Flow cytometric analysis of circulating microvesicles derived from myocardial ischemic preconditioning and cardioprotection of ischemia/reperfusion injury in rats
2015-05-22MiaoLIUYiluWANGManSHANGYaoWANGQiZHANGShaoxunWANGSuWEIKunweiZHANGChaoLIUYannaWUMinglinLIUJunqiuSONGYanxiaLIU
Miao LIU, Yi-lu WANG, Man SHANG, Yao WANG, Qi ZHANG, Shao-xun WANG, Su WEI, Kunwei ZHANG, Chao LIU, Yan-na WU, Ming-lin LIU, Jun-qiu SONG, Yan-xia LIU
1. Department of Pharmacology, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China; 2. Section of Endocrinology, Department of Medicine, Temple University School of Medicine, Philadelphia PA19140, USA; 3. Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia PA19104, USA
Flow cytometric analysis of circulating microvesicles derived from myocardial ischemic preconditioning and cardioprotection of ischemia/reperfusion injury in rats
Miao LIU1, Yi-lu WANG1, Man SHANG1, Yao WANG1, Qi ZHANG1, Shao-xun WANG1, Su WEI1, Kunwei ZHANG1, Chao LIU1, Yan-na WU1, Ming-lin LIU2,3, Jun-qiu SONG1, Yan-xia LIU1
1. Department of Pharmacology, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China; 2. Section of Endocrinology, Department of Medicine, Temple University School of Medicine, Philadelphia PA19140, USA; 3. Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia PA19104, USA
doi 10.13459/j.cnki.cjap.2015.06.007
Objective: To establish a fl ow cytometric method to detect the alteraon of phenotypes and concentration of circulating microvesicles (MVs) from myocardial ischemic preconditioning (IPC) treated rats (IPC-MVs), and to invesgate the ef f ects of IPC-MVs on ischemia/reperfusion (I/R) injury in rats. Methods: Myocardial IPC was elicited by three cycles of 5-min ischemia and 5-min reperfusion of the leanterior descending (LAD) coronary artery. Platelet-free plasma (PFP) was isolated through two steps of centrifugaon at room temperature from the peripheral blood, and IPC-MVs were isolated by ultracentrifugaon from PFP. PFP was incubated with an-CD61, an-CD144, an-CD45 and an-Erythroid Cells, and added 1, 2 μm latex beads to calibrate and absolutely count by fl ow cytometry. For funconal research, I/R injury was induced by 30-min ischemia and 120-min reperfusion of LAD. IPC-MVs 7 mg/kg were infused via the femoral vein in myocardial I/R injured rats. Mean arterial blood pressure (MAP), heart rate (HR) and ST-segment of electrocardiogram (ECG) were monitored throughout the experiment. Changes of myocardial morphology were observed aer hematoxylin-eosin (HE) staining. The acvity of plasma lactate dehydrogenase (LDH) was tested by Microplate Reader. Myocardial infarct size was measured by TTC staining. Results: Total IPC-MVs and dif f erent phenotypes, including platelet-derived MVs (PMVs), endothelial cell-derived MVs (EMVs), leucocyte-derived MVs (LMVs) and erythrocyte-derived MVs (RMVs) were all isolated which were idenf i ed membrane vesicles (<1 μm) with corresponding anbody posive. The numbers of PMVs, EMVs and RMVs were signif i cantly increased in circulaon of IPC treated rats (P<0.05, respecvely). In addion, at the end of 120-min reperfusion in I/R injured rats, IPC-MVs markedly increased HR (P<0.01), decreased ST-segment and LDH acvity (P<0.05,P<0.01). The damage of myocardium was obviously alleviated and myocardial infarct size was signif i cantly lowered aer IPC-MVs treatment (P<0.01). Conclusion: The method of fl ow cytometry was successfully established to detect the phenotypes and concentraon alteraon of IPC-MVs, including PMVs, EMVs, LMVs and RMVs. Furthermore, circulang IPC-MVs protected myocardium against I/R injury in rats.
microvesicles; myocardial ischemic preconditioning; myocardial ischemia/reperfusion injury; fl ow cytometry; cardioprotecon
Introduction
The morbidity and mortality of ischemic heart diseases (IHD) have been ascending trends year by year [1]. IHD is caused by an interruption in blood ff ow to the myocardium.rombolytic therapy, coronary artery bypass graft and percutaneous transluminal coronary angioplasty have emerged as main clinical treatment methods for patients with IHD. However, immediate reperfusion has been shown to acceleratecell death.is process is known as myocardial ischemic/reperfusion (I/R) injury [2]. Ischemic preconditioning (IPC) is a phenomenon in which brief episodes of sublethal myocardial ischemia followed by reperfusion that ultimately result in greater myocardial resistance to a subsequent ischemic injury. Such preconditioning protects the myocardium against I/R injury by reduction of myocardial infarct size, attenuation of post-ischemic arrhythmia and inhibition of myocardial apoptosis [3].e potential intracellular mechanisms of IPC in cardioprotection are more complex than initially thought. A humoral factor appears to be involved, although its identity is currently unknown.
