved ejection fraction is as prevalent as systolic heart failure or heart failure with reduced ejection fraction [29, 30].In patients with heart failure with preserved ejection fraction, the LV ejection fraction is normal or near normal but higher filling pressures are needed to obtain a normal end-diastolic volume of the left ventricle. Heart failure with preserved ejection fraction is increasing in prevalence with the aging of the population, and morbidity and mortality rates are comparable to those of heart failure with reduced ejection fraction [31–33]. Women are more often affected than men, especially patients with long-standing hypertension. Diastole in the cardiac cycle should not be regarded as a secondary process as it is as important as systole. Diastolic dysfunction tends to be chronic and can progress. It can be short term, as in the case of an acute myocardial infarction. Several techniques can be used to assess diastolic function,but echocardiography is the technique of choice.
There are many methods by which diastolic function can be assessed by echocardiography.This section focuses on the importance of mitral inf l ow, the changes with the Valsalva maneuver,pulmonary venous fl ow, tissue Doppler annular early and late diastolic velocities (tissue Doppler imaging), and isovolumic relaxation time (IVRT).Other means of assessing diastolic dysfunction including color M-mode fl ow propagation velocity, deformation measurements, LV untwisting,and diastolic stress test are beyond the scope of this article and further reading from the ASE guidelines is recommended.
Normal Diastolic Function and Mechanisms of Diastolic Dysfunction
Normal diastolic function is the complete and eff i -cient filling of the left ventricle at physiologic pressures. Diastole starts at aortic valve closure and includes LV pressure fall, rapid filling, diastasis,and atrial contraction. The following terms will be useful as they are universally used in describing diastolic function:
E wave: This is early LV diastolic filling. Following isovolumic relaxation, the mitral valve opens and most of LV filling occurs in the first third of diastole, during rapid early filling. This is due to elastic recoil and active relaxation of the left ventricle. It is measured by PW Doppler echocardiography.
A wave: Atrial systole contributes only a relatively small amount of LV filling. Therefore, the peak A wave velocity is less than that of the E wave in an individual with normal function.
Deceleration time: This is the interval from the peak of the E wave velocity to its extrapolation to the baseline. To evaluate E and A waves and the deceleration time, the PW Doppler sample volume is placed at the mitral valve leaf l et tips in the apical four-chamber view, and is then measured over at least three consecutive cardiac cycles (f i ve in atrial fi brillation).
E′ or e′: As the left ventricle expands to accommodate the inf l ow of blood, there is a simultaneously brisk motion of the mitral annulus. This process is recorded with tissue Doppler imaging. Only a minute amount of filling occurs in diastasis (mid diastole), the duration of which is dependent on heart rate. Mid diastole signifi cantly shortens or even disappears with increasing heart rate.
IVRT: This is the interval from aortic valve closure to mitral valve opening. It provides information on LA pressure and rate of early active LV relaxation. In general, it parallels the deceleration time. When relaxation is prolonged, mitral valve opening is delayed and IVRT is increased. Conversely, whenever LA pressure is elevated, mitral valve opening will occur earlier and IVRT will be shortened.
Pulmonary venous fl ow: The pulmonary vein systolic component (PVs) to pulmonary vein diastolic component (PVd) ratio is measured by PW Doppler echocardiography. Changes in the PVs/PVd ratio correlate with the stages of diastolic dysfunction. Further details regarding acquisition, obtaining measurements, normal values, clinical applications, and its limitations are discussed in more detail later.
Pulmonary venous fl ow: A – Ar is also measured by PW Doppler echocardiography. The difference in the duration of the two waves ref l ects LV filling pressure; where the retrograde pulmonary venous A wave duration (Ar) is typically shorter than the mitral A wave duration.
