Pulmonary Arterial Hypertension and the Failing Ventricle: Getting It Right
2015-05-22StacyMandrasMDSylviaOleckMDandHectorVenturaMD
Stacy A. Mandras, MD, Sylvia Oleck, MD and Hector O. Ventura, MD
1John Ochsner Heart and Vascular Institute, Department of Cardiovascular Diseases, Ochsner Clinical School-The University of Queensland School of Medicine, 1514 Jefferson Highway, New Orleans, LA 70121-2483, USA
Introduction
The World Health Organization has classif i ed the causes of pulmonary hypertension into fi ve groups(Table 1). Pulmonary arterial hypertension (PAH)is a progressive disease characterized by increased pulmonary vascular resistance (PVR) and pulmonary arterial pressures (PAP) [2]. These changes eventually lead to right ventricular (RV) failure(RVF), which is the main cause of death in PAH patients.
In this article, we focus on RVF in the setting of World Health Organization group 1 and group 4 PAH. We will review the pathophysiology of RVF,the noninvasive imaging techniques used to assess RVF, prognostic indicators in RVF, and the medical and surgical/interventional treatment options for RVF.
Pathophysiology of the Failing Right Ventricle
The normal right ventricle is thinner and more compliant than the left ventricle [3]. Because of the normally low vascular resistance in the lungs, RV stroke volume is the same as left ventricular (LV)stroke volume. The right ventricle initially responds to the increase in PVR in PAH by first increasing RV wall thickness, followed by RV contractile dysfunction [3]. Chamber dilation occurs to maintain preload and stroke volume in the face of a reduction in RV fractional shortening. As the right side of the heart gets weaker, clinical symptoms of RVFdevelop because of rising filling pressures, diastolic dysfunction, and a declining cardiac output [3].
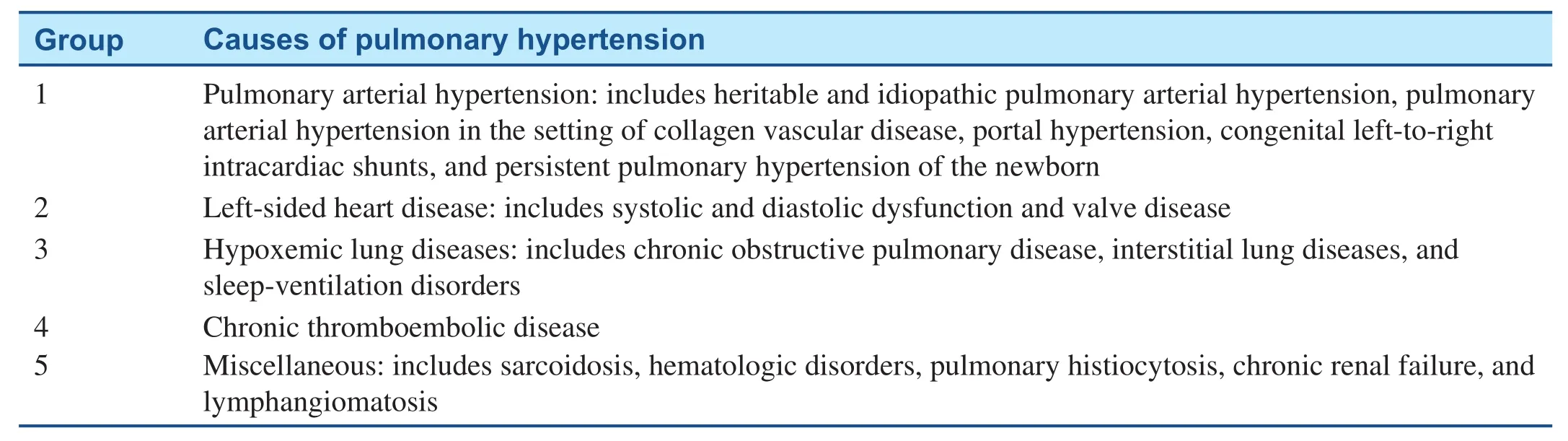
Table 1 World Health Organization Classification of Pulmonary Arterial Hypertension.
The earliest symptom of right ventricular heart failure is decreased exercise tolerance, which carries a strong prognostic predictive value of decreased survival [2, 4]. Subsequently, patients develop volume retention, syncope, renal disease, hyponatremia, and in more advanced cases cirrhosis [2].
The clinical decline is exacerbated by worsening tricuspid regurgitation, which occurs because of dilation of the tricuspid valve annulus and poor leaflet coaptation [3]. Interventricular dependence also occurs, in which the enlarged, pressure- overloaded right ventricle interferes with filling of the left ventricle.
RV function is dependent on the severity of the pulmonary vascular disease as well as the interaction of coronary perfusion, neurohormonal activation, immunologic activation, and myocardial metabolic effects [2]. These factors play a signif i cant role in affecting ventricular remodeling, contractility, afterload, preload, and ventricular interdependence of the right ventricle [2].
