Impacts of exposure to 900 MHz mobile phone radiation on liver function in rats
2015-05-22HuirongMAZhihongMAGuiyingWANGCuimiaoSONGXuelianMAXiaohuiCAOGuohongZHANG
Hui-rong MA, Zhi-hong MA, Gui-ying WANG, Cui-miao SONG, Xue-lian MA, Xiao-hui CAO,Guo-hong ZHANG
1. HeBei University of Chinese Medicine, ShiJiaZhuang 050091; 2. HeBei Medical University, ShiJiaZhuang 050017, China
Impacts of exposure to 900 MHz mobile phone radiation on liver function in rats
Hui-rong MA1, Zhi-hong MA1, Gui-ying WANG1, Cui-miao SONG1, Xue-lian MA1, Xiao-hui CAO2,Guo-hong ZHANG1
1. HeBei University of Chinese Medicine, ShiJiaZhuang 050091; 2. HeBei Medical University, ShiJiaZhuang 050017, China
doi 10.13459/j.cnki.cjap.2015.06.013
Objective: To study the impacts of exposure to electromagnec radiaon (EMR) on liver funcon in rats. Methods: Twenty adult male Sprague-Dawley rats were randomly divided into normal group and radiated group. The rats in normal group were not radiated, those in radiated group were exposed to EMR 4 h/ d for 18 consecuve days. Rats were sacrif i ced immediately aer the end of the experiment. The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and those of malondialdehyde (MDA) and glutathione (GSH) in liverssue were evaluated by colorimetric method. The liver histopathological changes were observed by hematoxylin and eosin staining and the protein expression of bax and bcl-2 in liver tissue were detected by immunohistochemical method. Terminal-deoxynucleotidyl transferase mediated nick and labelling (TUNEL) method was used for analysis of apoptosis in liver. Results: Compared with the normal rats, the serum levels of ALT and AST in the radiated group had no obvious changes (P>0.05), while the contents of MDA increased (P<0.01) and those of GSH decreased (P<0.01) in liverssues. The histopathology examinaon showed dif f use hepatocyte swelling and vacuolaon, small pieces and focal necrosis. The immunohistochemical results displayed that the expression of the bax protein was higher and that of bcl-2 protein was lower in radiated group. The hepatocyte apoptosis rates in radiated group was higher than that in normal group(allP<0.01). Conclusion: The exposure to 900 MHz mobile phone 4 h/d for 18 days could induce the liver histological changes, which may be partly due to the apoptosis and oxidave stress induced in liverssue by electromagnec radiaon.
electromagnetic radiation(EMR); mobile phone; rats; liver function; MDA; GSH; hepatocyte apoptosis
Introduction
With the development of social economy and the progress of science and technology, the holdings of mobile phones increase rapidly. The radiation of mobile phone belongs to the microwave field of electromagnetic radiation (EMR). It was found that different wavelengths of microwaves probably have dif f erent biological ef f ects, ionizing radiation and the high frequency EMR can cause liver lesion[1-4].e study also proved that 850-1900 MHz EMR frommobile phone could damage the liver function in mice[5], and our present study found that 900 MHz EMR could damage the liver function in pregnancy rats[6]. However, it remains largely unknown whether 900 MHz EMR damage the rat liver under the natural and physiological condition.e purpose of this study was to explore the impacts of EMR on the liver function in rats and related mechanisms.
Materials and Methods
Experiment animals
Twenty male Sprague-Dawley (SD) rats weighing 240±26.48 g were purchased from the Experimental Animal Center, Hebei Medical University (Shijiazhuang, China). All procedures were performed in according to the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China.
Experiment reagents
Group and radiated method
After 1 week of accustomization, all rats were weighed and randomized into two groups (n=10 in each group): normal group and radiated group.e wooden box was packed with fi ne wires in its surface, the radiated case with radiation source was selected as 900 MHz of the frequency and 370 μW/cm2of the average power density[6-8]. The radiation source was made by Physics Department of Hebei Normal University. Exposure parameters was detected by the EMR tester made in BeiJing Longzhentian Electronic Instrument Co., Ltd. (Product Model: LZT-1150).
Assessments of ALT and AST in serum
The levels of ALT and AST in serum were estimated by spectrophotometry-based methods using commercially available kits according to the instructions.
