A mini review: Tau transgenic mouse models and olfactory dysfunction in Alzheimer’s Disease
2015-05-22
Department of Pharmacology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou 510080, China
Review
A mini review: Tau transgenic mouse models and olfactory dysfunction in Alzheimer’s Disease
Yang HU, Wen-ting DING, Xiao-nan ZHU, Xue-lan WANG
Department of Pharmacology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou 510080, China
doi 10.13459/j.cnki.cjap.2015.06.001
Alzheimer’s Disease (AD) is a chronic neurodegenerative disease that usually takes many years from preclinical phase to prodromal phase characterized by mild symptoms before the onset of demena. Once diagnosed with AD, the brain is already severely damaged and the disease will process quickly to the most severe stages since there is no medicaons that reverse the neuronal injuries in the brain. Thus, simple, inexpensive, and widely available methods for detecng potenal AD paents during their preclinical phases are urgently needed. In such case, olfactory tesng may of f er a chance for early diagnosis of AD. However, there are limitaons in these olfactory tests due to the complexity of the brain areas it extends to and the frequently olfactory fague occurred in the behavioral olfactory tests. Great ef f orts have been done epidemiologically to invesgate the correlaon between olfactory funcons and possibility of developing AD. Dif f erent paerns of olfactory dysfuncon have been found in AD at early stages and even mild cognive impairment (MIC), but the cause of the dysfuncon remained unclear. Various kinds of AD animal models have been used in the fi eld to clarify the existence of olfactory dysfuncons and thus study the underling mechanism of the dysfuncon. In this review we discuss (1) the funcon of Tau physiologically and pathologically; (2) the genec background and biological characteriscs of the most commonly used Tau transgenic mice; (3) the structural and molecule basis of olfacon; (4) the possible relaonship between Tau pathology and olfactory dysfuncon. Finally, we suggest that the tau transgenic mouse models may be helpful in studying the possible mechanisms of the dysfuncon.
tau; olfactory dysfuncon; transgenic mouse; olfactory circuitry
Introduction
Alzheimer’s disease is a neurodegenerative disorder clinically characterized by progressive memory loss and cognitive decline [1]. The neuropathological hallmarks of AD are extracellular deposits of Aβ-amyloid, intraneuronal neurofibrillary tangles (NFTs) and the loss of neurons and synapses. In the tau pathology af f ected neurons, tau protein is hyperphosphorylated and becomes detached from the microtubules.e detached tau further accumulates in the somatodendritic compartment in forms of paired helical fi laments (PHFs) and straight fi laments. NFTsdeposition in AD patients correlates with cognitive decline and neuronal loss [2].
Tremendous efforts have been devoted into developing animal models to better understand AD pathology and its underlying mechanisms. Most of these models overexpress certain mutant genes which are discovered in familial AD (FAD) or frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) [3]. A majority of these models are typically generated by microinjecting complementary DNA (cDNA), inserted with an AD related transgene, into the founder male pronuclei [4, 5]. Each kind of the model is different based on the dif f erent mutant genes it carries. Although transgenic animal models are extremely useful that they provide good chance for researchers to explore the mechanisms of AD, these models do have their limits [6]. As mentioned above, since the foreign genes are induced into the mice using the technique of microinjection, neither the location nor the absolute copy numbers of the transgene can be determined. Thisleads to discrepancy in the expressions of certain transgenes within same line of mice and, thus, unpredictable changes in their behavioral performances [7]. Knock-in and knock-out models are generated to address such limitations [8, 9]. According to these limitations and differences that each model would have carried, it is of vital importance for researchers to considers their biological characteristics and make wise decision when choosing these mouse models. Despite limitations and dif f erences, animal models of AD still provide important tools for us to study AD.e fi rst part of this review summarizes the properties of Tau protein and the features of newer mouse AD models.
Studies have shown that the tau pathology correlates well with the process of AD. Braak indicates that the tau pathology occurs early in the development of AD [10]. Studies relating the tau pathology and the olfactory dysfunction are limited, but still they reveal some important features of the possible mechanisms underlie the olfactory dysfunction in AD [11].e second part of this review mainly discusses structural and molecule basis of olfaction and the possible relationship between Tau pathology and olfactory dysfunction. Thus, we suggest that future studies should be more focus on the Tau pathology mouse models and their better utilization on the investigation of olfactory def i cits.