Two types of RV remodeling are seen in patients with PAH. This is determined on the basis of morphometric and molecular characteristics and identif i ed as adaptive or maladaptive remodeling [2]. Adaptive remodeling involves concentric hypertrophy with a higher mass-to-volume ratio, whereas maladaptive is more eccentric in nature and is associated with severer systolic and diastolic dysfunction (seen in connective tissue disease and idiopathic PAH) [2].
“Ventriculoarterial coupling” is the term that describes the ability of the right ventricle to increase contractility to adapt to increased afterload [2]. This is measured via conductance catheterization, and is the ratio of the ventricular elastance and arterial elastance, which is assumed to normally be between 1.5 and 2 on the basis of initial studies involving the left ventricle. This corresponds to the mechanical work of the right ventricle and its associated oxygen consumption[2]. As right-sided heart failure advances, ventriculoarterial coupling worsens, and the contractility of the right ventricle overcomes the increasing afterload, which leads to the maladaptive remodeling previously described [3, 5].
There are several forces at play when the right ventricle is chronically overloaded and begins to display maladaptive remodeling, including changes in myocardial metabolism, increased levels of reactive oxygen particles, neurohormonal activation, a decrease in mitochondrial activity, and activation of apoptotic pathways [2]. All of these changes subsequently lead to increased fi brosis, diastolic and systolic dysfunction, and eventual failure, as shown in Figure 1.
Noninvasive Investigation of the Right Side of the Heart
There have been signif i cant advances in the noninvasive imaging modalities that are used to evaluate and assess the right ventricle. These different imaging modalities, including echocardiography, magnetic resonance imaging (MRI), computed tomography(CT), and positron emission tomography (PET),allow us to determine the global RV function and to evaluate both the cellular and molecular changes which can be seen in RV failure [6].
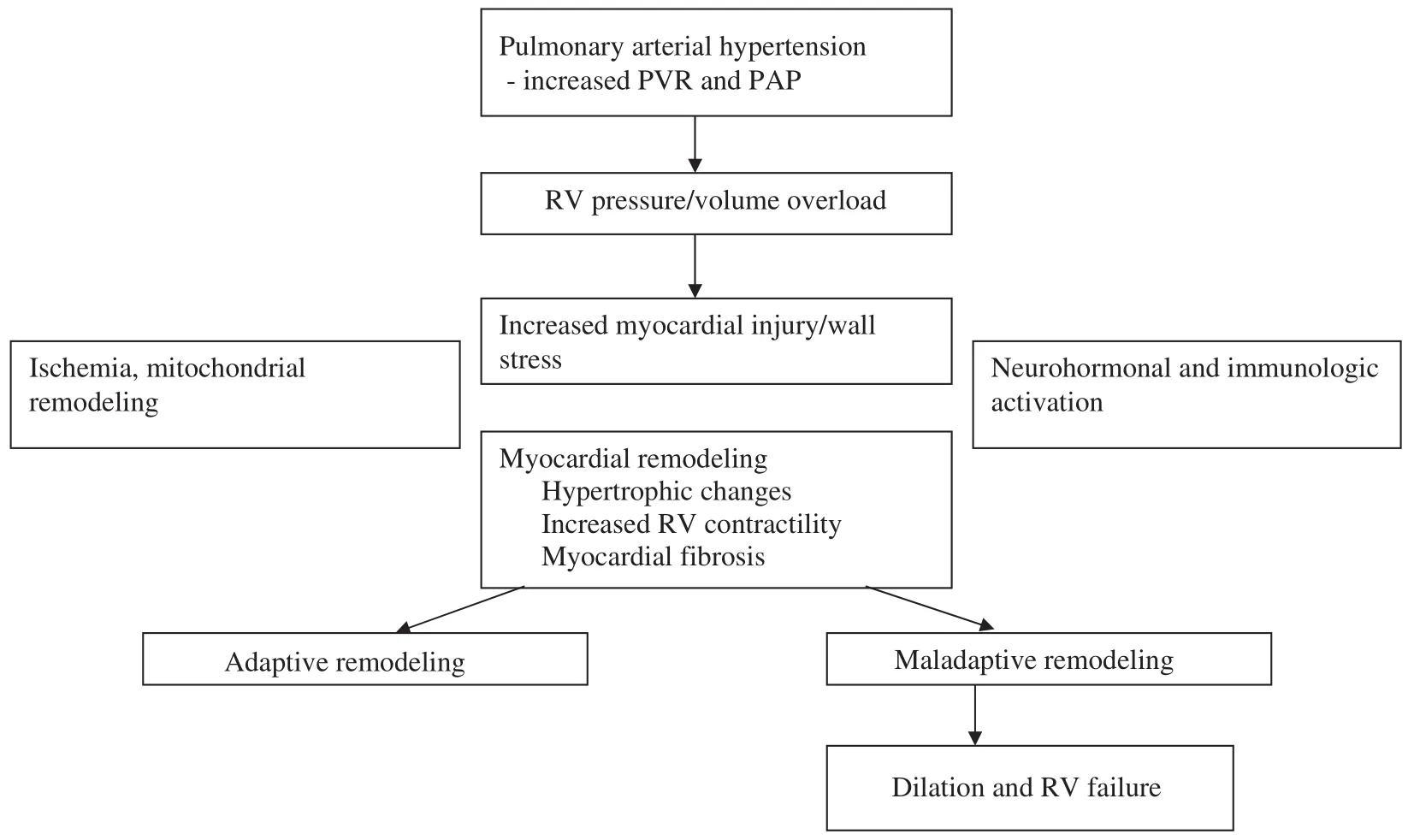
Figure 1 Pathophysiology of Right Ventricular (RV) Failure.