Microvesicles (MVs) are membrane fragments shed into extracellular space from cells under conditions of activation or apoptosis. MVs are defined as 0.1~1 μm in diameter typically. Believed to be formed by a variety of cell types, MVs release has been observed in cells of the vasculature, including platelets, endothelial cells, leukocytes and erythrocytes [4]. The detection of MVs is accomplished by ff ow cytometric analysis according to a subset of cell surface proteins derived from the plasma membrane of the original cells [5]. Recent studies indicated that MVs could be involved in the pathogenesis of various cardiovascular diseases [6]. One paper reported that MVs were collected from coronary perfusates of donor isolated hearts which were subjected to IPC, and exerted a protective ef f ect on I/R injury in recipient isolated hearts in rats via IPC-MVs treatment [7]. However, to date, whether the phenotypes and concentration of circulating IPCMVs changed, and ef f ects on I/R injuryin vivoin rats have not been elucidated.
In our study, IPC and I/R models of rats were establishedin vivo.e alteration of phenotypes and concentration of IPC-MVs were analyzed by flow cytometry, and the ef f ects of circulating IPC-MVs on I/R injury in rats were investigated.
Materials and Methods
Animals and materials
Healthy male Wistar rats weighing 240-260 g were purchased from Academy of Military Medical Sciences. All animals’ experiments were performed in adherence with the National Institutes of Health Guidelines for the Use of Laboratory Animals and approved by the local animal care and use committee. Flow Cytometry Size Calibration Kit was obtained from Molecular Probe. Anti-FITC-CD61, anti-FITC-CD45, anti-APC-Erythroid Cells, anti-FITCIgG1 and anti-APC-IgM were purchased from BD. Anti-PE-CD144 and anti-PE-IgG1 were purchased from Santa Cruz. LDH assay kit was obtained from JianCheng Bioengineering Institute. The available Optima L-100XP ultracentrifuge was purchased from Beckman Coulter and Accuri C6 ff ow cytometer was purchased from BD.
MVs isolation
Rats were anesthetized with an intraperitoneal injection of urethane (1 g/kg). A lead II electrocardiogram (ECG) and arterial blood pressure were monitored throughout the experiment. Aer tracheotomy, each rat was intubated to prepare for ventilation. Thoracotomy was performed on the leedge of sternum. The pericardium was opened and the heart was exposed.e leanterior descending (LAD) coronary artery was occluded with a silk suture between the arterial cone and the leauricle.e ends of the silk suture were threaded through a small vinyl tube to form a snare, and the chest was closed immediately. After achieving hemodynamic stability for 15 minutes, rats were divided into two groups randomly. (1) Sham group, rats were leuntreated for 30 min aer a silk ligature was placed around the LAD. (2) IPC group, rats received three cycles of 5-min ischemia and 5-min reperfusion of the LAD. The blood was drawn from abdominal aorta once the operation was finished. Successful occlusions were verified by observing the development of ST-segment elevation on ECG.
A quarter of the volume of circulating blood from rats was collected in sodium citrate tubes. Total rat blood volume was estimated using the formula: [0.06×body wt (g)+0.77] [8]. Blood samples were centrifuged at 2,600 g for 15 min at room temperature to obtain platelet-poor plasma (PPP), PPP was then isolated and centrifuged at 10,000 g for 5 min at room temperature to obtain platelet-free plasma (PFP). PFP was fi xed with paraformaldehyde (PFA) to a fi nal concentration of 1 % for 1 h at room temperature, snap-frozen in liquid nitrogen, and stored at -80°C until analysis. Remaining PFP was subjected to the ultracentrifugation at 33,000 rpm for 150 min at 4°C, pelleted MVs were resuspended in 100 μl 0.9 % sodium chloride and kept at -20°C.
Flow cytometric analysis
Fixed PFP was thawed in water bath at 37°C and then placed on ice. PFP was blocked with mouse serum and bovine serum albumin (BSA) for 30 minto combine with nonspecific protein sites. Eight EP tubes were divided into testing and control tubes. Each testing tube with 10 μl PFP was incubated with FITC-anti-CD61 5 μl, PE-anti-CD144 3 μl, FITC-anti-CD45 3 μl or APC-anti-Erythroid Cells 2.5 μl to detect different phenotypes of MVs, including PMVs, EMVs, LMVs and RMVs. The same fluorescent labeled isotype antibodies were added to control tubes in the same volume. All antibodies were incubated for 30 min at room temperature in the dark. After being fixed with PFA, samples were diluted in 300 μl PBS. For description of events, 1, 2 μm polystyrene beads were added to the PFP prior to analysis by flow cytometer. Dot plots of forward scatter (FSC)vsside scatter (SSC) were established. Gain settings were adjusted to place 2 μm beads in the upper log for scatter and 1 μm beads in the middle log for scatter. Events<1 μm in diameter with corresponding antibody positive were defined as MVs from various sources. MVs could be counted by 2 μm latex beads with known concentration. The absolute count of MVs was calculated with the formula: MVs/μl = [events counted by ff ow cytometer × (beads added / beads counted by ff ow cytometer)] / sample volume.
