Wnt/β-catenin pathway might underlie the MET in transdifferentiation from MSC to MSC-derived neuron
2015-05-22YingJINGJiachengZHANGSutingLIJihuaZHAOJinWANGXuefeiHANYingXING
Ying JING, Jia-cheng ZHANG, Su-ting LI, Ji-hua ZHAO, Jin WANG, Xue-fei HAN, Ying XING
1. Stem Cell Center, Zhengzhou University, Zhengzhou 450001; 2. Department of Physiology and Neurobiology, Basic Medical College of Zhengzhou University, Zhengzhou 450001, China
Wnt/β-catenin pathway might underlie the MET in transdifferentiation from MSC to MSC-derived neuron
Ying JING1,2, Jia-cheng ZHANG1,2, Su-ting LI1,2, Ji-hua ZHAO1,2, Jin WANG1,2, Xue-fei HAN1, Ying XING1,2
1. Stem Cell Center, Zhengzhou University, Zhengzhou 450001; 2. Department of Physiology and Neurobiology, Basic Medical College of Zhengzhou University, Zhengzhou 450001, China
doi 10.13459/j.cnki.cjap.2015.06.014
Objective: To observe MET-associated alteraon during the trans-dif f erenaon from MSCs to neuron-like cells, and to explore the possible molecular mechanism. Methods: Bone marrow MSCs were isolated from rat femur and purif i ed in connuous cell culture. Aer induced dif f erenaon to neuron-like cells by the combinaon of butylated hydroxyanisole(BHA)and dimethyl sulfoxide (DMSO), cells were tested by comparave polymerase chain reacon (PCR) for the relave expression of MET biomarkers and transcripon factors, and for cell cycle by fl ow cytometry. Meanwhile, target genes of Wnt/β-catenin pathway were also analyzed by comparave PCR to determine the possible involvement. Results: In MSC-induced neuron-like cells, MET-associated transcripon factors such as Snail, Slug, ZEB1, ZEB2, and Twist were signif icantly aenuated in expression level. The Mesenchymal marker Vimenn expression level was increased. Membrane protein E-cad was slightly down-regulated, while N-cad level was marginally elevated. Percentage of proliferang cells (S phase in cell cycle) markedly shrank from 40.42% for MSCs to 6.76% for MSC-derived neuron. Addionally, Wnt/β-catenin target genes β-catenin and c-myc were decreasingly expressed. Conclusion: Chemically induced trans-differentiation from MSC to neuron caused similar MET-featured alteraon in gene expression and proliferaon to known MET, which might be underlied by deacvaon of Wnt/ β-catenin pathway.
mesenchymal-epithelial transion (MET); mesenchymal stem cell (MSC); induced dif f erenaon; Wnt/ β-catenin pathway
Introduction
Epithelial-mesenchymal transition (EMT), together with its reversed process named mesenchymalepithelial transition (MET),is a common process during embryo development and in trauma healing. In pathological context, it is the profound mechanism related mainly to organ fi brosis and tumor metastasis [1]. At present, the known EMT are classified into 3 subtypes according to the context in which they function. Though they are distinct biological programs, a common set of genetic elements are taking roles, and causing subsequently general phenotypic alterations in, for instance, enhancedproliferating rate and elevated drug-resistance, etc [1]. Mesenchymal stem cells (MSC) are adult stem cells that distribute widely in umbilical cord, bone marrow, fat tissue and circulating blood. Our previous data have proved that mesoderm-originated MSCs were able to be induced to differentiate to ectodermoriginated neuron [2]. However, during the transdif f erentiation, it is unclear whether there exist MET-featured genetic and phenotypic alterations, and still unknown of the molecular mechanism underlying. Wnt/β-catenin pathway was reported to have effect on the tumor metastasisin vivo[3]. Hence, this study aims to observe MET-featured alteration during the trans-dif f erentiation from MSCs to neuron-like cells, and to explore the possible involvement of Wnt/ β-catenin pathway in this process.
Materials and Methods
Materials
Male SD rats (n=30), 4-6 weeks old and 100-120 gin body weight, were purchased from Laboratory Animal Center of Henan Province. Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were from Hyclone. Butylated hydroxyanisole (BHA), dimethyl sulfoxide (DMSO) , and prodium iodide (PI) were from Sigma-Aldrich. Total RNA kit was from Omega. RevertAid first strand cDNA synthesis kit and Maxima SYBR Green qPCR kit were fromermo Scientif i c. FACS calibur flow cytometer was from BD. Fast real-time PCR system 7 500 was from applied biosystem.