Histopathological examination for liver tissue
Immunohistochemical analysis of Bax and Bcl-2 protein expression in liver tissue
Conventional ABC method of immunohistochemistry staining according to the manufacturer’s instructions was used. In brief, paraff i n sections were washed with 0.01 mol/L phosphate buffered saline (PBS) for 5 minutes for three times. Endogenous peroxidase activity was inhibited by incubation with 0.3% hydrogen peroxide. After blocking sections with 5% (w/v) bovine serum albumin (BSA), the sections were then treated with primary rabbit bax and bcl-2 polyclonal antibodies in PBS containing 1% serum (overnight at 4°C), biotin-labeled secondary antibody (30 min at room temperature) and then avidin-biotin-peroxidase complex. The sections were stained using a diaminobenzidine (DAB) substrate kit and the nuclei were counterstained with hematoxylin.e protein expressions of the bax and bcl-2 in liver tissue were determined by microscopic observation of the brown peroxidase reaction product, using HMIAS-2000 highly clear Color medical image analysis system.
TUNEL assay
Sections from the rat liver were incubated for 1 h at 56°C, deparaffinized in xylene and rehydrated through an ethanol series. TUNEL staining was performed with the DeadEnd Colorimetric TUNEL System according to the manufacturer's protocol. Labeled DNA was visualized with a peroxidaseconjugated anti-digoxigenin antibody using 3,3'-diaminobenzidine (DAB) as the chromogen. Photographs were taken with the use of a microscope.e apoptosis rates was calculated by the ratio of the numbers of TUNEL positive cells to total cells in 10 microscopic visions.
Detection of MDA and GSH in hepatic tissues
Each liver tissue sample was weighed to prepare a 10% (w/v) buffered homogenate (100 mg tissue/ ml 50 mmol/L PBS, pH 7.2). The homogenate was centrifuged, and the supernatant was used for biochemical analyses. The protein concentration of the supernatant was determined by the Lowry method using bovine serum albumin (BSA) as a standard. The activities of GSH and the level of MDA in liver tissue were measured using spectrophotometry-based commercially available kits according to the instructions.
Statistical Analysis
Data analysis was conducted with SPSS software package for Windows (Version 11.5; SPSS Inc., Chicago, USA). Data distributions were examined for normality and homogeneity of variance. The values are expressed as means±SD if they are normally distributed. Comparison between two groups was performed using Independent samplet-tests or Chisquared test. Probability values less than 0.05 (P<0.05) were considered statistically signif i cant.
Results
Af f ects of EMR on the levels of ALT and AST in serum
As shown in Table 1, the levels of ALT and AST in serum had no obvious changes between the two group rats (P>0.05).
Af f ects of EMR on the levels of MDA and GSH in liver tissues
Indices of oxidative stress were detected by the contents of MDA and GSH in liver tissue. The results were shown in Table 2. Compared with the normal rats, the levels of MDA in radiated rats were significantly higher (t=6.174,P<0.01), while the contents of GSH were lower (t=4.298,P<0.01) .

Tab. 1 The levels of AST and ALT in serum (n=10).

Tab. 2 The contents of MDA and GSH in liver tissue (n=10).
Af f ects of EMR on liver histopathology
Rat liver sections stained with HE staining for morphological analysis were shown in Figure1. In the radiated rats, although hepatic lobule was arranged in neat rows, the liver structure appeared signif i cant abnormalities with small pieces and focal necrosis. Moreover, signif i cant dif f used swelling and vacuolation were found in hepatocytes.
Affects of EMR on the hepatocyte apoptosis in liver tissue
Hepatocyte apoptosis was reflected in A and B of Figure 2 and Table 3,e deeper color of bax protein expression and lighter color of bcl-2 protein expression were observed in the radiated rats.e protein expression of bax and bcl-2 were quantified by the reciprocal of average optical density. As shown in Tab. 3, the similar results occurred.e bax protein expression in the radiated hepatocytes were much stronger (P<0.01) and bcl-2 protein expression was lower obviously (P<0.01). The hepatocyte apoptosis rates in radiated group was higher than that in normal group.