Treatment with IPC-MVs on I/R injury in rats
Rats were treated with surgery including anesthesia, thoracotomy, LAD threading and steadiness of haemodynamics. Rats were divided into three groups randomly. (1) Sham group, rats were left untreated for 150 min aer a silk ligature was placed around the LAD. (2) I/R group, rats were subjected to 30 min ischemia and 120 min reperfusion of LAD. (3) IPC-MVs+I/R group, rats were subjected to 30-min ischemia and 120 min reperfusion of LAD. IPC-MVs 7 mg/kg was infused via the femoral vein within 1 min at 25 min during ischemia, while the same volume of 0.9 % sodium chloride was given to the other two groups. Mean arterial blood pressure (MAP), heart rate (HR) and ST-segment of ECG were monitored throughout the experiment.
HE staining
The anterior wall tissues of the left ventricle were fixed in 10 % formalin solution for 24 h. After the dehydration, wax infiltration and embedding, the myocardium was made into paraffin section with thickness of 5 mm to be stained with PE. Labeled myocardial tissues were visualized under an optical microscope, and photographs were taken under high-power magnif i cation (×400).
LDH activity assay
Blood samples (0.2 ml) were drawn from femoral vein after anesthesia, ischemia 30 min and reperfusion 120 min respectively in rats. Plasma was centrifuged at 18,000 g for 15 min at 4°C and then frozen at -20°C until further analysis. LDH activity was measured by a Microplate Reader according to the manufacturer’s instruction with the change in the rate of absorbance proportional to LDH activity.
Myocardial infarct size measurement
Statistical analysis
Flow cytometric results were analyzed by FlowJo 7.6 software. All values were presented as mean ± SD. Means between two groups were analyzed with the Student’st-test. One-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test was used.P<0.05 was regarded as statistically significant. Statistical evaluation was performed by using SPSS 17.0 soware.
Results
Characterization and phenotype identif i cation of IPCMVs
Dot plots of FSCvsSSC showed that 1, 2 μm standard beads were distributed in the appropriate region by modulating the photomultiplier tube (PMT) voltage. R1 area was gated under the region of 1 μm beads which was defined as IPC-MVs population (Fig.1Ai). IPC-MVs were gated in R1 which were conf i rmed by 1 μm beads (Fig.1Aii).
Different phenotypes of IPC-MVs in the R1 gate were further identified by fluorescent labeled antibodies. PFP incubated with FITC-IgG1 was detected aer establishing FITCvsSSC dot plots. Events were concentrated on the region of fluorescent negative, whereas positive region appeared the fluorescence signal after PFP incubated with FITC-CD61 was detected in testing tube. PMVs were identif i ed membrane vesicles smaller than 1 μm with CD61+. The signal peak labeled M1 showed that CD61+PMVs in the histogram (Fig.1B). In accordance with the same testing principle and representing method, EMVs, LMVs and RMVs were defined as CD144+, CD45+and Erythroid Cells+(Fig.1C-E).
Concentration alteration of IPC-MVs
Ef f ects of IPC-MVs on MAP, HR and ST-segment
MAP, HR and ST-segment were recorded during I/ R injury to evaluate the cardiac function of rats. In I/R and IPC-MVs+I/R groups, MAP and HR were significantly lowered versus Sham group at each time point during I/R injury. Compared with I/R group, IPC-MVs increased HR after 120-min reperfusion in myocardial I/R injured rats (331±9vs305±13 beats/min,P<0.01, respectively, Fig.3A-B). Additionally, ST-segment during ischemia was elevated dramatically and decreased gradually after reperfusion in I/R and IPC-MVs+I/R groups. IPCMVs decreased ST-segment at the end of 120-min reperfusion compared with I/R group (0.043±0.027vs0.097±0.056 mV,P<0.05, respectively, Fig.3C).
Ef f ects of IPC-MVs on myocardial morphology
In Sham group, cardiomyocytes showed a well-arranged myof i laments (Fig.4A). In I/R group, myocardium appeared remarkable damage associated with edematous separation of myofilaments. Meanwhile a mass of nuclei disappeared and myocardial fibers fractured with small amount of neutrophil fi ltration (Fig.4B). However, IPC-MVs treatment signif i cantly alleviated the damage of myocardium compared with I/R group (Fig.4C).
Effects of IPC-MVs on LDH activity and myocardial infarct size
Plasma LDH activity and myocardial infarct size were measured to assess myocardial damage after I/R injury. Compared with Sham group, LDH activity was distinctly increased at the end of 30 min ischemia and 120 min reperfusion both in I/R and IPC-MVs+I/R groups. Moreover, compared with I/ R group, the activitiy of plasma LDH was obviously decreased aer 120 min reperfusion in IPC-MVs+I/ R group (10.98±1.01vs12.91±1.11 U/ml,P<0.01, respectively, Fig.5A). Furthermore, compared with I/ R group, IPC-MVs treatment decreased IS/AAR % significantly (29.63±3.90vs40.50±2.54 %,P<0.01, respectively, Fig.5B).ere were no signif i cant dif f erences in AAR between these two groups.