MSC isolation and purif i cation
Rats were euthanized by cervical dislocation. Femurs were separated aseptically and DMEM medium was injected to rinse the bone marrow cavity.ereaer, all cells from femur bone marrow were collected and seeded into culture flask (recorded as passage 0 (P0)). Cells were maintained in DMEM medium supplemented with 10% FBS, and kept in incubator at 37°C, with 5% CO2and saturated humidity. Relatively pure MSCs were obtained aer continuous culture, and the passage 3-passage 5 (P3-P5) cells were selected for the following induce and test.
MSC induction to neuron
MSCs were allocated to 6-well plate in 1×106per well and randomly divided into (1) neuron group, in which MSCs were induced to differentiate to neuron-like cells; (2) MSC or control group, in which MSCs were not treated. In the neuron group, MSCs were pre-treated with extra fibroblast growth factor (bFGF) 10 ng/ml in DMEM medium plus 10% FBS for 24 hours, and then changed to inducing-medium which consisted of DMEM supplemented with 10% FBS, BHA 200 μmol/L and 2% DMSO. Induce lasted for 4-6 h and ceased when cells appeared neuronlike projections when observed under inverted microscope.
Gene expression analysis by comparative PCR
RNA abstract and cDNA synthesis were performed according to kit instructions. Briefly, MSC and MSC-derived neurons were respectively splitted in lysis buffer. Total RNA was first attached to the binding column and then eluted in DEPC treated water. RNA concentration was determined using the instrument Nanodrop. 2 μg RNA in either sample was reverse-transcripted to cDNA at 42°C for 60min, with the presence of primer Oligo(dT)18and reverse transcriptase. Then comparative PCR was carried out using 7 500 fast real-time PCR system and SYBR Green PCR kit. Reaction condition was as follow: (1) initial denaturation: 95°C,10 min. (2) 40 cycles of denaturation 95°C, 15 s; annealing 62°C, 45 s; extension 72°C, 1 min. Primer information was listed in table 1. GAPDH was used as the internal reference gene (standard control), and ROX as sampling control. Data were collected and analyzed with the soware AQ data analysis.
Cell cycle analysis by fl ow cytometry
Cell cycle was analyzed by PI staining and flow cytometry. MSC and MSC-derived neurons were respectively collected and suspended in cold PBS at 1x106/ml. 1ml cell suspension was centrifuged and fi xed in 70% ethanol for 2 h. Aer PBS washing, fi xed cells were re-suspended in PI solution (50 μg/ml) and incubated for 30 min at room temperature. Cell cycle was tested with BD FACS calibur ff ow cytometer, and data were analyzed by soware Modf i t.
Statistical analysis
All tests were repeated at least twice. Data were presented as mean±SD and statistical analysis was performed using SPSS software.t-test was used for the 2 samples comparison (a=0.05).
Results
Cell morphology change when MSCs were induced to neurons
Rat bone marrow MSCs (P3-P5) appeared spindleshaped, and were arranged in parallel when reaching above 80% conf f uence. Aer induction by BHA and DMSO for 4-6 h, cells extended long projections mimicking the axons of real neuron. As proved by our previous experiment [2], the cells were regarded as MSC-derived neuron-like cells.
EMT-associated genes were changed in the MSC-derived neuron
Comparative PCR data showed widely gene expression changes for EMT-associated transcriptional factors, mesenchymal biomarker and membrane proteins. In MSC-derived neurons which should have acquired the epithelial properties, transcription factors that were activated muchly in EMT including Snail, Slug, ZEB1, ZEB2 and Twist were significantly attenuated in expression. Especially for Snail, the expression level was about nine times lower (onetenth) in the induced neuron than that of the original MSCs (Fig.1). Cytoskeletal protein Vimentin, which functioned to form the intracellular network inviarous mesenchymal cells, was also low expressed in the MSC-derived neurons (Fig.2). However, the expression of specif i c cell surface markers E-cadherin (E-cad) and N-cadherin (N-cad) were inconsistent with most report. Membrane protein E-cad was down-regulated, while N-cad expression was enhanced (Fig.2).
Cell proliferation property was changed in MSC-derived neuron
As shown by flow cytometry data, cells were redistributed in cell cycle when MSC was induced into neuron-like cells. The percentage of S-phase was significantly decreased from 40.42% for MSCs to 6.76% for MSC-derived neurons (Fig.3 ).