Echocardiography
Transthoracic echocardiography is currently the most commonly used noninvasive imaging modality for the assessment of the right ventricle.Echocardiography allows visualization of RV morphology, hemodynamics, and function [7].
With use of multiple cross-sectional planes, the degree of right-sided chamber enlargement can be quantif i ed. In addition, assessment of the size and collapsibility of the inferior vena cava allows right atrial pressure to be estimated. PAP (systolic, mean,and diastolic) are estimated by Doppler measurements of the tricuspid regurgitant jet, pulmonic regurgitant jet, and RV outf l ow tract.
Because of the triangular shape of the right ventricle, quantif i cation of RV function is less accurate with echocardiography; however, with good acoustic windows, a qualitative assessment is possible.However, other echocardiographic parameters are used to estimate RV function; tricuspid annular plane systolic excursion (TAPSE), RV myocardial performance index (Tei index), RV fractional area of change (FAC), and RV strain.
TAPSE is usually obtained by tissue Doppler imaging or 2D speckle imaging and measures the displacement of the lateral annulus of the tricuspid valve toward the RV apex, and is a measure of RV systolic function. A normal TAPSE is greater than 20 mm. The sensitivity and specificity for RV dysfunction with a TAPSE of less than 15 mm are 100% and 41%, respectively [7].
The myocardial performance index is a measure of global ventricular function, both systolic and diastolic. It is the sum of the isovolumic contraction and relaxation times divided by the ejection time. In RV dysfunction, the myocardial performance index increases because of the lengthening of the isovolumic times and shortening of the contraction times.
One measures the RV FAC by tracing the endocardial border of the right ventricle in end systole and end diastole. It is normally less than 40% and is equal to 100 × [end-diastolic area – end-systolic area]/end-diastolic area] [7]. RV FAC is less precise than other measures of RV function because of imprecise identif i cation of endocardial borders owing to the trabecular nature of the right ventricle,but with improved echocardiography software and contrast agents the accuracy of FAC has improved.
RV strain and strain rate are obtained by tissue Doppler imaging or 2D speckle imaging and are measures of myocardial deformation. RV free wall strain may be used to estimate RV contractile function [7].
As the echocardiographic measurements of the right ventricle tend to be load dependent, it is important to use several of these variables together to determine a more accurate RV function [7].Three-dimensional echocardiography has also been used more recently to quantify more accurately RV volumes and ejection fraction [7].
Echocardiography is somewhat limited by difficulties in image acquisition and cardiac position in the presence of RVF [8]. As echocardiographic technology continues to advance, newer imaging techniques and parameters of RV function are being introduced and will require further investigation.
Magnetic Resonance Imaging
MRI is considered the gold standard when one is determining RV volumes and function. Multiplanar views are obtained to precisely calculate stroke volume index, RV ejection fraction, and indexed RV end-diastolic and end-systolic volumes. It is the most accurate method to evaluate RV mass, volume, and RV ejection fraction. Furthermore, MRI can calculate and quantify regurgitant volumes, cardiac output, shunt fractions, strain, perfusion, and pulmonary pulsatility [7].
MRI can also be used to identify RV damage or fi brosis. This is demonstrated by delayed gadolinium enhancement at the RV septal insertion points,and directly correlates with RV function. Often in PAH, this accounts for less than 10% of the ventricular volume, accounting for the RV recovery commonly seen after lung transplantation [2].
Additionally, the pulmonary circulation can be evaluated by phase contrast cine imaging and can be used to provide information about pulmonary hemodynamics and pulmonary arterial stiffness.Furthermore, MRI is an ideal reproducible tool to use when one is monitoring a patient’s response to certain medical therapies [9]. The EURO-MR study demonstrated that changes in the global function of the right ventricle after medical therapy had a direct correlation with the functional class and survival in patients with pulmonary hypertension [6, 10]. Specif i cally, an increase in stroke volume of greater than 10 mL, in response to certain medical therapies, has been shown to be clinically relevant [6].
MRI has several advantages over echocardiography. As mentioned above, its increased special resolution and 3D imaging capabilities allow precise measurement of RV volumes and function [8].However cardiac MRI is more expensive and timeconsuming, and poses technical challenges for PAH patients unable to perform breath holds and those with prostacyclin infusion pumps.
Computed Tomography
RV function and volumes can also be assessed by 64 slice-CT. However, this cannot be done simultaneously with evaluation of the left side of the heart or coronaries, and so additional radiation is required[7]. Another limitation of CT is the need for iodinated contrast medium, which is nephrotoxic. CT is reserved for when MRI is contraindicated, or as part of the PAH workup to evaluate the lung parenchyma or to rule out pulmonary emboli as a cause of RVF [6].