Tau pathology
The structure and physical function of tau protein
Microtubule-associated protein tau gene (MAPT) is genetically located in chromosome 17q21-22. In normal adult human brain, tau comprises six isoforms (Fig.1)viaalternative mRNA splicing at two different locations. These differences in splicing of exon 10 generate three isoforms with three microtubule binding repeats (3R) each and another three isoforms with four repeats (4R) each [12].e repeats contain 31 or 32 amino acids in length and are located near the carboxy-terminus. In addition, the inserts of 29 or 58 amino acids or no insert in the amino-terminus generates 1 N, 2 N or 0 N forms of each 3R and 4R tau with the molecular weight of 48-67 kD [13]. In physical condition, tau protein located in the neuron axon is able to combine with microtubules and promote microtubule assembly and maintain the stability of the microtulbules. Tau keeps these important features of microtubules to guarantee the physical functions of microtubules such as transporting the nutrients and chemical messengers.e repeats at carboxy-terminus are the binding sites of tau with microtubules, while that at amino-terminus is the binding site of tau with other components within the cell or the cell membrane [14].
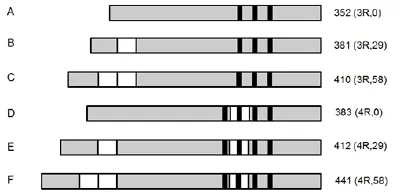
Fig. 1 Schematic representation of the six tau isoforms.
The abnormally phosphorylation of tau protein
In normal adult human brain, the longest isoform of tau (2N4R) consists of 441 amino acids which includes 80 Ser/Thr and 5 Tyr phosphorylation sites. Studies show that there are 40 of these Ser/r sites are related with AD pathology. Phosphorylation level is important in regulation of assembly of microtubules with tau in neuron. Hyperphosphorylated Tau is weak in the assembly ability [12]. In normal adult human brain each mole Tau protein contain 2-3 mole phosphate groups, while, in the brain of AD patient, the proportion of phosphorylated tau (including abnormally phosphorylated tau ) goes up to three times more than the normal brain. On the one hand, phosphorylated tau (P-Tau) is able to detach the normal tau or other microtubule-associated proteins (MAP1 or MAP2) from the microtubules thus lead to the depolymerization of the whole microtubule. On the other hand, P-Tau is able to aggregate with the detached tau or other P-Tau to form NFTs which can destruct the stability of microtubules which fails the axonal transport processes and thus lead to the neuronal loss [15].
The expression of tau mutant genes
Intraneuronal NFTs comprised of hyper-phosphorylated tau is a pathological feature of many neurodegenerative diseases, such as Pick’s disease, FTDP-17 and AD [16]. Typically, there are 18 mutations that are related to FTDP-17 and are commonly used in transgenic mouse models of tau pathology.irteen of these mutants are previously reviewed [17], which contributes to the understanding of tau related pa-thology. There are generally two forms of mutant gene that are associated with FTDP-17 [14]: mistranslation and selective changes in splicing.e former mutation results in the changes of tau structure and the impairment of its binding ability with microtubules, while the latter changes the ratio of wildtype 3R to 4R Tau, usually increase the 4R isoform, both mutations result in an increase of the detached Tau which facilitate the formation of NFTs.
Transgenic mouse models of Tau pathology
Details on the two major types tau transgenic mouse models are listed are presented in Table 1 and Table 2. Both of the two patterns of models express hyperphosphorylated tau, deposition of NFTs, neuronal loss and show age-dependent behavioral changes. Compare to mutant human tau mouse, the wild-type human tau mouse has milder changes in both pathology and behavior. Mouse models with mutant tau usually show AD-like symptom in a very young age that generates NFTs depositions and develops severe spatial learning and memory impairments. However, some of these mice develop motor deficits or even show symptoms of depression. These are all factors that can af f ect their performance in spatial learning and memory tests. In addition, all these models are not able to mimic the selective loss of cholinergic neurons in AD patients.
Since tau is one of the old statesmen of AD molecules, tremendous ef f orts are put into the study of tau transgenic mouse models [18,19].e fi rst decade of this history has been reviewed above and previously [20], so we focus here on the most recent advances on these models or newer tau models. A big advance in recent studies on tau lies in the species of tau [21]. The generation of regulatable tau transgenic mouse models becomes better tools in the study of toxic Tau. Early models show that expressing mutant tau is suffi cient to cause both Tau pathology and neuronal loss [22]. Dif f erent from the earlier models, these co-existing pathological changes are dissociated in the model of rTg4510 in which the expression of tau can be suppressed with doxycycline. Studies show that the repression of tau expressing prevents the rTg4510 mice from progressive age-related NFTs, neuronal loss, and behavioral impairments [23]. This study indicates that the insoluble NFTs may not be responsible to the neuronal and functional loss. On the contrary, accumulating evidences show that, in both the rTg4510 and hTau lines, neurons without tau aggregates are susceptible to death [24]. Similarly, Leora et al. investigate the response of cells in hippocampal circuits and they fi nd that soluble tau contributes to impairment of hippocampal function, while tangles do not prevent neurons from responding [25]. Katja et al. generate four regulatable tau transgenic mouse models to investigate the role of dif f erent species of tau (full-length tau / truncated tau or pro-aggregant tau / anti-aggregant tau) played in the progression of the disease.ey clarify that the aggregant full-length tau leads to a pre-tangle pathology, while the truncated tau causes massive formation of neurof i brillary tangles and neuronal loss in the hippocampus.is study provides direct evidences to the hypothesis that the tau pathology may be reversible [26].