Wnt/β-catenin pathway was involved in EMT
In order to explore whether Wnt/β-catenin pathway was involved in the molecular mechanism for above MET relevant change in MSC trans-differentiation, we tested the target genes of the pathway. As shown in Fig.4, c-myc and β-catenin were markedly low expressed in the MSC-derived neurons. Axin2 showed no detactable change in expression.
Discussion
EMT was first described by Hay to explain the phenomenon that tightly knitted epithelial cells suddenly become free and mobile, which was later found to play profound roles in both physiological and pathological conditions [4]. The knownEMT includes those occur: (1) during embryo implantation, embyogenesis, and organ development; (2) in tissue regeneration and fi brosis; (3) in cancer metastasis [1].ough EMT and MET are common phenomenon in vivo, little is reported about MET except for the well-examined model in kidney formation [5]. However, MET is frequently viewed as the reverse process of EMT and its occurrence is signaled by the subsidence of the EMT biomarkers [6], which we call MET-featured change.

Tab. 1 Primers for comparative PCR.
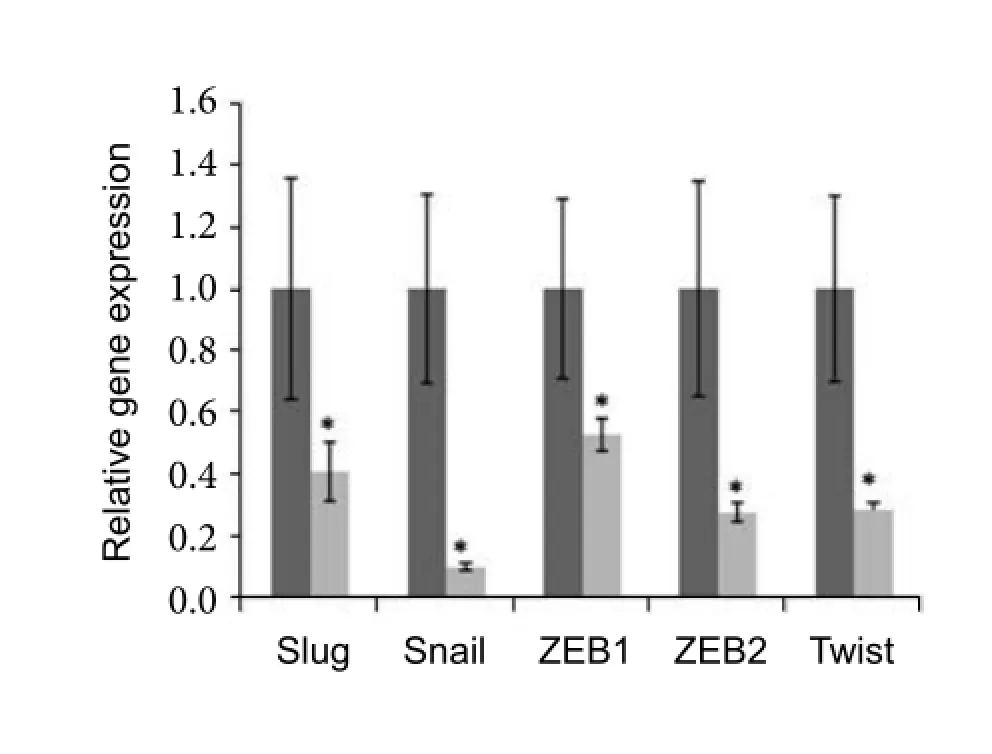