Positron Emission Tomography
More recently, PET has been used to assess RV and pulmonary metabolism and for apoptosis imaging.Similarly to what is seen in LV failure, myocardial fatty acid uptake is reduced and glucose uptake is increased in the hypertrophied right ventricle of patients with PAH [6].
A few studies have suggested that disease severity may correlate with the ratio of RV to LV glucose uptake; however, other studies have shown no correlation, and it remains unclear at this time if there is truly a “metabolic switch” in PAH patients with RVF as has previously been described in animal models [6].
Hybrid Imaging
Although all of these imaging modalities have proven useful in the assessment of RV function independently, the integration of multiple imaging techniques can provide the most complete assessment of patients with RVF. New hybrid single-photon-emission CT/CT, PET/CT, and PET/MRI systems are being investigated to gain a more in-depth understanding of the anatomic correlation of the right ventricle with perfusion and fl ow imaging [6]. The development of these hybrid imaging systems which assess both myocardial and vascular function will further enhance our understanding of the failing right ventricle [6].
Prognostic Factors of Right Ventricular Failure in Pulmonary Arterial Hypertension
Acute RVF in the setting of PAH is associated with high morbidity and mortality. Several clinical factors, biomarkers, and imaging parameters have been shown to have prognostic signif i cance, and are summarized in Table 2.
Clinical Factors and Biomarkers
In a prospective French study of 46 PAH patients admitted to the intensive care unit with acute RVF,mortality was 41% [11]. In this study, there was no difference in the baseline clinical characteristics between survivors and nonsurvivors, including cause of PAH or the factor triggering acute RVF.
Baseline diuretic dose, serum brain natriuretic peptide concentration, and creatinine concentration were higher in nonsurvivors, as was C-reactive protein concentration; however brain natriuretic peptide was the only independent predictor of mortality in the logistic regression analysis. Systemic arterial pressure was signifi cantly lower in the first 3 weeks of admission in nonsurvivors, and a hospital- acquired infection occurred in 14 of 19 nonsurvivors compared with six of 27 survivors.Progressive increases in dobutamine dose were also associated with worse survival. There was no association between PAH targeted therapy and survival in this study.
In another retrospective study, 27% of cases of acute RVF were triggered by infection, and in these cases, mortality was 50% [12]. All patients who presented with low cardiac output and low filling pressures in this study died.
In both of these studies, there was no association between troponin and survival; however, in more recent studies, high-sensitivity troponin did play a role in predicting outcomes in PAH [2, 12, 13].
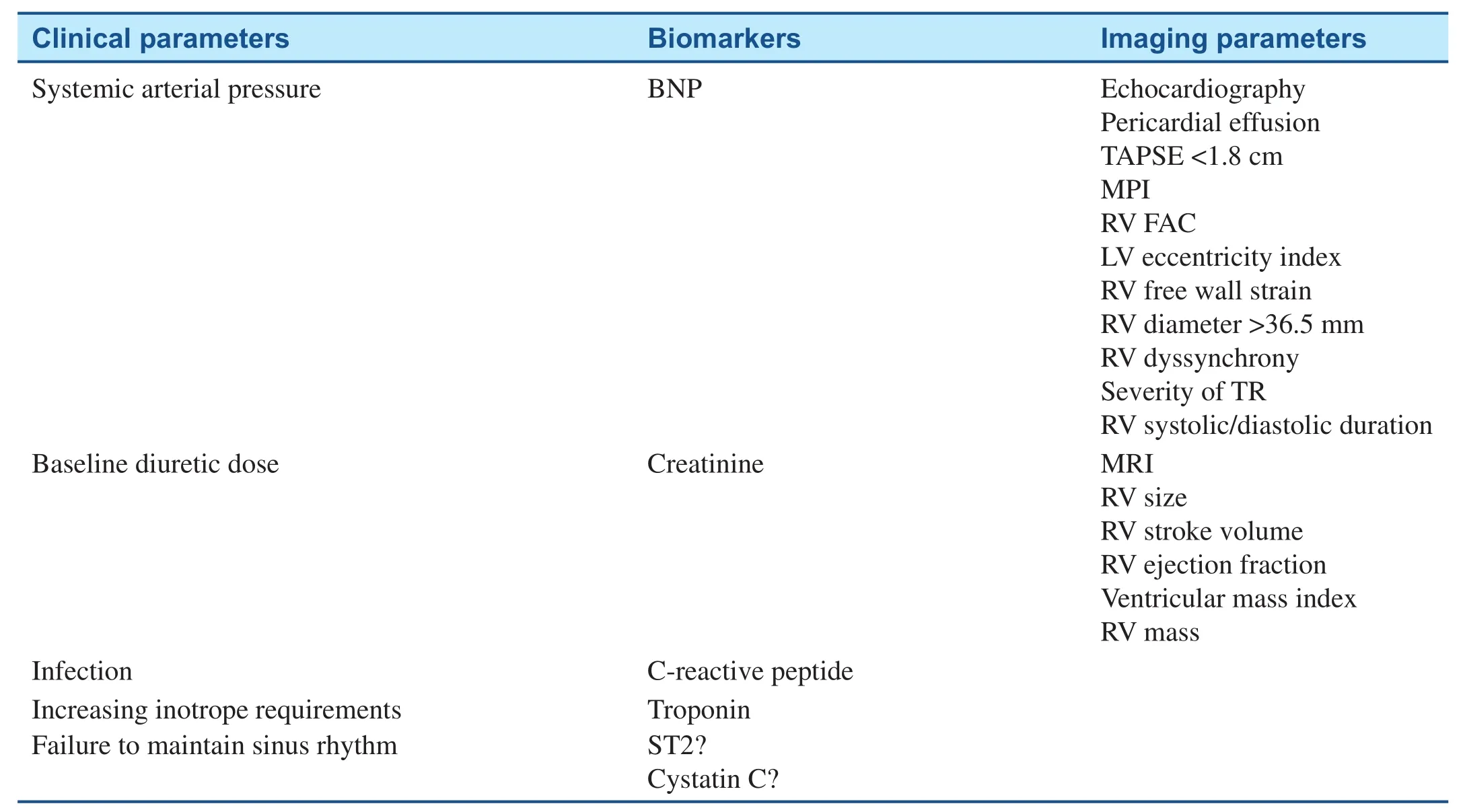
Table 2 Prognostic Factors in Right Ventricular (RV) Failure.