Olfaction
The structure of olfactory system
Food or ff owers can give of f special scents according to the different and unique structures of the scents.ese scents are called odorants that oen mixed in the air. When inhaling the air with nose, odorants are subsequently carried by the airf f ow which directs air superiorly toward the olfactory epithelium [39]. Odorants are next captured by olfactory sensory neurons (OSNs) which locate in the olfactory epithelium that distributed in the posterior and middle part of the cribriform plat. These primary OSNs are bipolar neurons which project a single dendrite with an olfactory knob extends to the cribriform plate at the roof of the nasal cavity and an axon that transmits the odorant signals to olfactory bulb (OB) or higher brain regions [40]. The primary olfactory system includes: piriform cortex, olfactory nucleus and tubercle, amygdala, entorhinal cortex, while secondary olfactory system includes: hippocampus, hypothalamus, thalamus, orbitofrontal cortex, and the cerebellum. These classifications are primarily determined according to the sequence of brain regions involved process of odorant perception [41]. Some researchers have been trying to study the specific function of each division, but more and more studies indicate that the process of olfactory perception is extremely complex that involves simultaneous stimulating of several brain areas.us, it seems to be pointless toseparate them.

Tab. 1 Genetic background of tau transgenic mouse models.
Neuronal circuitry in the OB and its linkage to other brain areas
In mouse, there are approximately 900 different odorant receptors (ORs) expressed in the OSNs, but each OSN expresses only one functional OR gene. Furthermore, OSNs expressing the same OR converge their axons to form glomeruli at stereotyped locations in the OB [42].ere are majorly four types of neurons in the OB: periglomerular cells (PGs), mitral cells (MC), tufted cells (TC) and superficial short-axon cells (SSAs). The axons of OSNs project to the OB and link to secondary sensory neurons, for example, PGs and external TCs. SSAs are closely related to PGs and a part of them may be located within the deep glomerular layer. Principal neurons include M/Cs and TCs link to the dendrites of inhibitory granule cells (Gr) through reciprocal connections in the external plexiform layer, subsequently receiving af f erent recurrent from OSNs and ef f erent current from central brain areas [43]. In contrast, M/ Ts can project out of the OB to several central brain areas.
Much has been elucidated regarding the functional organization of the axonal connection of olfactory sensory neurons to olfactory bulb (OB) glomeruli. However, the manner in which projection neurons of the OB process odorant input and send this information to higher brain centers remains unclear. Shin Nagayama et al. [43] inject a tracer into a single glomerulus to investigate the distribution of the axonal projections of M/Cs and TCs. They have successfully tracked the principal projections from mitral cells and tufted cells respectively from the lateroventral surface of mouse brain, including the anterior/posterior piriform cortex (PC) and olfactory tubercle (OT).ese two distinct projections are: PC-orienting axons and OT-orienting axons as they labeled. PC-orienting axons primarily projected axon collaterals broadly of the PC, while the OT-orienting axons projected axon collaterals primarily to the anterolateral OT (alOT). Further study reveals that the PC-orienting axon populations stem from MCs, while the OT-orienting axons in TCs. Their data provide us with clear evidence of a direct linkage between the OB and the central brain areas.