Fig. 1 Analysis of MET-associated transcription factors by comparative PCR. Total RNA was abstracted from both MSC and MSC-derived neurons. After reverse transcription, gene expression of MET-featured transcription factor was tested by comparative PCR. All the transcription factors tested showed significant down-regulation in MSC-derived neurons. The dark column stands for the MSC, while grey column for MSC-derived neuron-like cells. (*P<0.05)

Fig. 2 Analysis of marker proteins gene expression by comparative PCR. Total RNA was abstracted from both MSC and MSC-derived neurons. After reverse transcription, gene expression of MET-featured protein markers was tested by comparative PCR. Mesenchymal cytoskeletal protein Vimentin was significant down-regulated in MSC-derived neurons. Expression of E-cadherin (E-cad) was lower, while N-cadherin (N-cad) was higher in MSC-derived neurons compared to MSCs. The dark column stands for the MSC, while grey column for MSC-derived neuron-like cells. (*P<0.05)
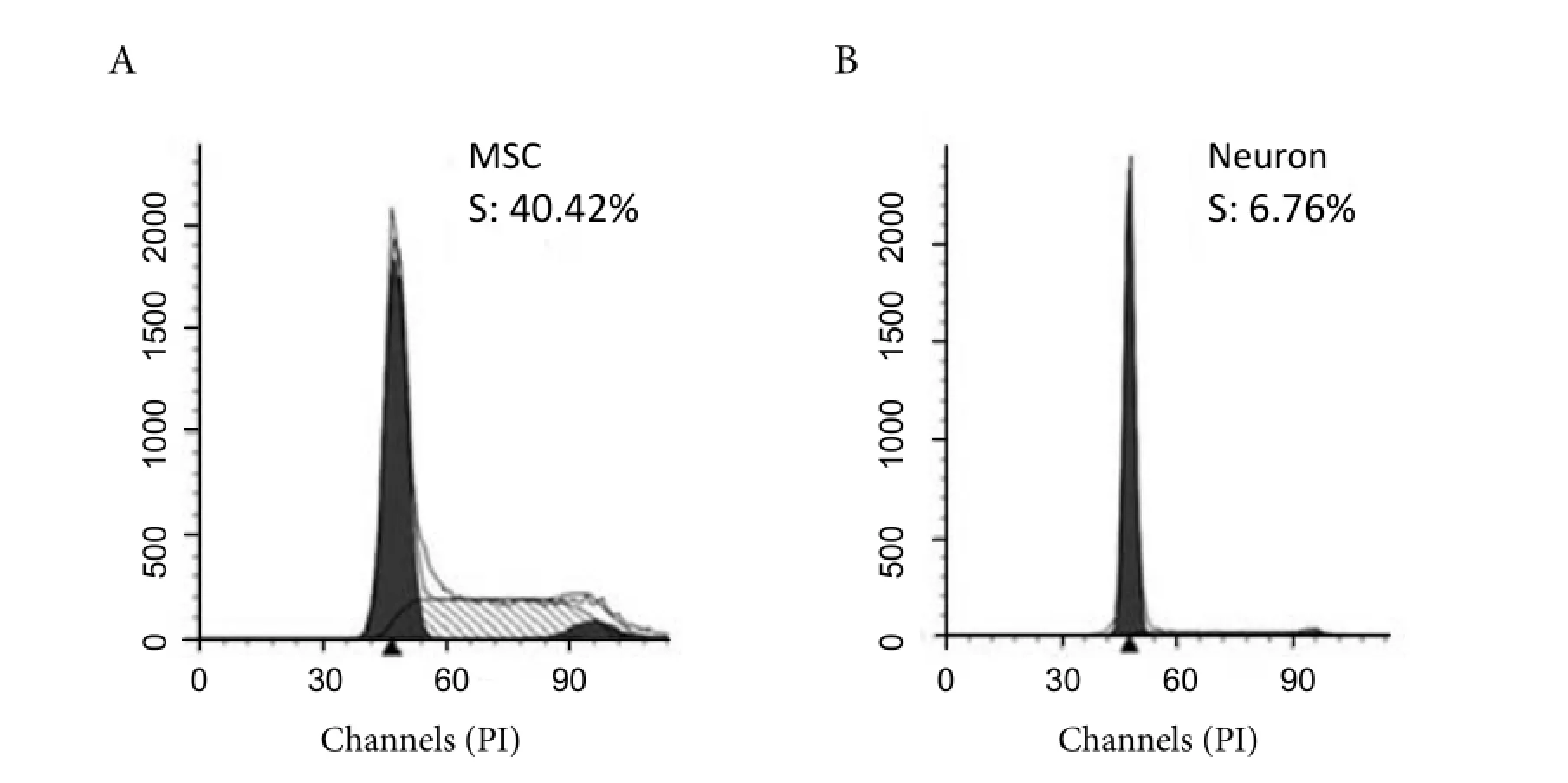
Fig. 3 Flow cytometry analysis of the cell cycle. Both MSC and MSC-derived neurons were fixed and stained in PI solution. Data analysis was carried out by Modfit software. A: MSC with the S-phase percentage of 40.42%; B: MSC-derived neuron with the S-phase percentage of 6.76%.