Another clinical factor which has been associated with worse outcomes is failure to restore sinus rhythm in patients with atrial arrhythmias [14, 15].
In addition to clinical factors which predict outcomes, it has been well documented that invasive hemodynamic measures are major predictors of mortality in PAH and RVF. Patients with an elevated mean right atrial pressure, mean PAP, and low cardiac index are at increased risk of death [2, 16].Conversely, patients who respond to vasodilator testing during right-sided heart catheterization have better outcomes, as do those who have normalization of their cardiac index with therapy.
Noninvasive Imaging Parameters
There are noninvasive imaging parameters that provide prognostic information in PAH and RVF. The presence of a pericardial effusion on echocardiography, reported in 54% of patients with severe idiopathic PAH, is a strong predictor of mortality [8].Larger effusions are associated with worse hemodynamics and RVF on echocardiography, impaired exercise tolerance, and poor 1-year prognosis [17].
TAPSE is strong prognostic indicator in PAH. A TAPSE of less than 1.8 cm in patients with PAH is associated with greater RVF (lower cardiac index and RV percentage area change) and decreased survival compared with a TAPSE of 1.8 of greater(88% and 94% vs. 50% and 60% at 1 and 2 years,respectively; hazard ratio 5.7, 95% conf i dence interval 1.3–24.9; P = 0.02) [18].
RV diameter greater than 36.5 mm has also been shown to be associated with increased mortality;however this increase in mortality is reduced if RV hypertrophy (wall thickness greater than 6.6 mm) is also present [8].
Other echocardiographic parameters with prognostic value include the myocardial performance index, RV FAC, the LV eccentricity index, RV dyssynchrony, RV free wall systolic strain, RV systolic to diastolic duration ratio, and the severity of tricuspid regurgitation [2, 7, 8].
Cardiac MRI may also be used to estimate prognosis in PAH patients with RVF. One study of 64 idiopathic PAH patients followed up for 1 year showed increased mortality with increased RV size and decreased RV stroke volume and RV ejection fraction [19]. In this study, a decline in RV stroke volume was associated with treatment failure.Increased ventricular mass index (ratio of RV to LV end-diastolic mass) and increased RV mass measured by MRI have also been shown to correlate with poor survival [8].
Despite the fact that RVF remains the main cause of death in PAH, there are few data on how the parameters of RV function affect long-term outcomes and response to treatment. Further research is needed to better assess RV function and treatment response. There are also novel biomarkers such as ST2 and cystatin C which may provide additional prognostic information. Lastly, multivariate studies are needed to validate a simplif i ed predictive score which incorporates RV imaging parameters [2].
Medical Management of Right Ventricular Failure in Pulmonary Arterial Hypertension
Before initiating treatment of acute RVF, one should first consider whether there is a reversible cause, such as withdrawal of use of pulmonary vasodilators or diuretics, infection (including sepsis and pneumonia), acute pulmonary embolism,RV ischemia, cardiac tamponade, and arrhythmia[11, 14]. Given that infection portends a very poor prognosis in acute RVF, preventative measures and prompt detection and treatment of infection play an important role in the treatment of patients with acute RVF [11].
A practical approach to the treatment of the patient with acute RVF is shown in Figure 2. The mainstay of treatment focuses on optimizing volume status,increasing RV contractility, and reducing RV afterload [14].
Patients who are hemodynamically unstable should be admitted to the intensive care unit. Placement of invasive hemodynamic monitors, including arterial lines, central venous catheters, and pulmonary arterial catheters, may be used to help guide therapy.