Discussion
Microvesicles derived from vascular cells can be detected in the circulating blood and have served as a new concept of biomarkers in cardiovascular diseases [9]. Accordingly, selecting the correct and appropriate testing method in the process of MVs detection is particularly necessary. MVs can be detected and analyzed by several approaches, such as flow cytometry, capture-based assays, immunoassays and electron microscopy.e most widespread approach is ff ow cytometry which best allows for enumeration among several methods [10]. However, the small size of MVs and background noise may affect the accuracy of measurement. Therefore, it is necessary to improve the detection sensitivity and eliminate the interferencing signals. In our study, beads with 1 μm diameter were used to def i ne the upper limit of MVs population in the detection because MVs are smaller than 1 μm. In addition, minimum value of FSC threshold was set at 30,000 in order that the small size of MVs could be detected, and background noise of PBS could be excluded clearly.
MVs shed by different cells contain a subset of membrane specific proteins derived from original cells. Circulating MVs has been observed in a variety of cells, including endothelium, leukocytes and erythrocytes, as well as platelets. It was known that cell-specific markers on platelets contain CD41, CD42a and CD61.e later was selected to identify PMVs generally. Because CD105 was not only expressed on endothelial cell, but also weakly expressed on leukocytes, CD144 was the most commonly used specif i c marker for EMVs. CD45 and Erythroid Cellspresented on leukocytes and erythrocytes respectively, and they were used to identify LMVs and RMVs [11]. In this study, PMVs, EMVs, LMVs and RMVs could be detected aer PFP was incubated with anti-CD61, anti-CD144, anti-CD45 and anti-Erythroid Cells. The phenotypes of circulating IPC-MVs in rats were elucidated clearly.