Further Understanding of Diastolic Dysfunction
The mitral E-wave velocity is related directly to the LA pressure and inversely to LV relaxation. In patients who have systolic heart failure, increased filling pressure (high LA pressure) and reduced LV relaxation coexist, so the E-wave velocity alone correlates poorly with mean LA pressure. One can more accurately estimate LA pressure (surrogate of mean wedge pressure) by correcting the E-wave velocity for any abnormal LV relaxation. The peak early diastolic mitral annular velocity (E′) on tissue Doppler imaging has been validated as measures of LV relaxation and has been combined with E-wave velocity to estimate pulmonary capillary wedge pressure [34]. Early diastolic motion of the mitral annulus is inf l uenced by the motion of longitudinally oriented myocardial fi bers. The lengthening of these fi bers in diastole results in mitral annular descent toward a relatively fi xed apex. The velocity of early mitral annular (E′) descent ref l ects LV relaxation and is independent of LA pressure. As the E-wave velocity is determined by the LV relaxation and LA pressure, correction of the E-wave velocity for LV relaxation is paramount. To obtainE′andA′ tissue velocities, images are recorded from the apical four-chamber view with the sample volume placed 1–2 cm within the mitral annulus; septal then lateral or vice versa. The ASE recommends use of the averageE′ velocity obtained from the septal and lateral sides of the mitral annulus for the prediction of LV filling pressures [35].
Normal Parameters
E/A ratio: Normal 1.1–1.5
Deceleration time: 160–240 ms. May be lower in the young.
E/E′ ratio: normal. A septalE/E′ ratio of less than 8 is associated with normal LV filling pressures [36].
IVRT: 76 ± 13 ms (>40 years); 69 ± 12 ms (<40 years)Pulmonary vein A wave fl ow reversal: <25 cm/s
PVs2 ≥ PVd (PVs2 may be lower than PVd in the young)
No anatomic abnormalities
Stages of Diastolic Dysfunction
Diastolic dysfunction progresses as a continuum from a mild (grade 1) stage to a more advanced stage, eventually becoming irreversible (grade 4) if the underlying disease process is left untreated. Not all patients progress from one grade to another linearly along the pathway. In many cases, reversal to a lower grade can be achieved with optimization of medical treatment,such as weight loss, good blood pressure control, or use of diuretic therapy to decrease preload. These stages have certain pathophysiologic characteristics,which are summarized below (Figures 8 and 9).
Abnormal Function
Three patterns or stages indicate abnormal diastolic filling. In addition, there is fourth stage, which has the same pattern as stage III but is termed “irreversible restrictive.”
Stage I: Impaired Early Left Ventricular Relaxation
Reduced LV filling in early diastole.
Signif i cance: Impaired (slow) early LV relaxation.
Signs and symptoms: None at rest.
Functional status: Mild impairment.
Left atrium: Usually normal dimension (may be hypercontractile).
Mitral fl ow velocity during atrial systole is increased. The auscultatory equivalent is S4.
Filling pressure,E/E′ ratio: usually normal at rest.
PW Doppler fi ndings:
E/Aratio less than 1.0 (A wave is now larger through a combination of increased atrial preload and a more forceful atrial contraction)
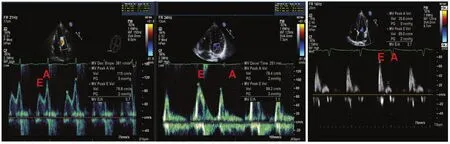
Figure 8 Transmitral Inf l ow Doppler Spectral Patterns.