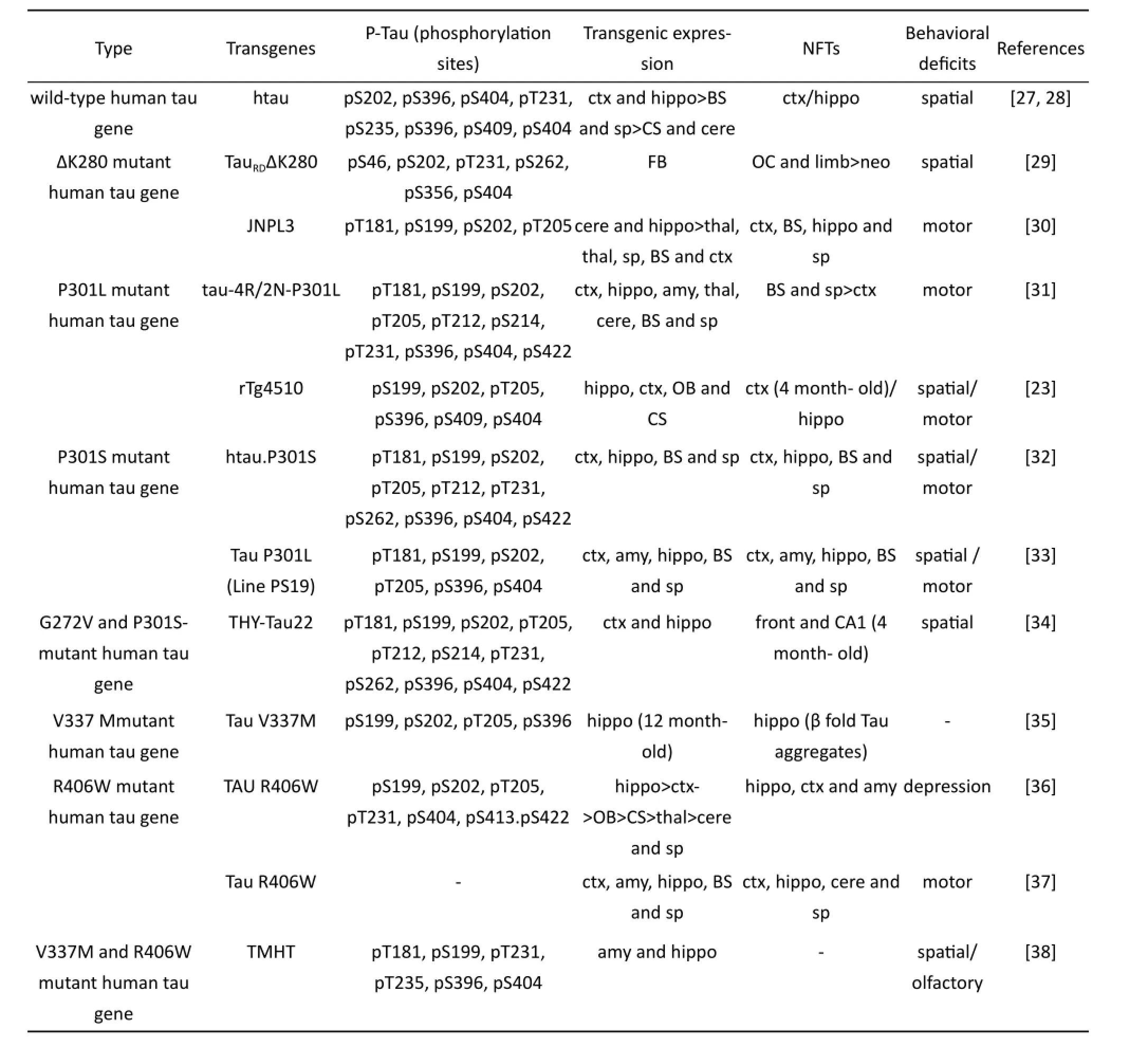
Tab. 2 Pathological and behavioral changes of tau transgenic mouse models.
Molecular mechanisms of olfactory learning and memory
Brain-derived neurotrophic factor (BDNF)
Signaling pathways relating to formation of olfactory or hippocampal memory, especially short term memory, often involves the neurogenesis in both adult brain and neonatal brain. BDNF plays an important role in the underling mechanism of neurogenesis. For example, Scharfmanet al. [44] infuses BDNF into the hippocampus of adult rats and fi nd that the number of adult-born granule cells is increased compared to control rats infused with saline vehicle or bovine serum albumin. In accordance with the study, other researchers also emphasize the role of BDNF in the survival of adult-born neurons. However, another research [45] suggests that the number of surviving new neurons in the hippocampus did not change in the brain of heterozygous BDNF knockout mice in spite of the significant increase of proliferation observed in the sub-granular zone (SGZ). Although it has been much less thoroughly studied in the olfactory system, some studies show that the expression of BDNF in neonatal OB is reduced during olfactory sensory deprivation. Furthermore, BDNF transcription is activated in OB and piriform cortex aer odor conditioning. Partial deprivation or structural changes of BDNF can cause olfactory behavioral changes in mice. For example, Bathet al. [46] fi nds out that both deheterozygous BDNF knockout mice andBDNF(Val66Met) mice exhibit reduced secretion of BDNF and impairments in olfactory discrimination.
Norepinephrine (NE)
Odorant cues can trigger the release of NE from nucleus ceruleus (LC) and trigger rapid increases in NE levels in OB as well as the accessory olfactory bulb (AOB) in behaving animals. In addition, in the olfactory system, NE modulates both bulbar and cortical processing. Doucetteet al. [47] demonstrate that aer blocking the α and β adrenergic receptors, the discrimination between perceptually similar odorants is impaired. NE modulation may have a long term ef f ect on odor specif i c neural habituation related olfactory memory. Shea et al. [48] use stimulation of LC that precisely coordinate NA surges with odor exposure on anesthetized mice and demonstrate that the response to pairing odors is reduced due to NA release. Increasing NE in the OB can modulate the detection of low concentration odorants. A computational model of the olfactory bulb and piriform cortex suggest that NE play an important role in modulating the detection and associative learning of very low odor concentrations. Their results also demonstrate that bulbar norepinephrine serves to pre-process odor representations to facilitate cortical learning, but not recall [49].