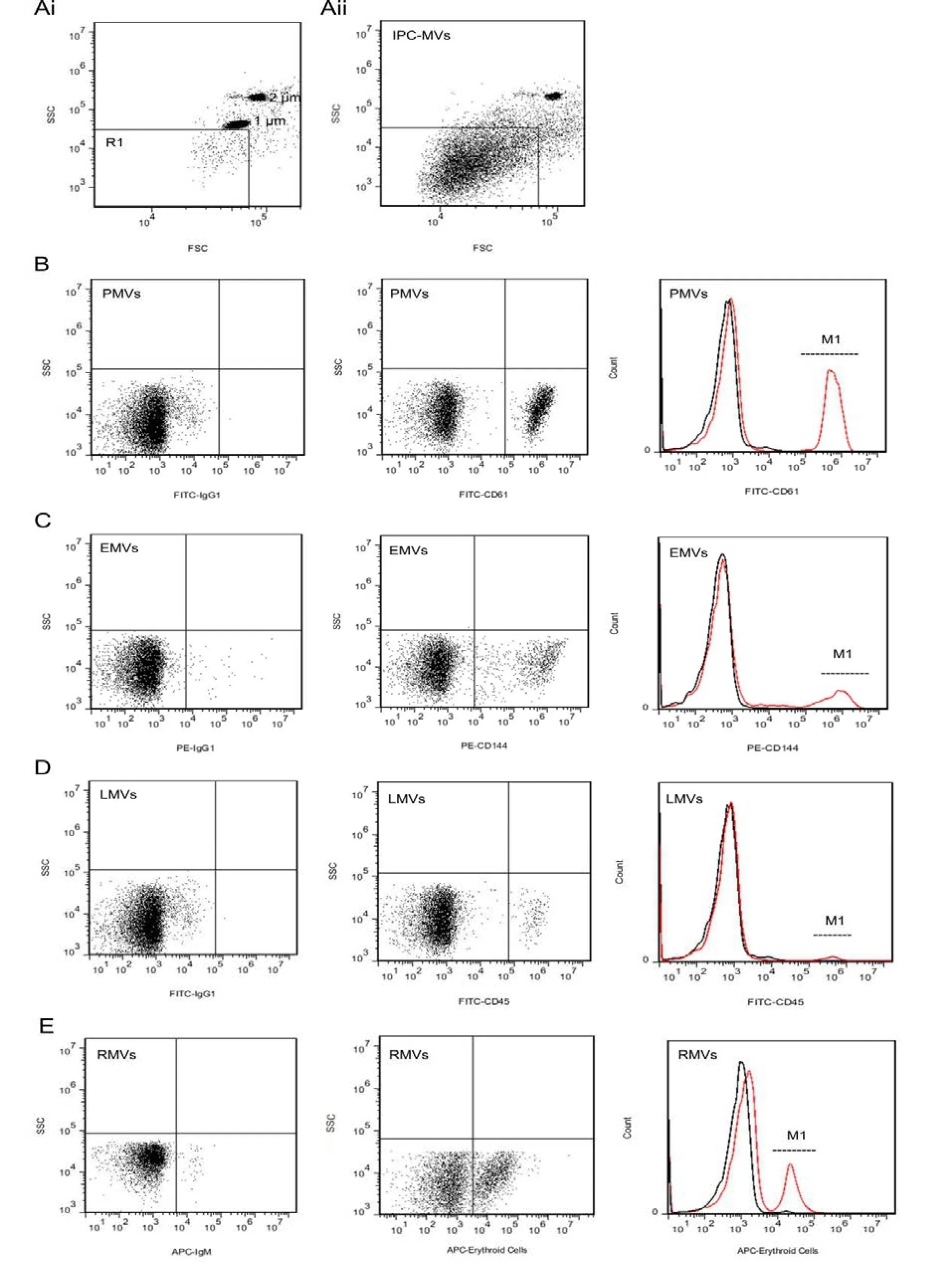
Fig. 1 Characterization and phenotype detection of IPC-MVs. Ai: Representative dot plots of forward scatter (FSC)vsside scatter (SSC) for evaluation of 1, 2 μm latex beads. MVs were identified as events with size less than 1 μm within the gate R1. Aii: Representative dot plots for IPC-MVs in gate R1. B: Representative dot plots of FITCvsSSC for evaluation of IgG1 and CD61 labeled PFP, and representative histogram for CD61+PMVs. C: Representative dot plots of PEvsSSC for evaluation of IgG1 and CD144 labeled PFP, and representative histogram for CD144+EMVs. D: Representative dot plots of FITCvsSSC for evaluation of IgG1 and CD45 labeled PFP, and representative histogram for CD45+LMVs. E: Representative dot plots of APCvsSSC for evaluation of IgM and Erythroid Cells labeled PFP, and representative histogram for Erythroid Cells+RMVs. Signal peak of isotype controls---black lines; Signal peak of cell-surface markers---red lines; Signal peak of IPC-MVs of different phenotypes---M1. IPC-MVs, microvesicles derived from myocardial ischemic preconditioning; PMVs, platelet-derived microvesicles; EMVs, endothelial cell-derived microvesicles; LMVs, leucocyte-derived microvesicles; RMVs, erythrocyte-derived microvesicles.
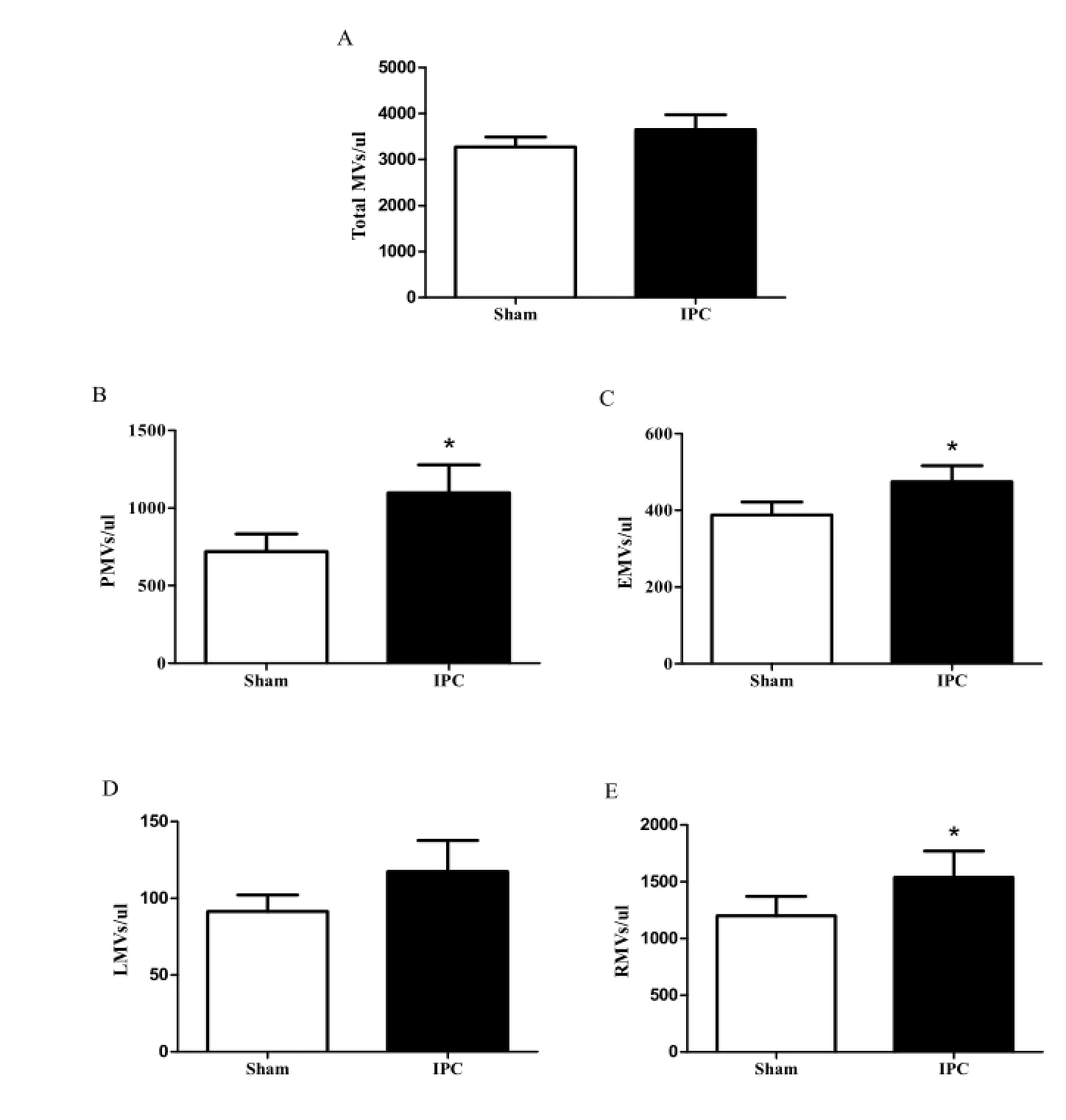
Fig. 2 MVs levels in two groups. A: Total MVs; B: PMVs; C: EMVs; D: LMVs; E: RMVs. PMVs, platelet-derived microvesicles; EMVs, endothelial cell-derived microvesicles; LMVs, leucocyte-derived microvesicles; RMVs, erythrocyte-derived microvesicles.*P<0.05vssham.