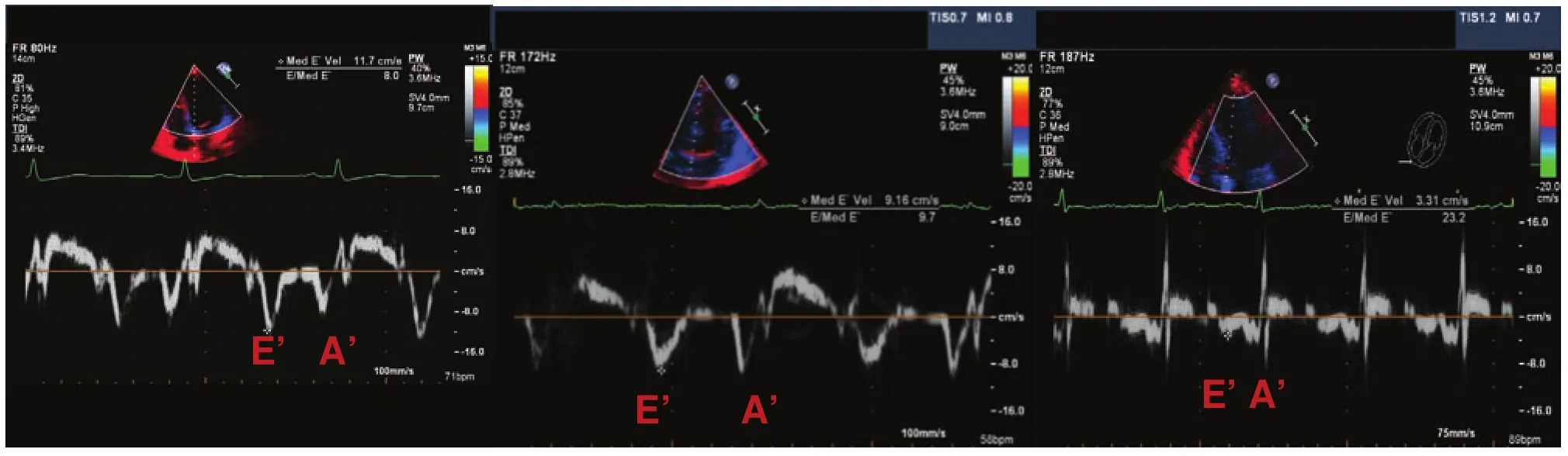
Figure 9 Mitral Annulus Tissue Doppler Imaging Patterns, where E′ is the Early Annulus Velocity and A′ the is Late Annulus Velocity.
Deceleration time longer than 240 ms
IVRT longer than 90 ms
Pulmonary vein atrial (PVa) velocity less than 25 cm/s
PVs2 >> PVd
Stage II: Pseudonormalization
Signif i cance: Suggests impaired (slow) early LV relaxation with decreased LV compliance.
Signs and symptoms: Exertional dyspnea.
Functional status: May have moderate impairment.
*Left atrium: Enlarged and hypocontractile.1An asterisk indicates echocardiographic features which can differentiate normal from pseudonormal.
*E′ is usually less than 7 cm/s.
*Filling pressures, septalE/E′: may be increased.A ratio greater than 15 is associated with increased filling pressures [36].
PW Doppler fi ndings:
E/Aratio 1.0–1.5
Deceleration time 160–240 ms
IVRT 76 ± 13 ms (>40 years), 69 ± 12 ms (< 40 years)
*PVa velocity greater than 25 cm/s; may be large
*PVs2 < PVd
At stage II, the effects of impaired early LV relaxation on early diastolic filling are now opposed by the elevated left atrial (LA) pressure, and the early diastolic transmitral pressure gradient and mitral fl ow velocity pattern return to normal. This stage is referred to as “ pseudonormalization,” indicating that although the mitral inf l ow pattern appears normal, LV filling pressures are elevated. In addition,in many patients, LV end- diastolic filling pressure is elevated and there is 2D echocardiographic evidence of structural heart disease such as an increase in LA size, decreased ejection fraction, or LV hypertrophy. A decrease in preload such as that resulting from the patient performing the Valsalva maneuver may be able to unmask the underlying impaired LV relaxation in patients with true stage II diastolic dysfunction.
Stage III: “Restrictive” Filling Pattern(Reversible)
Signif i cance: Severe decrease in LV compliance and impaired (slow) early LV relaxation
Signs and symptoms: Dyspnea on minimal exertion
Functional status: Marked impairment
Left atrium: Enlarged and hypocontractile
Filling pressures,E/E′: Markedly increased
PW Doppler fi ndings:
E/A ratio greater than 2.
Deceleration time less than 160 ms.
IVRT less than 60 ms.
PVa velocity 35 cm/s or greater. This occurs because pulmonary venous fl ow during systole is greatly reduced compared with diastolic fl ow (variable,depending on atrial systolic function).
PVs2 << PVd.
Ar- A greater than 30 ms. Retrograde pulmonary venous A-wave duration (Ar) is typically longer than the mitral A-wave duration, consistent with elevated filling pressures.