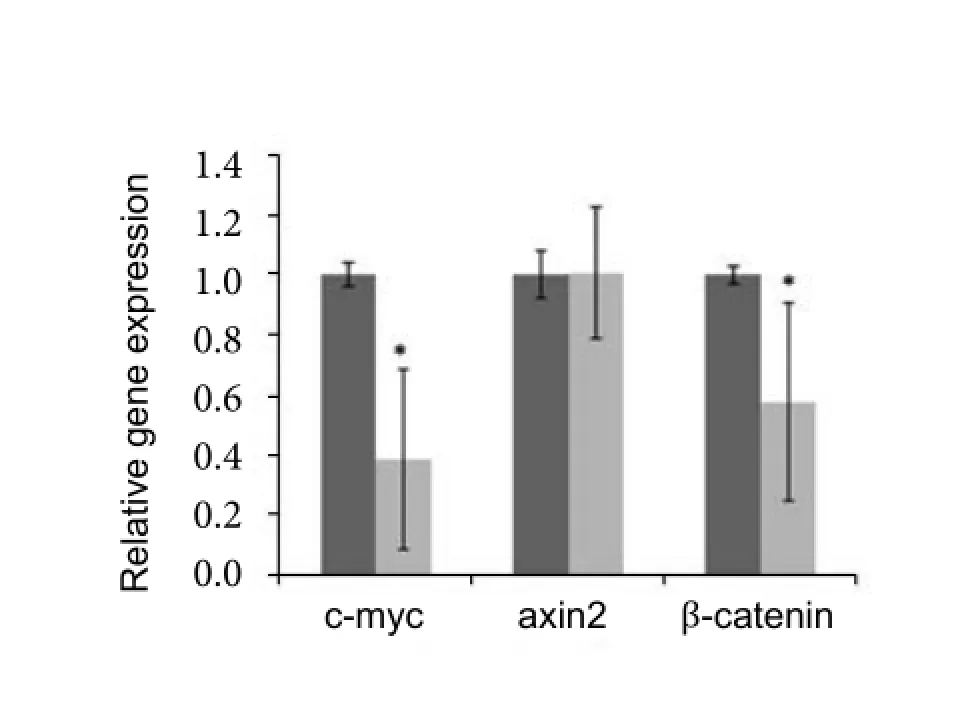
Fig. 4 Analysis of Wnt/β-catenin pathway target genes by comparative PCR. Total RNA was abstracted from both MSC and MSC-derived neurons. After reverse transcription, target genes of Wnt/β-catenin pathway were tested by comparative PCR. Among the 3 tested genes, c-myc and β-catenin showed significantly lower expression. The dark column stands for the MSC, while grey column for MSC-derived neuron-like cells. (*P<0.05).
Though the subtypes of EMT are involved in different biological processes, they are all based on the same genetic and phenotypic programs [1,3,7], that is, the common set of transcription factors are used [8,9]. The present study discovered MET-featured genetic changes in MSC-derived neurons, suggesting that this trans-differentiation might share similar molecular mechanism to the in vivo MET. Data showed that MSC-derived neurons induced by combination of 2 chemical reagents resembled the known MET change in the depression of transcription factors including Snail, Slug, ZEB1, ZEB2 and Twist. Meanwhile, down-regulation of the mesenchymal marker vimentin in MSC-derived neuron added support for the formation of MET conversion. However, expression variation of membrane protein E-cad and N-cad does not follow the common rules. There was slightly increased expression of N-cad in MSC-derived neurons, and decrease of E-cad. This might be explained by the characterization of neurons, which originate from primary epithelium but dif f erent with other epithelial derivatives that form tight connection to each orther. Neurons normally do not form continuous singlelayered sheet. In stead, neurons connect into network and form connections only at the specialized sites of synapse, where N-cad is enriched for the sake of proper connection of pre- and post- synaptic neurons [9].
Interestingly, the epigenetic change of MSC-derived neurons in this study fits well to the MET feature, too. Functionally cells undergoing EMT obtained the stem cells capability including loss of cellpolarity, promoted cell mobility and enhanced proliferation [7,8]. On the contrary, MET might lower down the proliferating ability. Our cell cycle analysis come to the similar conclusion that when MSCs are gradually losing their mesenchymal nature, only were small percentage of the MSC-derived neurons lein the proliferating phase (S-phase in cell cycle).
