Leptin receptor of the hind brain nuclei is involved in the conditioned taste preference of rats
2015-05-22CaixiaLINShaoyunZHANGKeCHENXiaoLUOBoSUNYumingKANGJianqunYAN
Cai-xia LIN, Shao-yun ZHANG, Ke CHEN, Xiao LUO, Bo SUN, Yu-ming KANG, Jian-qun YAN
Department of Physiology and Pathophysiology, Xi'an Jiaotong University, School of Basic Medical Science, Xi'an 710061, China
Leptin receptor of the hind brain nuclei is involved in the conditioned taste preference of rats
Cai-xia LIN, Shao-yun ZHANG, Ke CHEN, Xiao LUO, Bo SUN, Yu-ming KANG, Jian-qun YAN
Department of Physiology and Pathophysiology, Xi'an Jiaotong University, School of Basic Medical Science, Xi'an 710061, China
doi 10.13459/j.cnki.cjap.2015.06.010
Objective: Condioned taste preference (CTP) is a taste learning ref l ex by which an animal learns to prefer a substance which tastes not well and has been studied with much interest in recent years. However, the neural substrates of CTP are less known. This study aimed to determine the possible neural pathways of CTP and whether serum lepn level and the lepn receptor (OB-Rb) in the hind brain are involved following CTP formaon. Methods: We established CTP of quinine in rats with a 2-bole preference test. The serum lepn concentraons were detected, the expression of c-fos in the rat brain was tested to determine the nuclei in relaon with establishment of CTP. Finally, the OB-Rb mRNA expression was examined by RT-qPCR assay in parabrachial nucleus (PBN) and the nucleus of the solitary tract (NST) of the hind brain. Results: Compared with control group, the level of serum lepn was higher in the CTP group (4.58 ± 0.52vs1.67 ± 0.25 μg/L,P<0. 01); increased c-fos posive cells were found in the anterior hypothalamus (AH, 221.75 ± 4.96vs.178.50±6.63 cells/mm2,P<0.05), the basal lateral amygdala (BLA, 70.75±6.17vs56.50±3.62 cells/ mm2,P<0.05) and the nucleus of the solitary tract (NST, 41.25±1.32vs32.50±1.02 cells/mm2,P<0.05). But in ventromedial nucleus of the hypothalamus (VMH, 20.75±2.73vs38.5±1.54 per 1 mm2,P<005), PBN (21.50 ±2.24vs36.25±1.49 cells/mm2,P<0.05) and the central nucleus of the amygdala (CeA, 22.25±1.53vs35.50 ±2.11 cells/mm2,P<0.05) , the number of c-fos posive cells was decreased in the CTP group. In addion, we found OB-Rb mRNA expression in PBN of CTP group rats was higher than that of control group (0.95±0.055vs0.57± 0.034,P<0.05), while there was no significant difference of OB-Rb mRNA expression in NST between the two groups. Conclusion: Nuclei AH, BLA, NST, VMH, PBN and CeA parcipate in the formaon of CTP. Lepn and its receptor in PBN may be involved in the formaon and maintenance of CTP.
condioned taste preference; c-fos; lepn; OB-Rb
Introduction
Taste is critical for nutrients intake and living for all animals [1]. Animals, including humans, choose their source of nutrition based on the nutritive value and the taste of available foods [2]. It is unsurprising that food preference plays a crucial role in animals’ feeding behavior. What an animal tends to consume is not only in accordance with congenital taste preference, but also relative to itsacquired taste experiences which means learning is important for animals’ feeding. Animals’ taste preference varying with feeding experiences is an important character for animals to adapt to the changes of living environment and improve their abilities of survival and reproduction. Conditioned taste preference (CTP) is a taste learning reflex by which an animal learns to prefer a substance which not tastes well [2, 3]. Experimentally, one neutral flavor (the conditioned stimulus; e.g quinine) is paired with the sweet and highly preferred taste of glucose.e acquisition of the learned preference is then assessed with a 2-bottle preference test [4,5]. Although CTP learning is known, the relative neural substrates of it are still unclear. In recent years, possible gustatory associative brain nuclei of CTP have been discussed in some studies [4]. Besides, lesion studies have demonstrated that parabrachialnuclei (PBN) and insular cortex integrity are critical for developing nutrient conditioned preference [6, 7]. Some researchers found the lateral hypothalamus (LH) ablation affects learning paradigms such as conditioned taste preference (CTP) and conditioned taste aversion (CTA), and they concluded that LH appears to be central to the circuit for CTP [8]. Touzani and Sclafan found that amygdale-ablated rats failed to display a preference in the taste preference test[7], suggesting that amygdala may be vital for establishing the taste-related learning behavior.
c-fos is one of the immediate early genes. When animals receive various taste stimuli, the formative taste signals would transmit to the gustatory associative brain nuclei, inducing c-fox expression in these regions [10]. c-fos mRNA and FOS protein are quickly synthesized in response to trans-synaptic activities throughout the brain [3] . In other words, c-fos serves as a marker of neuronal responses to the sensory stimuli.us, we can fi nd CTP associated brain nuclei via observing c-fos expression in the brain of CTP animals.