It was known that the concentration of PMVs, EMVs, LMVs and RMVs may change following different releasing stimuli and surroundings. Several reports showed that PMVs represented approximately 50%-60% of circulating MVs which played a major biological role in inflammation, coagulation, angiogenesis, and thrombosis [12]. The concentration of them was increased in old patients with essential hypertension complicating with coronary heart disease [13]. EMVs constituted an emerging marker of endothelial dysfunction [14].e levels of EMVs were increased in coronary artery disease patients and were increased obviously which derived from remote ischemic conditioning in rats [8, 15]. Moreover, RMVs were elevated in patients with myocardial infarction treated with primary angioplasty [16]. All above papers indicated that the functions of different original MVs in plasma were closed related with cardiovascular diseases. IPC serve as an endogenous myocardial protective mechanism against severe I/ R injury. However, little is known about the concentration alteration of MVs in plasma derived from myocardial IPCin vivo.e present study first demonstrated that PMVs, EMVs and RMVs were all significantly increased in circulation from IPC treated rats, while LMVs remained unchanged.
To date, the functions of circulating IPC-MVs on I/R injury were unknown. Numerous data have examined that MVs were involved in the occurrence, development and outcome of various cardiovascular diseases, including IHD. One report showed that MVs derived from hypoxia stimulation treated-human bone marrow mesenchymal stem cells could protect cardiac tissues from ischemic injury at least by means of promoting blood vessel formation [17]. However, our previous study demonstrated that EMVs induced by A23187, a calcium ionophore, exerted a pro-apoptotic effect on H9c2 cardiomyocytes [18]. Hence, the participation of MVs is likely to produce beneficial effect, also may produce adverse effect, and it depends on both different releasing stimuli and cell origins of MVs. Current study determined that IPC-MVs treatment decreased plasma LDH activity and myocardial infarct size which indicated an amelioration of cardiac function and cardioprotection of I/R injury in rats.
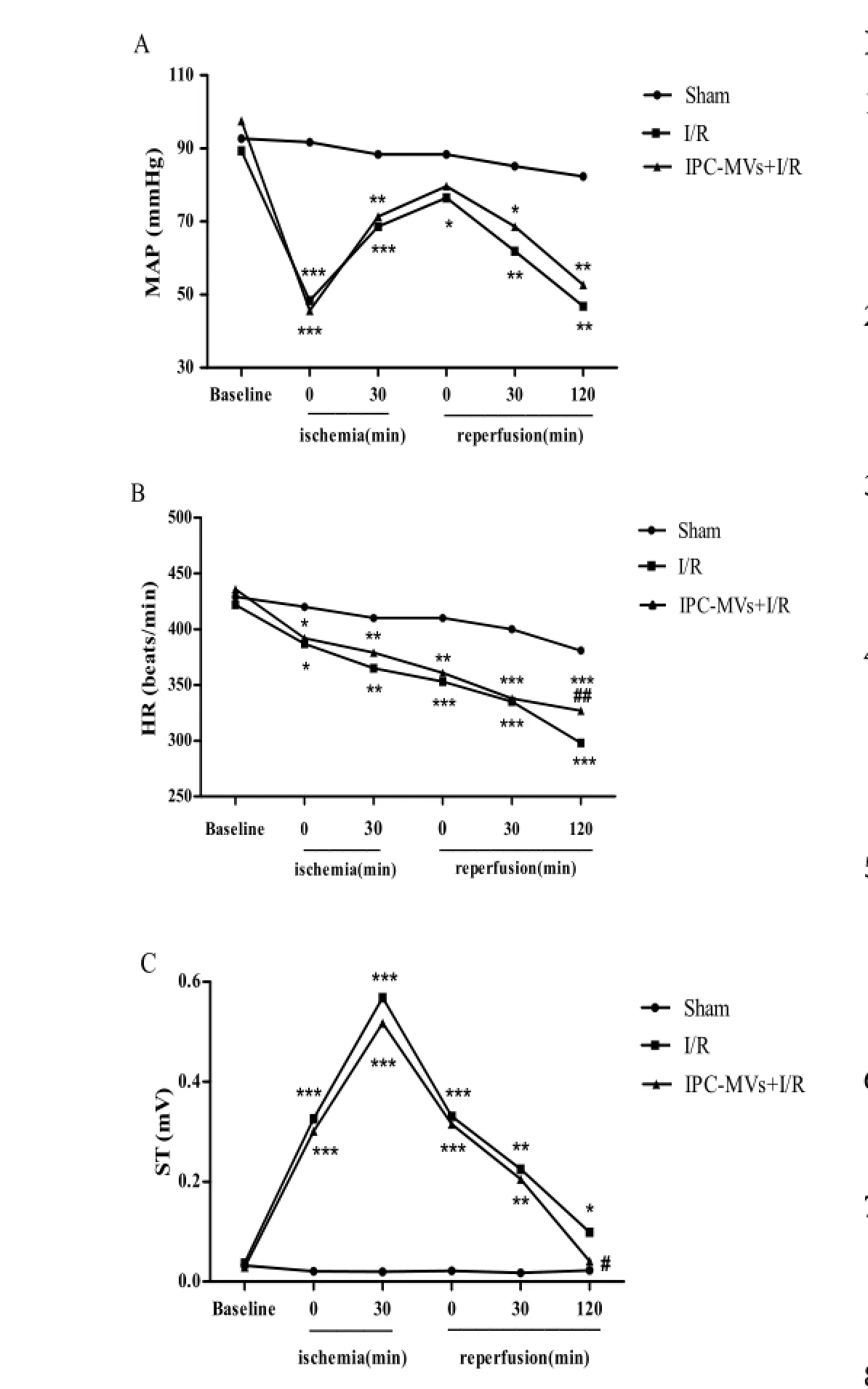
Fig. 3 Effects of IPC-MVs on MAP, HR and ST-segment. A: MAP; B: HR; C: ST-segment. MAP, mean arterial blood pressure; HR, heart rate; IPC-MVs, microvesicles derived from myocardial ischemic preconditioning.*P<0.05,**P<0.01,***P<0.001vsSham;#P<0.05,##P<0.01vsI/R.