In patients requiring mechanical ventilation,care should be taken to avoid excessive tidal volumes and positive end-expiratory pressure as they increase PAP, right atrial pressure, and RV afterload [14]. Permissive hypercapnia should also be avoided as it leads to vasoconstriction, thereby worsening RVF [14]. Hyperventilation can be used to acutely reduce PAP in the short term and reverse acidosis-induced vasoconstriction, but care must be taken with tidal volumes in this setting [14].
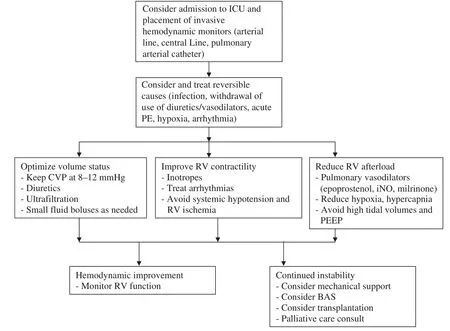
Figure 2 Practical Approach to Management of Acute Right Ventricular (RV) Failure.
As the right ventricle is preload dependent, care must be taken to avoid overdiuresis leading to a drop in cardiac output. At the same time, RV volume overload leads to interventricular dependence and compression of the left ventricle by the right ventricle. Diuretics should be used to remove volume, with small fl uid boluses when filling pressures fall too low. Invasive monitoring with a pulmonary arterial catheter can assist in maintaining this balance [14].
Inotropes increase RV contractility and cardiac output via the cyclic adenosine monophosphate pathway. Digoxin marginally improves cardiac output in patients with RVF due to severe PAH in the short term and can also be used to assist with rate control in patients with atrial arrhythmias [15].
Dobutamine increases contractility via the β1receptor and leads to vasodilatation via the β2receptor, thereby reducing RV afterload. It is the inotrope of choice in RVF in the setting of PAH, because of its positive effect on right ventricle–pulmonary artery coupling and the lower incidence of tachycardia seen with dobutamine than with dopamine[20, 21]. Hypotension may occur with the addition of dobutamine, which may be mitigated by the addition of a vasopressor, such as norepinephrine[11, 14].
Milrinone acts as both an inotrope and a vasodilator via inhibition of phosphodiesterase 3. Its use may be limited by systemic hypotension, which is exacerbated in the setting of renal insuff i ciency.Both milrinone and dobutamine may be combined with inhaled nitric oxide to improve cardiac output and reduce PVR [14].
Norepinephrine is the vasopressor of choice, as it increases RV contractility via the β1receptor, and RV perfusion pressure and cardiac output via the α1receptor [14].
Pulmonary vasodilators are essential to reduce RV afterload. Inhaled nitric oxide acts via cyclic guanosine monophosphate and is inactivated by hemoglobin in the capillaries of the lung, leading to pulmonary vasodilatation without systemic hypotension. Inhaled nitric oxide has been well studied in patients with acute RVF and has been shown to improve cardiac output, oxygenation, and PVR [22]. Care must be taken to monitor patients for methemoglobinemia when inhaled nitric oxide is used, and its use should be withdrawn slowly to avoid hemodynamic decompensation from rebound pulmonary hypertension.
The intravenous prostacyclins epoprostenol and treprostinil act via the cyclic adenosine monophosphate pathway to result in potent pulmonary vasodilatation and inhibition of platelet aggregation. With a half-life of 6 min, epoprostenol is the prostacyclin of choice for critically ill patients with acute RVF. Epoprostenol therapy is started at low doses(1–2 ng/kg/min) and is uptitrated as tolerated, with caution in patients who are hypoxemic or hemodynamically unstable. Titration is often limited by dose-dependent side effects, mainly hypotension,nausea/vomiting/diarrhea, and headache.
The inhaled prostacyclins iloprost and treprostinil may be given in acute RVF as well to reduce PVR and improve cardiac output, with fewer systemic side effects. Although treprostinil may also be given subcutaneously, its unpredictable absorption and longer half-life make it less desirable in hemodynamically unstable patients.
The oral pulmonary vasodilators in the endothelin receptor antagonist and phosphodiesterase 5 inhibitor classes may also be used to reduce PAP and improve cardiac output in patients with RVF; however, their use in critically ill patients has not been well studied. Care must be taken to avoid systemic hypotension with both drug classes, hepatotoxicity with endothelin receptor antagonists, and thrombocytopenia with phosphodiesterase 5 inhibitor s [14].
Surgical and Interventional Treatment Options
In PAH patients with RVF in whom medical therapy has failed, there are few options for surgical or percutaneous intervention.
Interventional Therapies
Balloon atrial septostomy (BAS) is indicated for idiopathic PAH patients with syncope or refractory RVF to decompress the right heart chambers and improve cardiac output via creation of a right-toleft shunt [23]. Stepwise balloon dilatation is preferred over blade-balloon atrial septostomy, and should be performed only in experienced centers.Mortality associated with BAS is approximately 5%, and spontaneous closure of the defect occasionally necessitates repeated BAS. BAS is contraindicated in patients with very high right atrial pressure(>20 mmHg), oxygen saturation greater than 90%on room air, severe RVF requiring cardiorespiratory support, PVR index greater than 55 U/m2, and LV end- diastolic pressure greater than 18 mmHg [13, 14,23]. BAS may be used as a bridge to transplantation or as palliation, and has a role in developing countries in which targeted PAH therapy is not available.