The role of Tau in structural mechanisms of olfactory learning and memory
Tau in synaptic plasticty
Tau transgenic mouse overexpressing mutant tau is associated with spine loss. Spine density is reduced in the pyramidal cells and the hippocampal circuits response is impaired of rTg4510 mice compares to the control group. Also in 4.5-month old rTg4510 mice, mislocalization of htau occurs in spines and leads to memory loss and synaptic plasticity def i cits, which is prior to overt synaptic or neuronal degeneration [50]. Recent studies on tau and synaptic plasticity pull Aβ, tau, and dendritic spine loss together. For example, Roberson et al. found that Aβ, tau, and Fyn together impair synaptic network function APP23 mice, pR5 transgenic and tau-/-mice [51]. Ittner et al. indicated that tau has a dendritic function in targeting the Src kinase Fyn to dendritic spines. Fyn phosphorylates NMDA receptor NR2 subunits and mediates their interaction with the postsynaptic scaf f olding protein PSD95.us, through disruption of this interaction with tau, Fyn prevents Aβ toxicity in hAPPJ9 and hAPPJ20 mice [52].
Tau in adult neurogenesis
Adult neurogenesis involves a multistep process that includes the production of cell progenitors, their migration, their dif f erentiation and maturation into fully integrated neurons. The differentiation of adult-born neurons within OB and its role olfactory learning and memory are extensively studied and reviewed elsewhere [53]. Recently, Mariana et al. unveil a direct causal relationship between the adult neurogenesis and the function of the olfactory bulb circuit. In their study, activation of adult-born neurons facilitates on both odor learning and memory using odor discrimination tests, yet only when the task is difficult [54]. The phosphorylation of Tau involves in adult neurogenesis. Three-repeat tau is expressed and highly phosphorylated in adult-born granule cells in the dentate gyrus [55]. An enhanced proliferation induces the adult neurogenesis within hippocampus, but the data regarding OB is missing. On the contrary, in one strain of tau knockout mice, adult neurogenesis is severely reduced [55]. However, adult tau knockout mice showed no deficits in a variety of learning and memory paradigms, thus the importance of tau in adult neurogenesis in learning and memory is still lack of data and under debate.
AD/ Tau pathology and olfactory dysfunction
Olfactory dysfunction in AD
Olfactory dysfunction is a common feature of many neurodegenerative disease, including Parkinson’s disease (PD), FTDP-17, amyotrophic lateral sclerosis (ALS) and Huntington’s disease (HD). In particular, studies show that approximately 90% of AD patients have impairments in olfaction. According to Jianli Wang et al. [56], in the fMRI test, compare to the healthy control group, AD samples show signif i cantly weaker activations within the primary olfactory cortex (POC), hippocampus, and insula, which receive projections from the OB and are involved in olfactory-associated processing.e results of the fMRI test are correlated with the def i cits in odor identif i cation ability detected by University of Pennsylvania Smell Identif i cation Test (UPSIT) and the odor identif i cation ability is correlated with olfactory and neurocognitive dementia measures. Patients with amnestic mild cognitive impairment (aMCI) and non-amnestic MCI (naMCI) do not dif f er signif i cantly in olfactory identification tested by Motol Hospital Smell Test (MHST) and both performed signif i cantly worse than controls but better than patients with mild AD.Patients with aMCI mainly progress to AD dementia, while patients with naMCI may convert more frequently to non-AD dementias, especially to FTLD, PD and Lewy body disease (LBD) where olfactory identif i cation def i cits are frequently found [57].us, olfactory identification may be similarly impaired in aMCI and naMCI patients.e study suggests an early involvement of odor identification deficits in AD patients.
Role of tau pathology in olfactory dysfunction
Deposits of hyperphosphorylated tau was found in olfactory epithelium, OB and anterior olfactory nucleus in AD patients. For example, 72% of tested AD patients have P-Tau deposits while 23.9% of them have Aβ deposits [11]. Mackninet al. [58] demonstrate that the five-month-old Ta1-3RT mice which express wild-type human tau protein show impairment in olfaction using habituation/dishabituation test. Furthermore, immunohistochemisty results show that positive immunostains are found in six -month-old Ta1-3RT mice, but in the silver staining test the results are similar to non-transgenic mice controls. Given the olfaction is related to several brain regions, the olfactory dysfunction in AD patients indicates that the tau pathology can be detected in primary and secondary olfactory regions. Indeed, Cassanoet al. demonstrate that NFTs are observed in piriform cortex, entorhinal cortex, orbotal frontal cortex and hippocampus [59].