Mitral annulusE′ is reduced to less than 7 cm/s and may even be as low as 5 cm/s or less.
E/E′ is usually greater than 15.
Stage III represents a severe decrease in LV chamber compliance as there is further deterioration in diastolic function. Diastolic filling pressures are elevated. Patients are markedly symptomatic and demonstrate a severely reduced functional capacity. Despite the presence of impaired LV relaxation, the markedly elevated LA pressure results in a high velocity of early diastolic filling, which stops abruptly because of an abnormally rapid rise in ventricular pressure and atrial dysfunction. In some patients, this stage may be reversible. The stage could revert to impaired LV relaxation or the pseudonormalization stage by any intervention which lowers the LA pressure and reduces the LA-LV pressure gradient such as preload reduction with diuresis. As expected,there is 2D echocardiographic evidence of structural heart disease.
Stage IV: “Restrictive” Filling Pattern(Irreversible)
The diastolic pattern can become irreversible, but technically speaking with the advent of the continuous-f l ow LVADs and their improvement in systolic and diastolic function [37, 38], this pattern could become reversible after some time. This is due to the impact of continuous-f l ow LVADs on myocardial unloading and remodeling improving hemodynamics over a period of time [39–41].
In the grade 4 pattern, preload reduction or Valsalva maneuver no longer leads to an improvement in the filling pattern. Volume management in these patients is diff i cult as maintaining a balance between volume overload and hypoperfusion provides a challenge that is tedious for even the most seasoned clinician. If the restrictive filling pattern does not change with the Valsalva maneuver, reversibility cannot be excluded because the Valsalva maneuver may not be adequate or filling pressure may be too high to be altered by the maneuver.
Pulmonary Venous Flow Patterns
Measurement of the pulmonary vein fl ow velocity is performed in the apical four-chamber view with PW Doppler echocardiography. It aids in the assessment of LV diastolic function [42]. It is recorded at the junction of the pulmonary veins and left atrium with color fl ow imaging being useful for the proper location of the sample volume in the right upper pulmonary vein. A 2 to 3-mm sample volume is placed about 5 mm into the pulmonary vein and superior angulation is often required for optimal recording of the spectral waveforms. Measurements should be taken over three consecutive cycles at the end of expiration.
Measurements
Pulmonary venous fl ow consists of three main components: a peak systolic anterograde (PVs) wave,a peak anterograde diastolic (PVd) velocity and the peak retrograde wave (Ar) in late diastole, corresponding to atrial systole. The ratio of the peak anterograde velocities in systole and diastole is reported as the PVs/PVd ratio (the normal value is greater than 1). There are two systolic velocities(PVs1 and PVs2), which are most noticeable when there is a prolonged PR interval as in the case of bradycardia or first-degree atrioventricular block.In such cases, PVs2 should be used to compute the ratio of peak systolic to peak diastolic velocity [43,44]. A decrease in LA compliance and an increase in LA pressure decrease the PVs velocity and increase the PVd velocity, resulting in a PVs/PVd ratio less than 1. Shortening of the deceleration time of the PVd velocity to less than 150 ms occurs as pulmonary capillary wedge pressure increases [45]. The peak velocity and duration of the pulmonary vein atrial fl ow reversal increase with higher LV enddiastolic pressure [46]. For patients in atrial fi brillation, there is blunting of the PVs wave (as PVs1 becomes lost and PVs2 is usually smaller than that of the PVd wave) and absence of the Ar velocity.
Normal Values and Clinical Applications
Pulmonary venous inf l ow velocities (Figure 10) are inf l uenced by age. Normal young subjects younger than 40 years usually have prominent PVd velocities, ref l ecting their mitral E waves.
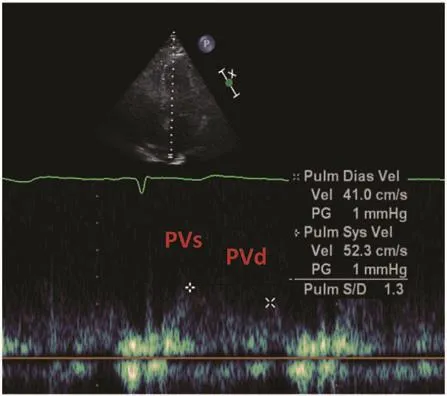
Figure 10 Pulmonary Venous Flow by Doppler Echocardiography, where PVs is Peak Systolic Anterograde Velocity and PVd is Peak Diastolic Anterograde Velocity.