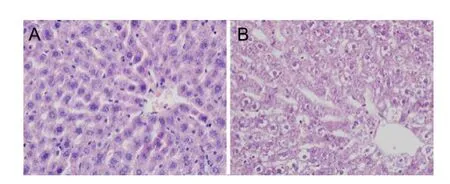
Fig.1 Affects of EMR on liver histopathology. Representative microscopic photographs of liver stained with HE (magnification 400×). Livers were obtained from the normal group (A) and radiated group (B).
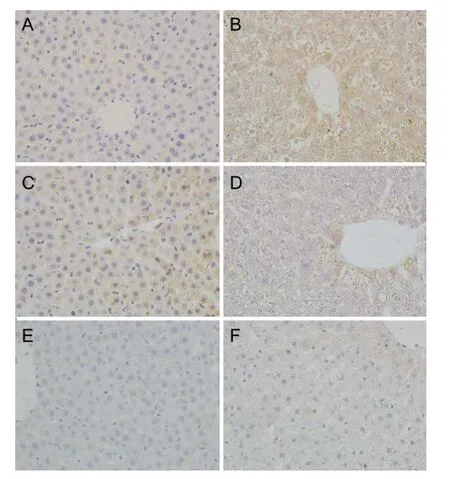
Fig.2 Affects of EMR on the hepatocyte apoptosis in liver tissue. A-D: Representative microscopic photographs of liver immunohistochemical staining (magnification 400×). A-B: bax; C-D: bcl-2; E-F: Representative microscopic photographs of hepatocyte apoptosis by TUNEL (magnification 400×). Livers were obtained from the normal group (A,C,E) and radiated group (B,D,F).*P<0.01vsnormal group. TUNEL: Terminal deoxynucleotidy transferase (TdT) mediated dUTP nick end labeling.
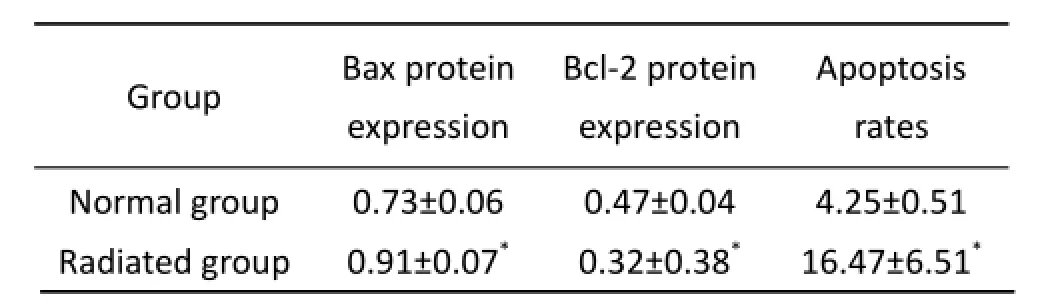
Tab. 3 The value of average optical density bax and bcl-2 protein and apoptosis rates (mean±SD,n=10).
Discussion
Because the liver plays a key role in metabolism of a variety of endogenous compounds and xenobiotics, it is highly susceptible to tissue injury. The results of our present study revealed that EMR exposure of 4 h/d for 18 days could damage the liver structure, enhance the oxidative stress and hepatic apoptosis. Based on other studies and our previous study[6-8], exposure to EMR from mobile phone with 900 MHz and 370 μW/cm2could damage the liver function in the pregnant rats. In this study, we went on observing the impact of EMR on the rat liver function under the natural and physiological condition. 900 MHz and 370 μW/cm2at an intensity similar to mobile phone emissions were selected as the radiated conditions in this study.
The serum levels of ALT and AST are two important markers ref f ecting the liver function. Our present study found that the serum levels of ALT and AST in radiated rats were not increased markedly, however, the liver histopathology under the optical microscope appeared significant abnormalities. This results proved that EMR exposure caused abnormal histopathology in rat liver. Due to the partial separation of function and injury, a number of liver diseases are not initially discovered because of decreased liver function, but rather through evidence of increased liver injury. For example, some patients generally show normal liver function, but their liver structures are found in abnormal state by biopsy[9-10]. Accumulating evidence shows that the damage degree increased with the exposure time to EMR in the mice. Exposure to 850-1900 MHz EMR 1 h/d for 10 days caused hepatocytic vacuolation in mice, and with the time increased to 12 h/d for 10 days, it resulted in hepatocytic vacuolation and inf f ammatory cellular inf i ltration around the hepatic vein[5]. It was consistent with the opinions that repeated exposure to the lower frequency EMR could induced hepatic tissue damage[11-12].