According to Braak stages, abnormally hyperphosphorylated Tau occurs earlier in the entorhinal cortex than other brain regions such as temporal lobe, hippocampus and striatum. Furthermore, NFTs and neuropil threads are observed in the olfactory tract (OT) as early as they are in the entorhinal cortex. Age-related axonal transport reductions in the OT are detected using manganese-enhanced MR imaging in AD rat and mouse models. Similar changes in axonal integrity are also observed in the OT of MCI. In a study using PET and18F-FDG combined with diffusion tensor imaging (DTI), Crosset al. [60] shows that the fi ber tract integrity in the olfactory tract correlates with cortical glucose metabolism in olfactory processing structures (in brain areas located within cortex and thalamus) of MCI patients. As microtubule-associated protein (MAP), Tau protein plays an important role in stabilizing microtubules (as mentioned above in section 1). In AD or other tauopathy, Tau is disabled through the process of (abnormally) hyperphosphorylation. But it remains unknown why the olfactory areas are vulnerable to these changes. In our previous study [61], NO concentration (measured by NO metabolites) is decreased in several brain areas of the human P301L tau transgenic mice. Furthermore, the decrease in NO concentration signif i cantly correlates with the scores of odor discrimination test. NO is an important regulator in olfactory dependent learning and memory. NO may have some subtle inf f uence on our tau transgenic mice. However, since no similar studies are found and the data in our study is very limited, whether there is an interaction between the NO and pathological tau remained unknown.
Conclusion
Despite the impressive progress researchers have made in their attempts to mimic many aspects of AD, no model fully reflects the biochemical hallmarks combined with the progressive cognitive and behavioral symptoms present in AD accurately. However, transgenic mice remains the most important tool for research of the neuropathology in AD, since these models are excellent that mimics specific facets of AD. Olfactory dysfunction is a very important symptom occurred in early AD patients that may be very helpful in early diagnosis. Tau pathology occurs early in the olfactory regions in the brain of AD patients and disabled tau can be harmful to the nutrients transport within neurons and signal transmissions in odorant perception or formation of olfactory memory. Alix de Calignonet al. [62] develop a transgenic mouse model, labeled model of early AD, which restrictedly overexpresses human P301L tau in the second layer of entorhinal cortex (EC-II). Furthermore, they demonstrate that in their model tau pathology can progress from EC transgene-expressing neurons to neurons without detectable transgene expression, from EC neighboring cells to downstream neurons in the dentate gyrus and CA fi elds of the hippocampus, and cingulate cortex. Although this is an advanced model in studying the relationship of tau pathology and olfactory deficits, neither in this study nor in their later studies do they provide data relating behavioral olfactory test. Thus, better and proper utilization of our existing Tau or other transgenic models is very important in future study of olfactory dysfunction in AD.
1. Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer’s disease[J]. Trends Mol Med, 2015, 21(6): 394-402.
2. Arshavsky YI. Alzheimer disease and cellular mechanisms of memory storage[J]. J Neuropathol Exp Neurol, 2014, 73(3): 192-205.
3. Ferrari R, Grassi M, Salvi E,et al. A genomewide screening and SNPs-to-genes approach to identify novel genetic risk factors associated with frontotemporal dementia[J]. Neurobiol Aging, 2015, 36(10): 2904-2913.
4. Boluda S, Iba M, Zhang B,et al. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains[J]. Acta Neuropathol, 2015, 129(2): 221-237.
5. Xu H, Rosler TW, Carlsson T,et al. Memory def i cits correlate with tau and spine pathology in P301S MAPT transgenic mice[J]. Neuropathol Appl Neurobiol, 2014, 40(7): 833-843.
6. Platt TL, Reeves VL, Murphy MP. Transgenic models of Alzheimer’s disease: better utilization of existing models through viral transgenesis[J]. Biochim Biophys Acta, 2013, 1832(9): 1437-1448. 7. Balducci C, Forloni G. APP transgenic mice: their use and limitations[J]. Neuromolecular Med, 2011, 13(2): 117-137.
8. Gilley J, Seereeram A, Ando K,et al. Agedependent axonal transport and locomotor changes and tau hypophosphorylation in a“P301L” tau knockin mouse[J]. Neurobiol Aging, 2012, 33(3): 621.
9. Saito T, Matsuba Y, Mihira N,et al. Single App knock-in mouse models of Alzheimer’s disease[J]. Nat Neurosci, 2014, 17(5): 661-663.
10. Braak H, Alafuzof f I, Arzberger T,et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry[J]. Acta Neuropathol, 2006, 112(4): 389-404.
11. Attems J, Walker L, Jellinger KA. Olfactory bulb involvement in neurodegenerative diseases[J]. Acta Neuropathol, 2014, 127(4): 459-475.
12. Morris M, Maeda S, Vossel K,et al. The many faces of tau[J]. Neuron, 2011, 70(3): 410-426.
13. Wang JZ, Xia YY, Grundke-Iqbal I,et al. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration[J]. J Alzheimers Dis, 2013, 33 (Suppl 1): S123-S139.
14. Ghetti B, Oblak AL, Boeve BF,et al. Invited review: frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging[J]. Neuropathol Appl Neurobiol, 2015, 41(1): 24-46.