With increasing age, the PVs/PVd ratio increases.In normal subjects, Ar velocities can increase with age but usually do not exceed 35 cm/s. A higher value suggests increased LV end-diastolic pressure[47]. The Ar – A duration difference is useful as it is the only age-independent indication of LV A-wave pressure increase [47]. This increase in LV enddiastolic pressure is the first hemodynamic abnormality seen with diastolic dysfunction.
The Left Atrium
In the assessment of LV diastolic function, measurement of LA size and volume is important (Table 2).As the left atrium remodels and enlarges, in many cases diastolic dysfunction becomes more evident.LA size should be measured at the end of LV systole in the cardiac cycle, when it is at its greatest dimension. The most widely used linear dimension is the LA anteroposterior (AP) measurement in the parasternal long-axis view with M-mode echocardiography or, preferably, 2D echocardiography.Measurement of LA size can provide prognostic information [48–51]. When one is acquiring images to measure LA size and volumes, care should be taken to avoid foreshortening of the left atrium. One corrects the effect of different body surface areas on LV volume with the use of an indexed valueby dividing LA volume by the patient′s body surface area. The newly recommended upper normal indexed LA volume, regardless of age and sex, is 34 mL/m2[4]. An LA volume index of 34 mL/m2or greater is an independent predictor of death, heart failure, atrial fi brillation, and ischemic stroke [52].LA volume ref l ects the cumulative effects of filling pressures over time. The most accurate measurements are obtained via the apical four-chamber view (Figure 11) and two-chamber view [4].

Table 2 Normal Measurements of the Left Atrium.
When one is assessing the LA size, measurement of LA volume is recommended over measurement of LA size as LA volume has been shown to be a better prognostic variable in a variety of cardiac disease states than measurement of LA anteroposterior diameter [53, 54] or LA area. As the biplane disk summation technique incorporates fewer geometric assumptions, it should be the preferred method to measure LA volume in clinical practice. The ASE recommends use of this method [52].
Right Atrial Measurements
The right atrium can be visualized in several views. Its size and function are not as well studied as those of the other chambers. It is commonly enlarged in patients with disorders which cause RV volume and pressure overload and RV failure.For clinical purposes, the visual comparison of RA to LA size in the apical four-chamber view is most commonly used to assess if there is RA enlargement. Several recent studies have provided normal values of RA dimensions for men and women [55, 56]. Measurement of RA volume can be performed, but its value is underestimated with 2D echocardiographic techniques compared with 3D echocardiography [57, 58]. The ASE recommends the single-plane area-length or disk summation techniques in a dedicated apical fourchamber view (Figure 11) when one is assessing RA size and RA volume [4]. The normal ranges for 2D echocardiographic RA volume are 25 ±7 mL/m2in men and 21 ± 6 mL/m2in women.
In patients presenting with heart failure symptoms who are found to have a preserved ejection fraction, valvular heart disease, constrictive pericarditis (diastolic pericardial heart failure), and restrictive cardiomyopathy should be considered as well.
Conclusion and Take-Home Message
Heart failure is a complex syndrome characterized by progressive decline in the function of the left ventricle, low exercise tolerance, and increased mortality and morbidity. LV diastolic dysfunction plays a major role in heart failure and in the progression of most cardiac diseases.
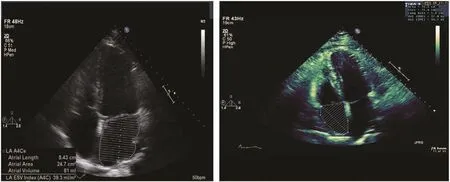
Figure 11 Volume Measurement of the Left Atrium (left) and Right Atrium (right) in the Apical Four-chamber View.