Hepatic apoptosis occurs during cell development and maturation response to varying toxin stimuli. Bax and bcl-2 are the most concerned gene family controlling apoptosis. Bax and bcl-2 can form polymer to regulate the permeability of mitochondria membrane, finally control cellular apoptosis and survival, over-expression of anti-apoptotic proteins such as bcl-2 can protect the cell against apoptosis. However, bax is thought to be the main executioner and plays an essential role in apoptosis[13].e results of present study showed that there was stronger expression of bax protein and weaker expression of bcl-2 protein in liver of the radiated rat. As the balance between bcl-2 and bax are related with the cellular survival, therefore, the liver damage in radiated rats may be induced by upregulating the bax protein expression and down-regulating the bcl-2 protein expression in liver tissue. EMR of 4 h/d for 20 days caused hepatic apoptosis in pregnant rats[14]. In the present study, our results were consistent with these researches.e hepatocyte apoptosis rates in radiated group was higher than that in normal group.
Oxidative stress has been implicated in the induction of hepatocyte apoptosis that results from a variety of forms of liver injury. The organism produces oxygen free radical under the physical and pathogenic conditions, if the active oxygen free radicals could not be scavenged in time, it will attack the polyunsaturated fatty acid of biomembrane and lead to lipid peroxidation forming the lipid peroxidation[14]. MDA is an end-product of lipid peroxidation, and its content can ref f ect the degree of peroxidation indirectly[15]. Among the antioxidants, the tripeptide GSH plays a central role, especially in the liver. It has been found that 1 800 MHz EMR significantly increased the MDA contents in the liver of guinea pigs[16]. In another study, an acute EMR exposure was found to increase MDA and decrease GSH levels in the liver signif i cantly[17]. EMR of 15 min/d for 4 days induced serum GSH level in rat[12], Exposure to EMR of 50 Hz for 5 min every other day over a period of 6 months decreased the GSH levels in the liver of rats[18]. Oxidative stress is not only an important inducer of cell apoptosis, and but also are critical mediators of apoptosis of hepatocytes[19].e present results suggested that EMR increased the hepatic oxidative damage. Some studies proved that exposure to EMR can modify the oxidative process[20-21], and bcl-2 played a role in regulating cellular redox pathways associated with apoptosis[22]. Therefore, the hepatic apoptosis induced by EMR in this study may be due to the increased oxidative stress.
Our findings indicate that EMR leads to disruption of the normal structure, increased apoptosis andoxidative stress of liver in rats.is may be one of the mechanisms by which the exposure to ERM impacts on the liver damage. Moreover, our data imply that long-term mobile phone users should pay attention to EMR, and take the necessary measures to prevent the EMR.
Acknowledgements
This work was supported by the Natural Science Foundation of Hebei Province (201220123) and Youth Fund Project of HeBei University of Chinese Medicine (QNZ2014015).
1. Trosić I, Pavicić I, Milković-Kraus S,et al. Ef f ect of electromagnetic radiofrequency radiation on the rats' brain, liver and kidney cells measured by comet assay[J]. Coll Antropol, 2011, 35(4): 1259-1264.
2. Ono T, Saito Y, Komura J,et al. Absence of mutagenic effects of 2.45 GHz radiofrequency exposure in spleen, liver, brain, and testis of lacZ-transgenic mouse exposed in utero[J]. Tohoku J Exp Med, 2004, 202(2): 93-103.
3. Wang F, Zuo YH, Wang XL,et al. Study of dif f erential protein expression in human normal liver cells exposed to radiation[J]. Radiation Protect J, 2009, 29(6): 385-340.
4. Tian ZJ, Shen N, Lv SJ,et al.e ef f ects of high power microwave radiation on SOD, HSP70 in serum and lipid peroxidation, mitochondrial swelling in the liver of female rats[J]. Sichuan J Zoology, 2009, 28(4): 528-531.
5. Al-Glaib B, Al-Dardf i M, Al-Tuhami A,et al. A technical report on the ef f ect of electromagnetic radiation from a mobile phone on mice organs[J]. Libyan J Med, 2008, 3(1): 8-9.