In conclusion, our findings demonstrated that the alteration of phenotypes and concentration of circulating IPC-MVs could be characterized by flow cytometry. Moreover, IPC-MVs generated a protective ef f ect on I/R injury in rats. Further studies should be carried out to clarify the underlying cardioprotective mechanisms.
Acknowledgements
1. Cui Y, Zheng L, Jiang M,et al. Circulating microparticles in patients with coronary heart disease and its correlation with interleukin-6 and C-reactive protein [J]. Mol Biol Rep, 2013, 40(11): 6437-6442.
2. Zhu Y, Wu J, Yuan SY. MCT1 and MCT4 expression during myocardial ischemic-reperfusion injury in the isolated rat heart [J]. Cell Physiol Biochem, 2013, 32(3): 663-674.
3. Lai CC, Tang CY, Chiang SC,et al. Ischemic preconditioning activates prosurvival kinases and reduces myocardial apoptosis [J]. J Chin Med Assoc, 2015, 78(8): 460-468.
4. Ishida K, Taguchi K, Hida M,et al. Circulating microparticles from diabetic rats impair endothelial function and regulate endothelial protein expression [J]. Acta Physiol (Oxf), 2015, 3(8): 1-10.
5. Stepien E, Gruszczynski K, Kapusta P,et al. Plasma centrifugation does not influence thrombin-antithrombin and plasmin-antiplasmin levels but determines platelet microparticles count [J]. Biochem Med(Zagreb), 2015, 25(2): 222-229.
6. Franca CN, Izar MC, Amaral JB,et al. Microparticles as potential biomarkers of cardiovascular disease [J]. Arq Bras Cardiol, 2015, 104(2): 169-74.
7. Giricz Z, Varga ZV, Baranyai T,et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles [J]. J Mol Cell Cardiol, 2014, 68: 75-78.
8. Jeanneteau J, Hibert P, Martinez MC,et al. Microparticle release in remote ischemic conditioning mechanism [J]. Am J Physiol Heart Circ Physiol, 2012, 303(7): H871-877.
9. Sluijter JP, Verhage V, Deddens JC,et al. Microvesicles and exosomes for intracardiac communication [J]. Cardiovasc Res, 2014, 102(2): 302-311.
10. Jayachandran M, Miller VM, Heit JA,et al. Methodology for isolation, identif i cation and characterization of microvesicles in peripheral blood [J]. J Immunol Methods, 2012, 375(1-2): 207-214.
11. Van der Heyde HC, Gramaglia I, Combes V,et al. Flow Cytometric Analysis of Microparticles [J]. Methods Mol Biol, 2011, 699: 337-354.
12. Viera AJ, Mooberry M, Key NS,et al. Microparticles in cardiovascular disease pathophysiology and outcomes [J]. J Am Soc Hypertens, 2012, 6(4): 243-252.