Use of echocardiography to assess diastolic dysfunction in these patients can assist with guiding their medical treatment. In addition, understanding how the right side of the heart functions is crucial. Many of these patients will develop pulmonary hypertension over a period of time; the echocardiogram is a reliable noninvasive tool for this assessment, but it is time to go beyond the LV ejection fraction.
Conflict of interest
The authors declare no conf l ict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or notfor-profit sectors.
1. Amaki M, Nakatani S, Kanzaki H,Kyotani S, Nakanishi N, Shigemasa C, et al. Usefulness of threedimensional echocardio graphy in assessing right ventricular function in patients with primary pulmonary hypertension. Hypertens Res 2009;32(5):419–22.
2. Freixa X, Portillo K, Paré C,Garcia-Aymerich J, Gomez FP,Benet M, et al. Echocardiographic abnormalities in patients with COPD at their first hospital admission. Eur Respir J2013;41(4):784–91.
3. Karas MG, Kizer JR. Echocardiographic assessment of the right ventricle and associated hemodynamics. Prog Cardiovasc Dis 2012;55(2):144–60.
4. Lang RM, Badano LP, Mor-Avi V,Af i lalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantif i cation by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28(1):1–39.
5. Maffessanti F, Muraru D, Esposito R, Gripari P, Ermacora D, Santoro C, et al. Age-, body size-, and sexspecific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging2013;6(5);700–10.
6. Le Tourneau T, Piriou N, Donal E,Deswarte G, Topilsky Y, Lamblin N,et al. Imaging and modern assessment of the right ventricle. Minerva Cardioangiol2011;59(4):349–73.
7. Rudski LG, Lai WW, Af i lalo J,Hua L, Handschumacher MD,Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology,and the Canadian Society of Echocardiography. J Am Soc Echocardiogra 2010;23(7):685–713.
8. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease,part I: Anatomy, physiology, aging,and functional assessment of the right ventricle. Circulation2008;111(17):1436–48.
9. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease,part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation2008;117(13):1717–31.
10. Naeije R, Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev 2014;23(134):476–87.
11. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM,Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J echocardiogra 2011;12(3):167–205.
12. Scalia GM, McCarthy PM, Savage RM, Smedira NG, Thomas JD.Clinical utility of echocardiography in the management of implantable ventricular assist devices.J Am Soc Echocardiogr 2000;13:754–63.
13. Saxena N, Rajgopalan N, Edelman L, Lopez-Candales A. Tricuspid annular systolic velocity: a useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography. 2006;23(9):750–5.
14. Badesch DB, Champion HC,Sanchez MA, Hoeper MM, Loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54 Suppl 1:S55–66.
15. McLaughlin VV, Archer SL,Badesch DB, Barst RJ, Farber HW,Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association:developed in collaboration with the American College of Chest Physicians, American Thoracic Society,Inc., and the Pulmonary Hypertension Association. Circulation 2009:119(16):2250–94.
16. Ristow B, Ahmed S, Wang L, Liu H, Angeja BG, Whooley MA,et al. Pulmonary regurgitation end- diastolic gradient is a Doppler marker of cardiac status: data from the Heart and Soul Study. J Am Soc Echocardiogr2005;18(9):885–91.
17. Dabestani A, Mahan G, Gardin JM,Takenaka K, Burn C, Allf i e A, et al.Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol1987:59(6):662–8.
18. Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA,Lester SJ. Echocardiographic determination of mean pulmonary artery pressure. Am J Cardiol 2003;92(11):1373–6.
19. Faris R, Coats AJ, Henein MY.Echocardiography-derived variables predict outcome in patients with nonischemic dilated cardiomyopathy with or without a restrictive filling pattern. Am Heart J 2002;144(2):343–50.
20. Whalley GA, Doughty RN,Gamble GD, Wright SP, Walsh HJ,Muncaster SA, et al. Pseudonormal mitral filling pattern predicts hospital re-admission in patients with congestive heart failure. J Am Coll Cardiol2002;39(11):1787–95.
21. Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET,et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation 2002;105(16):1928–33.