15. Hernandez F, Lucas JJ, Avila J. GSK3 and tau: two convergence points in Alzheimer’s disease[J]. J Alzheimers Dis, 2013, 33 (Suppl 1): S141-S144.
16. Cooper-Knock J, Kirby J, Ferraiuolo L,et al. Gene expression prof i ling in human neurodegenerative disease[J]. Nat Rev Neurol, 2012, 8(9): 518-530.
17. Rao AT, Degnan AJ, Levy LM. Genetics of Alzheimer disease[J]. AJNR Am J Neuroradiol, 2014, 35(3): 457-458.
18. Barten DM, Cadelina GW, Hoque N,et al. Tau transgenic mice as models for cerebrospinal ff uid tau biomarkers[J]. J Alzheimers Dis, 2011, 24 (Suppl 2): 127-141.
19. Matarin M, Salih DA, Yasvoina M,et al. A genome-wide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology[J]. Cell Rep, 2015, 10(4): 633-644.
20. Gotz J, Deters N, Doldissen A,et al. A decade of tau transgenic animal models and beyond[J]. Brain Pathol, 2007, 17(1): 91-103.
21. Sahara N, DeTure M, Ren Y,et al. Characteristics of TBS-extractable hyperphosphorylated tau species: aggregation intermediates in rTg4510 mouse brain[J]. J Alzheimers Dis, 2013, 33(1): 249-263.
22. Cook C, Kang SS, Carlomagno Y,et al. Tau deposition drives neuropathological, inflammatory and behavioral abnormalities independently of neuronal loss in a novel mouse model[J]. Hum Mol Genet, 2015, 24(21): 6198-6212.
23. Santacruz K, Lewis J, Spires T,et al. Tau suppression in a neurodegenerative mouse model improves memory function[J]. Science, 2005, 309(5733): 476-481.
24. Kayed R. Anti-tau oligomers passive vaccination for the treatment of Alzheimer disease[J]. Hum Vaccin, 2010, 6(11): 931-935.
25. Fox LM, William CM, Adamowicz DH,et al. Soluble tau species, not neurofibrillary aggregates, disrupt neural system integration in a tau transgenic model[J]. J Neuropathol Exp Neurol, 2011, 70(7): 588-595.
26. Hochgrafe K, Sydow A, Mandelkow EM. Regulatable transgenic mouse models of Alzheimer disease: onset, reversibility and spreading of Tau pathology[J]. FEBS J, 2013, 280(18): 4371-4381.
27. Andorfer C, Kress Y, Espinoza M,et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms[J]. J Neurochem, 2003, 86(3): 582-590.
28. Polydoro M, Acker CM, Duff K,et al. Agedependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology[J]. J Neurosci, 2009, 29(34): 10741-10749.
29. Mocanu MM, Nissen A, Eckermann K,et al. The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous tau in inducible mouse models of tauopathy[J]. J Neurosci, 2008, 28(3): 737-748.
30. Lewis J, McGowan E, Rockwood J,et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein[J]. Nat Genet, 2000, 25(4): 402-405.
31. Terwel D, Lasrado R, Snauwaert J,et al. Changed conformation of mutant tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of tau-4R/2N transgenic mice[J]. J Biol Chem, 2005, 280(5): 3963-3973.
32. Allen B, Ingram E, Takao M,et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein[J]. J Neurosci, 2002, 22(21): 9340-9351.
33. Takeuchi H, Iba M, Inoue H,et al. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating[J]. PLoS One, 2011, 6(6): e21050.
34. Schindowski K, Bretteville A, Leroy K,et al. Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits[J]. Am J Pathol, 2006, 169(2): 599-616.
35. Tanemura K, Murayama M, Akagi T,et al. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau[J]. J Neurosci, 2002, 22(1): 133-141.
36. Tatebayashi Y, Miyasaka T, Chui DH,et al. Tau filament formation and associative memory def i cit in aged mice expressing mutant (R406W) human tau[J]. Proc Natl Acad Sci U S A, 2002, 99(21): 13896-13901.
37. Zhang B, Higuchi M, Yoshiyama Y,et al. Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy[J]. J Neurosci, 2004, 24(19): 4657-4667.
38. Flunkert S, Hierzer M, Löffler T,et al. Elevated levels of soluble total and hyperphosphorylated tau result in early behavioral def i cits and distinct changes in brain pathology in a new tau transgenic mouse model[J]. Neurodegener Dis, 2013, 11(4): 194-205.
39. Barber CN, Coppola DM. Compensatory plasticity in the olfactory epithelium: age, timing, and reversibility[J]. J Neurophysiol, 2015, 114(3): 2023-2032.
40. Gordus A, Pokala N, Levy S,et al. Feedback from network states generates variability in a probabilistic olfactory circuit[J]. Cell, 2015, 161(2): 215-227.