Cardiac resynchronization therapy has been proven to improve morbidity and mortality in patients with LV failure [24]. It restores mechanical synchrony in the failing left ventricle, leading to improved hemodynamics and reverse remodeling. There are some data from animal studies and small case series suggesting that RV pacing results in acute short term hemodynamic improvement in patients with RVF in the setting of PAH, but there are no data showing long-term clinical improvement in this patient population [24].
Another method of creating a shunt to decompress the right ventricle is to create a Potts anastomosis between the left pulmonary artery and the descending aorta [23]. This has been performed surgically in the past, and most recently has been attempted percutaneously, with some promise.
Percutaneous temporary mechanical support devices including the TandemHeart®( Cardiac Assist,Pittsburgh, PA, USA) and Impella®( Abiomed,Danvers, MA, USA) pumps have also been used in anecdotal reports; however, such use is off-label and requires further investigation.
Surgical Therapies
In patients with chronic thromboembolic pulmonary hypertension, pulmonary thromboendarterectomy is the preferred treatment option for those with proximal disease and with PVR less than 1000–1200 dyn s/cm5. Pulmonary thromboendarterectomy has been shown to improve exercise capacity,functional status, quality of life, gas exchange,hemodynamics, RV function, and survival [14, 23].Postoperative outcomes correlate directly with surgeon and center experience, concordance between the anatomic disease and PVR, preoperative PVR,absence of comorbidities (specifically splenectomy and ventricular-atrial shunt) and postoperative PVR less than 500 dyn s/cm5[23]. Operative mortality in an experienced center is between 4% and 7%,and surgery should not be delayed in candidates for operation in favor of medical therapy [23].
Mechanical circulatory support may be used as a bridge to lung transplantation or heart-lung transplantation (HLT). Although LV assist devices have been shown to reduce PAP in patients with left ventricular heart failure awaiting heart transplantation, there are few data to support the use of these devices in RVF, and they may in fact be harmful by increasing preload without a reduction in RV afterload [14, 25].
Venoarterial extracorporeal membrane oxygenation is another option for awake patients with severe hypoxemic respiratory failure who are awaiting transplantation in whom there is no irreversible endorgan damage [26]. Extracorporeal membrane oxygenation may also be used for patients with acute RVF in the setting of massive pulmonary embolism,for support after lung transplantation, and for treatment of patients with reperfusion lung injury after pulmonary thromboendarterectomy [23]. Complications of extracorporeal membrane oxygenation include bleeding, stroke, infection, and other thromboembolic events [23].
Ultimately, the def i nitive treatment of RVF in PAH patients with class IV symptoms or class III symptoms receiving combination medical therapy is bilateral lung transplantation (BLT) or HLT [23].
Most patients with severe RVF due to PAH receive BLT. It remains unclear at which point the right ventricle is beyond recovery; however, in most cases, the right ventricle is resilient and lung transplant alone is suff i cient. The choice between BLT and HLT is determined by organ donation rates,local allocation protocols, and center preference[23]. Patients with Eisenmenger’s syndrome may undergo BLT with repair of simple shunts (e.g.,atrial septal defect) at the time of surgery, or HLT,which has been shown to have a survival benef i t in this patient population [27].
Estimated survival after BLT is 52%–75% at 5 years and 45%–66% at 10 years [13]. Although 3-month survival is lowest in PAH patients compared with other BLT recipients, 5- and 10-year survival are similar to those of other BLT recipients, and those idiopathic PAH patients who survive to 1 year have greater 10-year survival than those who received a transplant for chronic obstructive lung disease or pulmonary fi brosis [23]. Survival is worse in patients with RVF, as well as in those with renal and hepatic failure [23]. Early referral before the development of end-organ dysfunction is key.
Conclusion and Take-Home Message
RVF remains the primary cause of death in patients with PAH. Our understanding of the pathophysiology of RVF continues to grow with the advancement of noninvasive imaging techniques and recognition of new prognostic factors which help stratify patients by risk. Despite this progress,mortality remains high in PAH patients with RVF.
Treatment is often palliative and focuses on optimization of volume status and RV contractility while reducing RV afterload. Transplant is the def i nitive therapy, but is not without risk and many PAH patients will die while on the waiting list.Strategies to prevent the development of RVF and early detection of RVF in patients with PAH will be important if outcomes in this patient population are to improve.
Conflict of Interest
Stacy A. Mandras has accepted consulting and speaking fees from Actelion, consulting fees from Bayer, and educational grants from Actelion, United Therapeutics, Gilead, Pf i zer, and Bayer Pharmaceuticals. Hector O. Ventura and Sylvia Oleck have no conf l icts of interest.
1. Simonneau G, Gatzoulis MA,Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–41.
2. Vonk Noordegraaf A, Haddad F,Chin K, Forf i a PR, Kawut SM,Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology.J Am Coll Cardiol2013;62 Suppl 25:D22–33.
3. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD,Meldrum DR, et al. Right ventricular function and failure, report of a National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Circulation 2006;114:1883–91.
4. Miyamoto S, Nagaya N, Satoh T,Kyotani S, Sakamaki F, Fujita M,et al. Clinical correlates and prognostic signif i cance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000;161(2 Pt 1):487–92.
5. Naeije R, Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev 2014;23(134):476–87.
6. van de Veerdonk MC, Marcus TM,Bogaard HJ, Vonk Noordegraaf A.State of the art: advanced imaging of the right ventricle and pulmonary circulation in humans.Pulm Circ 2014;4(2):158–68.
7. Selton-Suty C, Juillière Y. Noninvasive investigations of the right heart: how and why? Arch Cardiovasc Dis 2009:102(3):219–32.
8. Noordegraaf AV, Galie N. The role of the right ventricle in pulmonary arterial hypertension.Eur Respir Rev 2011;20(122):243–53.
9. Iwasawa T. Diagnosis and management of pulmonary arterial hypertension using mr imaging. Magn Reson Med Sci 2013;12(1):1–9.
10. Peacock AJ, Crawley S, McLure L. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. CircCardiovasc Imaging 2014;7(1):107–14.
11. Sztrymf B, Souza R, Bertoletti L,Jaïs X, Sitbon O, Price LC, et al.Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 2010;35:1286–93.
12. Kurzyna M, Zyłkowska J,Fijałkowska A, Florczyk M,Wieteska M, Kacprzak A, et al.Characteristics and prognosis of patients with decompensated right ventricular failure during the course of pulmonary hypertension.Kardiol Pol 2008;66:1033–9.
13. Galie N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al.Updated treatment algorithm of pulmonary arterial hypertension.J Am Coll Cardiol 2013;62 Suppl 25:D60–72.
14. Lahm, T, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY,Obeidat OS, et al. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol 2010;56(18):1435–46.
15. Rich S, Seidlitz M, Dodin E,Osimani D, Judd D, Genthner D, et al. The short-term effects of digoxin in right ventricular dysfunction from pulmonary hypertension. Chest 1998;114(3):787–92.
16. Agarwal R, Gomberg-Maitland M. Current therapeutics and practical management strategies for pulmonary arterial hypertension.Am Heart J 2011;162:201–13.
17. Hinderliter AL, Willis PW 4th,Long W, Clarke WR, Ralph D,Caldwell EJ, et al. Frequency and prognostic signif i cance of pericardial effusion in primary pulmonary hypertension. PPH Study Group.Primary pulmonary hypertension.Am J Cardiol 1999;84:481–4.
18. Forf i a PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–41.
19. van Wolferen SA, Marcus JT,Boonstra A, Marques KM,Bronzwaer JG, Spreeuwenberg MD,et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007;28:1250–7.
20. Kerbaul F, Rondelet P, Demester JP, Fesler P, Huez S, Naeije R,et al. Effects of levosimendan versus dobutamine on pressure loadinduced right ventricular failure.Crit Care Med2006;34:2814–9.
21. Leier CV, Heban PT, Huss P, Bush CA, Lewis RP. Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation 1978;58:466–75.
22. Bhorade S, Christenson J,O’Connor M, Lavoie A, Pohlman A, Hall JB. Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Care Med 1999;159:571–9.
23. Keogh AM, Mayer E, Benza RL,Corris P, Dartevelle PG, Frost AE,et al. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol2009;54:S67–77.
24. Rasmussen JT, Thenappan T,Benditt DG, Weir EK, Pritzker MR.Is cardiac resynchronization therapy for right ventricular failure in pulmonary arterial hypertension of benef i t? Pulm Circ 2014;4(4):552–9.
25. Liden H, Haraldson A, Ricksten SE, Kjellman U, Wiklund L. Does pretransplant left ventricular assist device therapy improve results after heart transplantation in patients with elevated pulmonary vascular resistance? Eur J Cardiothor Sur 2009;35:1029–34.
26. Fuehner T, Kuehn C, Haedm J,Wiesner O, Gottlieb J, Tudorache I, et al. Extracorporeal membrane oxygenation in awake patients as a bridge to lung transplantation. Am J RespCrit Care Med 2012;185:763–8.
27. Waddell TK, Bennett L, Kennedy R, Todd TR, Keshavjee SH. Heartlung or lung transplantation for Eisenmenger syndrome. J Heart Lung Transplant 2002;21:731–7.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Mechanical Circulatory Support for the Failing Heart: Which Device to Choose
- Continuous Flow Left Ventricular Assist Device Therapy: A Focused Review on Optimal Patient Selection and Long-Term Follow-up Using Echocardiography
- Cardiac Resynchronization Therapy in 2015:Lessons Learned
- The Evaluation of the Heart Failure Patient by Echocardiography: Time to go beyond the Ejection Fraction
- Noninvasive Hemodynamic Monitoring for Heart Failure: A New Era of Heart Failure Management
- Epidemiological Study of Heart Failure in China