22. Dini FL, Michelassi C, Micheli G, Rovai D. Prognostic value of pulmo nary venous fl ow Doppler signal in left ventricular dysfunction: contribution of the difference in duration of pulmonary venous and mitral fl ow at atrial contraction. J Am Coll Cardiol 2000;36(4):1295–302.
23. Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M,et al. Usefulness of tissue Doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study). Am J Cardiol 2005;96(2):257–62.
24. Hillis GS, Moller JE, Pellikka PA,Gersh BJ, Wright RS, Ommen SR,et al. Noninvasive estimation of left ventricular filling pressure by E/E′is a powerful predictor of survival after acute myocardial infarction.J Am Coll Cardiol2004:43(3):360–7.
25. Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, et al. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J Hypertens2005;23(1):183–91.
26. Sharma R, Pellerin D, Gaze DC,Mehta RL, Gregson H, Streather CP, et al. Mitral peak Doppler E-wave to peak mitral annulus velocity ratio is an accurate estimate of left ventricular filling pressure and predicts mortality in end-stage renal disease. J Am Soc Echocardiogr2006; 19(3):266–73.
27. Okura H, Takada Y, Kubo T, Iwata K, Mizoguchi S, Taguchi H, et al.Tissue Doppler-derived index of left ventricular filling pressure,E/E′, predicts survival of patients with non-valvular atrial fi brillation.Heart2006;92(9);1248–52.
28. Bruch C, Klem I, Breithardt G,Wichter T, Gradaus R. Diagnostic usefulness and prognostic implications of the mitral E/E′ ratio in patients with heart failure and severe secondary mitral regurgitation. Am J Cardiol 2007;100(5):860–5.
29. Redf i eld MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community:appreciating the scope of the heart failure epidemic. J Am Med Assoc 2003;289(2):194–202.
30. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC.Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol2006:47(1);76–84.
31. Heart Failure Society of America,Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, et al.HFSA 2010 comprehensive heart failure practice guideline. J Card Fail 2010:16(6);1–194.
32. Owan TE, Hodge DO, Herges RM,Jacobsen SJ, Roger VL, Redf i eld MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med2006:355(3):251–9.
33. Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al.Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood institute.Circulation2009:119(24);3070–7.
34. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA.Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol1997:30(6);1527–33.
35. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr.2009;22(2):107–33.
36. Ommen SR, Nishimura RA,Appleton CP, Miller FA, Oh JK,Redf i eld MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study.Circulation. 2000; 102(15):1788–94.
37. Drakos SG, Wever-Pinzon O,Selzman CH, Gilbert EM,Alharethi R, Reid BB, et al.Magnitude and time course of changes induced by continuousflow left ventricular assist device unloading in chronic heart failure:insights into cardiac recovery. J Am Coll Cardiol2013:61(19);1985–94.
38. Nair N, Thotakura S, Gongora E. Do continuous fl ow LVADs improve diastolic dysfunction.Journal of Organ Transplant Surgery 2014:4;23–28.
39. Gupta S, Woldendorp K, Muthiah K, Robson D, Prichard R,Macdonald PS, et al. Normalisation of haemodynamics in patients with end-stage heart failure with continuous-f l ow left ventricular assist device therapy. Heart Lung Circ2014:23(10);963–9.
40. Topilsky Y, Oh JK, Atchison FW,Shah DK, Bichara VM, Schirger JA, et al. Echocardiographic fi ndings in stable outpatients with properly functioning HeartMate II left ventricular assist devices. J Am Soc Echocardiogr 2011:24(2);157–69.
41. John R, Liao K, Kamdar F, Eckman P, Boyle A, Colvin-Adams M.Effects on pre- and posttransplant pulmonary hemodynamics in patients with continuous-f l ow left ventricular assist devices. J Thorac Cardiovasc Surg2010:140(2);447–52.
42. Appleton CP, Jensen JL, Hatle LK, Oh JK. Doppler evaluation of left and right ventricular diastolic function: a technical guide for obtaining optimal fl ow velocity recordings. J Am Soc Echocardiogr 1997:10(3):271–92.