41. Foster SR, Roura E, Thomas WG. Extrasensory perception: odorant and taste receptors beyond the nose and mouth[J]. Pharmacol Ther, 2014, 142(1): 41-61.
42. Takeuchi H, Sakano H. Neural map formation in the mouse olfactory system[J]. Cell Mol Life Sci, 2014, 71(16): 3049-3057.
43. Nagayama S, Enerva A, Fletcher ML,et al. Dif f erential axonal projection of mitral and tued cells in the mouse main olfactory system[J].Front Neural Circuits, 2010, 4. pii: 120.
44. Scharfman H, Goodman J, Macleod A,et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats[J]. Exp Neurol, 2005, 192(2): 348-356.
45. Sairanen M, Lucas G, Ernfors P,et al. Brainderived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus[J]. J Neurosci, 2005, 25(5): 1089-1094.
46. Bath KG, Mandairon N, Jing D,et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination[J]. J Neurosci, 2008, 28(10): 2383-2393.
47. Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination[J]. Learn Mem, 2007, 14(8): 539-547.
48. Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories[J]. J Neurosci, 2008, 28(42): 10711-10719.
49. de Almeida L, Reiner SJ, Ennis M,et al. Computational modeling suggests distinct, location-specific function of norepinephrine in olfactory bulb and piriform cortex[J]. Front Comput Neurosci, 2015, 9: 73.
50. Hoover BR, Reed MN, Su J,et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration[J]. Neuron, 2010, 68(6): 1067-1081.
51. Roberson ED, Halabisky B, Yoo JW,et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease[J]. J Neurosci, 2011, 31(2): 700-711.
52. Ittner LM, Ke YD, Delerue F,et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models[J]. Cell, 2010, 142(3): 387-397.
53. Gheusi G, Lepousez G, Lledo PM. Adult-born neurons in the olfactory bulb: integration and functional consequences[J]. Curr Top Behav Neurosci, 2013, 15: 49-72.
54. Alonso M, Lepousez G, Sebastien W,et al. Activation of adult-born neurons facilitates learning and memory[J]. Nat Neurosci, 2012, 15(6): 897-904.
55. Hong XP, Peng CX, Wei W,et al. Relationship of adult neurogenesis with tau phosphorylation and GSK-3beta activity in subventricular zone[J]. Neurochem Res, 2011, 36(2): 288-296.
56. Wang J, Eslinger PJ, Doty RL,et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease[J]. Brain Res, 2010, 1357: 184-194.
57. Vyhnalek M, Magerova H, Andel R,et al. Olfactory identification in amnestic and nonamnestic mild cognitive impairment and its neuropsychological correlates[J]. J Neurol Sci, 349(1-2): 179-184.
58. Macknin JB, Higuchi M, Lee VMY,et al. Olfactory dysfunction occurs in transgenic mice overexpressing human tau protein[J]. Brain Res, 2004, 1000(1-2): 174-178.
59. Cassano T, Romano A, Macheda T,et al. Olfactory memory is impaired in a triple transgenic model of Alzheimer disease[J]. Behav Brain Res, 2011, 224(2): 408-412.
60. Cross DJ, Anzai Y, Petrie EC,et al. Loss of olfactory tract integrity affects cortical metabolism in the brain and olfactory regions in aging and mild cognitive impairment[J]. J Nucl Med, 2013, 54(8): 1278-1284.
61. Hu Y, Ding W, Zhu X,et al. Olfactory dysfunctions and decreased nitric oxide production in the brain of human P301L tau transgenic mice[J]. Neurochem Res, 2015.
62. de Calignon A, Polydoro M, Suarez-Calvet M,et al. Propagation of tau pathology in a model of early Alzheimer’s disease[J]. Neuron, 2012, 73(4): 685-697.
Xue-lan WANG, Professor, Department of Pharmacology, Zhongshan School of Medicine, Sun-Yat Sen University, Zhongshan 2nd Rd, Guangzhou 510080, China. Tel: 86-20-87330561, Fax: 86-20-87330561; E-mail: mdswxl@mail. sysu.edu.cn
Received 2015-11-12; accepted 2015-11-20
杂志排行
中国应用生理学杂志的其它文章
- Ethical inspection about laboratory animals
- Better parameters of ventilation-CO2output relationship predict death in CHF patients
- Association between endothelial nitric oxide synthase (ENOS) G894T polymorphism and high altitude (HA) adaptation: a meta-analysis
- Flow cytometric analysis of circulating microvesicles derived from myocardial ischemic preconditioning and cardioprotection of ischemia/reperfusion injury in rats
- Synergisms of cardiovascular effects between iptakalim and amlodipine, hydrochlorothiazide or propranolol in anesthetized rats
- Effects of curcumin on sodium currents of dorsal root ganglion neurons in type 2 diabetic neuropathic pain rats