The apparent down-regulation of transcription factors, mesenchymal biomarkers and proliferation in MSC-derived neurons fi ts well to the prof i le in MET. In order to further investigate whether Wnt/β-catenin pathway was involved in MET-featured trans-dif f erentiation, target genes were tested. Among the three target genes tested, expression of two (c-myc and β-catenin) were markedly decreased, suggesting the less activity of the Wnt/β-catenin pathway during this process. This is consistent to previous report that activation Wnt/β-catenin pathway might enhance the EMT.ough TGFβ was to date estimated to be closely relevant to EMT/MET, Wnt/β-catenin pathway was also reported to work in the EMT and tumor metastasis.e pathway might cause the cell dissemination by interrupting β-catenin stability, but it is not excluded of other mechanism [3,5]. Our data further hinted Wnt/β-catenin pathway functions in MET change of MSC dif f erentiation in vitro. For sure, more experiments are required to conf i rm the possible caudal relationship.
EMT is esteemed the pivotal mechanism for the tumor metastasis. Considering that more than 90% of all the tumor victims die of re-currence and metastasis, it is of extraordinary meaning to understand the mechanism and stop its happening. Prevention of metastasis by inhibiting or even reverting EMT is then of extremely importance [6]. Our study demonstrated the combination of BHA and DMSO caused MSC cell to undergo the conversion consistent to MET.ough more ef f ort is needed to test the ef f ect of this combination on tumor cells other than MSC, and to explore specific molecular sites, the successful induction of MET at less show the possiblility of EMT reverse.
In a summary, our findings have shown that trans-differentiation of MSCs to MSC-derived neurons demonstrated MET-featured changes in gene expressions and cell proliferation properties. Moreover, Wnt/β-catenin pathway might underlie the MET as shown to lower down in activity during MET. By comparing the similarity of MSC trans-differentiation and known MET, it hints the possibility of inducing the process of MET or the reverse of the malignant EMT.
1. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition [J]. J Clin Invest, 2009, 119 (6): 1420-1428.
2. Li J,Zhao TC, Duan P,et al. Aβ25-35 causes Tau protein hyperphosphorylation of BMSCs differentiated neuron-like cells [J]. J Apoplexy Nerv Dis, 2011, 28(7): 580-582.
3. Scheel C, Eaton EN, Li SH,et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast [J]. Cell, 2011, 145(6): 926-940.
4. Hay ED. An overview of epithelio-mesenchymal transformation [J]. Acta Anat, 1995, 154(1): 8-20.
5. Park JS, Valerius MT, McMahon AP. Wnt/ β-catenin signaling regulates nephron induction during mouse kidney development [J]. Development, 2007, 134(13): 2533-2539.
6. Velpula KK, Dasari VR, Tsung AJ,et al. Cord blood stem cells revert glioma stem cell EMT by down regulating transcriptional activation of Sox2 and Twist1 [J]. Oncotarget, 2011, 2(12): 1028-1042.
7. Yu M, Bardia A, Wittner BS,et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition [J]. Science, 2013, 339 (6119): 580-584.
8. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms [J]. Cell, 2011, 147 (2): 275-292.
9. Sakane F, Miyamoto Y. N-cadherin regulates the proliferation and dif f erentiation of ventral midbrain dopaminergic progenitors [J]. Dev Neurobiol, 2013, 73(7): 518-529.
10. Moreno-Bueno G, Peinado P, Molina P,et al.e morphological and molecular features of the epithelial-to-mesenchymal transition [J]. Nat Protoc, 2009, 4 (11): 1591-1613.
Ying XiNG, PhD, Professor, (1) Stem Cell Center, (2) Department of Physiology and Neurobiology, Zhengzhou University, 100 Kexue Road, Zhengzhou 450001, China. Tel: 13525516617; E-mail: xingy@zzu.edu.cn
Received 2015-10-12; accepted 2015-11-20
杂志排行
中国应用生理学杂志的其它文章
- A mini review: Tau transgenic mouse models and olfactory dysfunction in Alzheimer’s Disease
- Ethical inspection about laboratory animals
- Better parameters of ventilation-CO2output relationship predict death in CHF patients
- Association between endothelial nitric oxide synthase (ENOS) G894T polymorphism and high altitude (HA) adaptation: a meta-analysis
- Flow cytometric analysis of circulating microvesicles derived from myocardial ischemic preconditioning and cardioprotection of ischemia/reperfusion injury in rats
- Synergisms of cardiovascular effects between iptakalim and amlodipine, hydrochlorothiazide or propranolol in anesthetized rats