6. Ma HR, Li JJ, Zhao YN,et al. Experimental study on the liver in SD pregnant rats exposed by 900 MHz radiofrequency of cellular phone[J]. J Environment Hygiene, 2013, 3(5): 394-397.
7. Tang GH, Li SL. Investigation of the wave-radiation from handheld mobile phone in different intensity of signals[J]. Chin Public Health, 2001, 17(5): 424-425.
8. Zeng QL. Electromagntic radiation[J]. Chin J Prevent Med, 2004, 38(1): 49.
9. Huang ZX, Zhang HQ, Pan YQ. The relation between the value of serum enzyme and hepatic pathological change in chronic hepatitis[J]. Zhejiang J Prev Med, 2004, 16(8): 70.
10. Li Y, Zhai WR, Zhang QB. The relationship between pathologic classification and clinical features in chronic hepatitis[J]. Chin J Hepatology, 2000, 5(1): 2-4.
11. Ai ZW, Zhang SH,Wei JH. Primary study on the influence of electromagnetic radiation emitted by mobile phone on rats[J]. Beijing Biomed Eng, 2005, 24(3): 211-213.
12. Elhag MA, Nabil GM, Attia AM. Ef f ects of electromagnetic fi eld produced by mobile phones on the oxidant and antioxidant status of rats[J]. Pak J Biol Sci, 2007,10 (23): 4271-4274
13. Lei X, Chen Y, Du G,et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c releaseviaa conformational change in Bcl-2[J]. FASEB J, 2006, 20(12): 2147-2149.
14. He M, Zhan M, Liu RM,et al. Systematic review on reduced glutathione in the treatment of druginduced liver disease[J]. China Pharmacy, 2010, 21(32): 3049-3052.
15. Sies H. Oxidative stress: oxidants and antioxidants[J]. Experimental physiology, 1997, 82(2): 291-295.
16. Ozgur E, Güler G, Seyhan N. Mobile phone radiation-induced free radical damage in the liver is inhibited by the antioxidants N-acetyl cysteine and epigallocatechin-gallate[J]. Int J Radiat Biol, 2010, 86(11): 935-945.
17. Shirazi A, Mihandoost E, Ghobadi G,et al. Evaluation of radio-protective ef f ect of melatonin on whole body irradiation induced liver tissue damage[J]. Cell J, 2013, 14(4): 292-297.
18. Baltaci AK, Mogulkoc R, Salbacak A,et al. The role of zinc supplementation in the inhibition of tissue damage caused by exposure to electromagnetic fi eld in rat lung and liver tissues[J]. Bratisl Lek Listy, 2012, 113(7): 400-403.
19. Esmekaya MA, Ozer C, Seyhan N. 900 MHz pulse-modulated radiofrequency radiation induces oxidative stress on heart, lung, testis and liver tissues[J]. Gen Physiol Biophys, 2011, 30(1): 84-89.
20. Wang J, Zhu H, Liu X,et al. Oxidative stress and Ca2+signals involved on cadmium-induced apoptosis in rat hepatocyte[J]. Biol Trace ElemRes, 2014, 161(2): 180-189.
21. Ciejka E, Goraca A. Influence of low magnetic field on lipid peroxidation[J]. Pol Merkur Lekarski, 2008, 24 (140): 106-108.
22. Voehringer DW, Meyn RE. Redox aspects of Bcl-2 function[J]. Antioxid Redox Signal, 2000, 2(3): 537-550.
Guo-hong ZHANG, MD, HeBei University of Chinese Medicine, Shijiazhuang 050091, China. Tel: 86-15081167038; E-mail: 1226462769@qq.com
Received 2015-10-15; accepted 2015-11-20
杂志排行
中国应用生理学杂志的其它文章
- A mini review: Tau transgenic mouse models and olfactory dysfunction in Alzheimer’s Disease
- Ethical inspection about laboratory animals
- Better parameters of ventilation-CO2output relationship predict death in CHF patients
- Association between endothelial nitric oxide synthase (ENOS) G894T polymorphism and high altitude (HA) adaptation: a meta-analysis
- Flow cytometric analysis of circulating microvesicles derived from myocardial ischemic preconditioning and cardioprotection of ischemia/reperfusion injury in rats
- Synergisms of cardiovascular effects between iptakalim and amlodipine, hydrochlorothiazide or propranolol in anesthetized rats