43. Smiseth OA, Thompson CR,Lohavanichbutr K, Ling H, Abel JG, Miyagishima RT, et al. The pulmonary venous systolic fl ow pulse – its origin and relationship to left atrial pressure. J Am Coll Cardiol1999:34(3);802–9.
44. Keren G, Bier A, Sherez J, Miura D, Keefe D, LeJemtel T. Atrial contraction is an important determinant of pulmonary venous fl ow. J Am Coll Cardiol1986:7(3);693–5.
45. Yamamuro A, Yoshida K, Hozumi T, Akasaka T, Takagi T, Kaji S,et al. Noninvasive evaluation of pulmonary capillary wedge pressure in patients with acute myocardial infarction by deceleration time of pulmonary venous fl ow velocity in diastole. J Am Coll Cardiol 1999:34(1);90–4.
46. Yamamoto K, Nishimura RA,Burnett JC Jr, Redf i eld MM.Assessment of left ventricular enddiastolic pressure by Doppler echocardiography: contribution of duration of pulmonary venous versus mitral fl ow velocity curves at atrial contraction. J Am Soc Echocardiogr1997:10(1);52–9.
47. Klein AL, Tajik AJ. Doppler assessment of pulmonary venous fl ow in healthy subjects and in patients with heart disease. J Am Soc Echocardiogr1991:4(4);379–92.
48. Benjamin EJ, D’Agostino RB,Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study.Circulation1995:92(4);835–41.
49. Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population.Stroke1999:30(10);2019–24.
50. Dini FL, Cortigiani L, Baldini U,Boni A, Nuti R, Barsotti L, Micheli G. Prognostic value of left atrial enlargement in patients with idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. Am J Cardiol2002;89(5):51–24.
51. Rusinaru D, Tribouilloy C, Grigioni F, Avierinos JF, Suri RM, Barbieri A, et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to fl ail leaf l ets:results from a large international multicenter study. Circ Cardiovasc Imaging2011:4(5);473–81.
52. Abhayaratna WP, Seward JB,Appleton CP, Douglas PS, Oh JK,Tajik AJ, et al. Left atrial size:physiologic determinants and clinical applications. J Am Coll Cardiol 2006:47(12);2357–63.
53. Tsang TS, Barnes ME, Bailey KR,Leibson CL, Montgomery SC,Takemoto Y, et al. Left atrial volume: important risk marker of incident atrial fi brillation in 1655 older men and women. Mayo Clinic Proc 2001:76(5):467–75.
54. Pritchett AM, Jacobsen SJ,Mahoney DW, Rodeheffer RJ,Bailey KR, Redf i eld MM. Left atrial volume as an index of left atrial size: a population-based study.J Am Coll Cardiol2003:41(6):1036–43.
55. Kou S, Caballero L, Dulgheru R,Voilliot D, De Sousa C, Kacharava G, et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 2014:15(6);680–90.
56. Peluso D, Badano LP, Muraru D, Dal Bianco L, Cucchini U,Kocabay G, et al. Right atrial size and function assessed with threedimensional and speckle-tracking echocardiography in 200 healthy volunteers. Eur Heart J Cardiovasc Imaging 2013:14(11);1106–14.
57. Aune E, Baekkevar M, Roislien J,Rodevand O, Otterstad JE. Normal reference ranges for left and right atrial volume indexes and ejection fractions obtained with realtime three-dimensional echocardiography. Eur J Echocardiogr 2009:10(6);738–44.
58. Quraini D, Pandian NG, Patel AR. Three-dimensional echocardiographic analysis of right atrial volume in normal and abnormal hearts: comparison of biplane and multiplane methods. Echocardiography2012:29(5);608–13.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Mechanical Circulatory Support for the Failing Heart: Which Device to Choose
- Continuous Flow Left Ventricular Assist Device Therapy: A Focused Review on Optimal Patient Selection and Long-Term Follow-up Using Echocardiography
- Cardiac Resynchronization Therapy in 2015:Lessons Learned
- Pulmonary Arterial Hypertension and the Failing Ventricle: Getting It Right
- Noninvasive Hemodynamic Monitoring for Heart Failure: A New Era of Heart Failure Management
- Epidemiological Study of Heart Failure in China