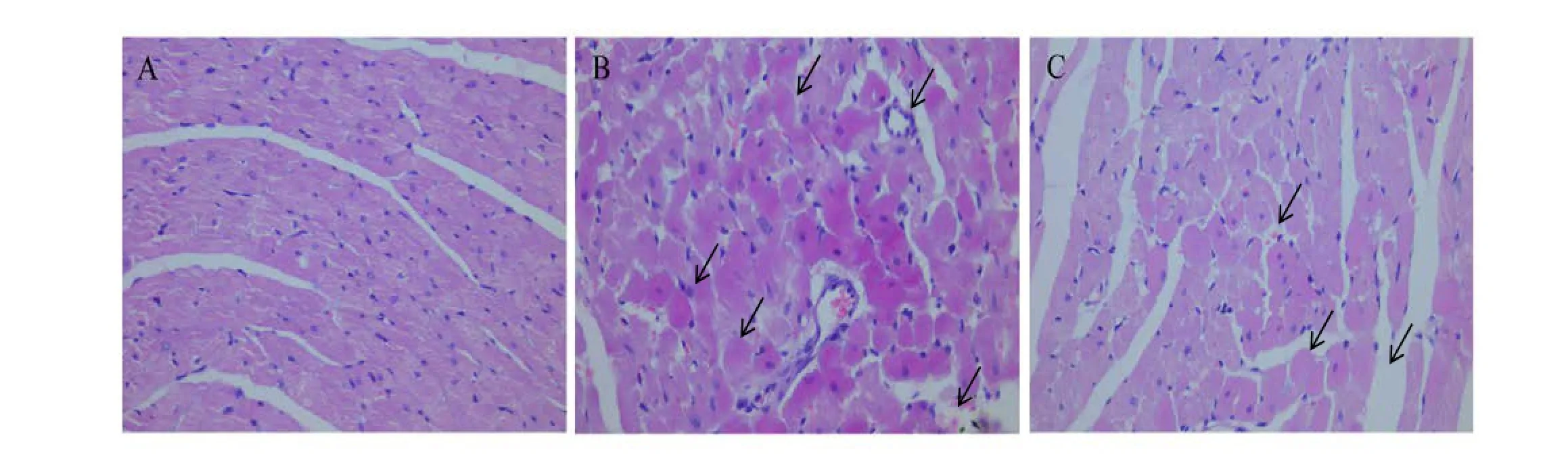
Fig. 4 HE staining of myocardial tissues (original magnification ×400). A: Sham; B: I/R; C: IPC-MVs+I/R. IPC-MVs, microvesicles derived from myocardial ischemic preconditioning.
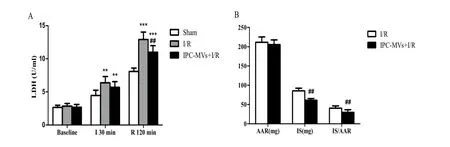
Fig. 5 Effects of IPC-MVs on LDH activity and myocardial infarct size. A: Plasma LDH activity. B: Myocardial infarct size which was expressed as percentage of IS to AAR (IS/AAR %). IPC-MVs, microvesicles derived from myocardial ischemic preconditioning; AAR, area at risk; IS, infarct size; I, ischemia; R, reperfusion.**P<0.01,***P<0.001vssham;##P<0.01vsI/R.
13. Deng XL, Cao J. The concentration of platelet microparticles is increased in old patients with essential hypertension complicating with coronary heart disease [J]. Chin Med J (Engl), 2012, 125(14): 2602.
14. Jansen F, Yang X, Baumann K,et al. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism [J]. J Cell Mol Med, 2015, 19(9): 2202-2214.
15. Horn P, Amabile N, Angeli FS,et al. Dietary flavanol intervention lowers the levels of endothelial microparticles in coronary artery disease patients [J]. Br J Nutr, 2014, 111(7): 1245-1252.
16. Giannopoulos G, Oudatzis G, Paterakis G,etal. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty [J]. Int J Cardiol, 2014, 176(1): 145-150.
17. Bian S, Zhang L, Duan L,et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model [J]. J Mol Med (Berl), 2014, 92(4): 387-397.
18. Shang M, Zhang Q, Zhang MX,et al. Effects of endothelial microvesicles induced by A23187 on H9c2 cardiomyocytes [J]. Chin J Appl Physiol, 2013, 29(6): 559-564.
Yan-xia LIU, MD, PhD, Professor; Junqiu SONG, MD, PhD, Associate Professor, Department of Pharmacology, School of Basic Medical Sciences, Tianjin Medical University, 22 Qixiangtai Road, Heping District, Tianjin 300070, China. Tel/Fax: 86-22-83336835; E-mail: liu_yanxia126@126. com; song_junqiu@126.com
Received 2015-11-12; accepted 2015-11-20
杂志排行
中国应用生理学杂志的其它文章
- A mini review: Tau transgenic mouse models and olfactory dysfunction in Alzheimer’s Disease
- Ethical inspection about laboratory animals
- Better parameters of ventilation-CO2output relationship predict death in CHF patients
- Association between endothelial nitric oxide synthase (ENOS) G894T polymorphism and high altitude (HA) adaptation: a meta-analysis
- Synergisms of cardiovascular effects between iptakalim and amlodipine, hydrochlorothiazide or propranolol in anesthetized rats
- Effects of curcumin on sodium currents of dorsal root ganglion neurons in type 2 diabetic neuropathic pain rats