It is well known that leptin, the product of the ob gene, is mainly produced in adipose tissue and then transferred to the brain via blood [11-14]. Leptin, as a modulator of animals’ taste sensitivity, can regulate food intake, energy expenditure, and body weight. Some specific receptors of leptin have been discovered in many brain regions such as hypothalamus and amygdala [11,15]. When taste bud cells are stimulated by tastants, the depolarization or hyperpolarization of the cell membrane is caused by different signal transduction pathways, and then taste af f erent nerve endings will be stimulated to generate action potentials, which transmit the taste signals to some regions of the brain, where leptin will combine with some of its receptor. As a result, some neuropeptides and neurotransmitters would be produced which participate in the long-term regulation of appetite and animals’ response to taste stimulus via JAKSTAT and Ras-MARK signal transduction pathways, etc [11,16].erefore, leptin acts as a feedback signal to gustatory brain regions in taste-related behaviors.
In view of the above fi ndings, possible CTP associative brain nuclei have been found, in which PBN and insular cortex may be critical for the establishment and maintenance of CTP. However, it has not been well illustrated whether other brain neuclei are essential to CTP. As mentioned above, leptin acts as a modulator of taste sensitivity and a feedback signal to gustatory brain regions of taste behaviors; it is still unclear whether leptin and its receptor in these CTP related brain nuclei play a role in the neural pathways of its learning process.is study was undertaken to investigate the gustatory associative brain nuclei of CTP and the possible changes of serum leptin level and the leptin receptor expression accompanied with learning and maintaining of CTP.
Materials and Methods
Animals
The establishment of CTP
Two day before the CTP establishment, all rats drank water freely at 10:00-10:20, and then at 15:40-16:00 the quinine preference is assessed with a 2-bottle preference test (one bottle was 0.04 mmol/ L quinine and the other was water); rats were waterdeprived the rest of the time.is quinine preference is the basic preference (day 0).
From the 1st day of the CTP establishment, the CTP group received the following treatment: at 10:00, the rats drank 0.04 mmol/L quinine solution for 20 min and the next 20 min the rats drank 0.2 mol/L sucrose solution, and then at 15:40-16:00 the learning quinine preference is assessed with a 2-bottle preference test; the rats were water-deprived the rest of the time.e control group received the following treatment: the rats respectively drank 0.04 mmol/L quinine solution and water for 20 min one aer another, and then at 15:40-16:00 the quinine preference is assessed with a 2-bottle preference test; rats were also water-deprived the rest of the time.e training continued for 5 days.
Preference ratios were calculated for each group with the following formula:
PR = F1E/ (F2E + F2W)
Here, PR = preference ratio, F1Q = intake of quinine, and F2W = intake of water.
The assay of serum leptin
All the rats were anaesthetized with 10% chloral hydrate (3 ml/kg, ip). 2 ml blood was collected fromthe abdominal aorta and put into dry tube allowing to clot 1h. Serum was separated by centrifugation (2 000 r/min) for 10 min.en the serum leptin was measured with leptin (LEP) ELISA kits (CUSABIO).
Immunohistochemistry
Eight rats were randomly selected from each group and were perfusedviathe ascending aorta with 200 ml of 0.01 mol/L phosphate-buffered saline (PBS) followed by 400 ml of paraformaldehyde (PFA, 4 g/ L, pH 7.4). Brains were dissected out and post-f i xed in the same fixative for 3 hours at 4°C, and then transferred into 30% sucrose solution (4°C) for at least 24h until the brains completely sank .en the regions including hypothalamus, the amygdala, PBN, CeA and cortex of each brain were cut coronally at a thickness of 20 μm in the freezing microtome. Endogenous peroxidase activity was blocked with 0.01 mol/L PBS containing 0.3 ml/L hydrogen peroxide.en the sections were rinsed with 0.01 mol/L PBS, and incubated with 15 ml/L blocking serum in PBS for 1 h.e sections were incubated at 4°C for 40 h with rabbit polyclonal antiserum against c-fos (antiserum 1500 with dilution at 1: 400, Santa-Cruz Biotechnology, CA, USA). Aer incubation, the sections were immunostained by the avidin-biotin complex method (rabbit ABC staining system, 1300, PIERCE Biotechnology).e sections were mounted on slide glasses, dried and dehydrated in a graded ethanol series, and covered with balata. Then the strained sections were observed under the laser scanning confocal microscope and the expression of c-fos immunoreactivity was quantif i ed by counting positive cells in the nuclei of six adjacent brain sections.
Real-time PCR
Eight rats of each group were sacrif i ced quickly, and fresh samples were obtained fresh from relevant nuclei of rat brains, and stored in 1.5 ml Eppendorf’s tubes at -70°C until RNA extraction. RNA isolation was performed using Total RNA Extraction Reagent Kit according to the manufacturer’s instructions. Concentration of RNA obtained was measured using NanoDrop BioPhotometer D (260 nm) /D (280 nm) (R). Reversed transcription System (20 μl) utilizing PrimeScriptTMRT Reagent Kit (No. DRR037A, TaKaRa BIO) was performed in order to obtain cDNA and the reaction condition was: 37°C last 15 min, and then 85°C 5 s. The obtained cDNA was saved at -20°C for further analysis. OB-Rb mRNA expression was measured using real-time PCR. The RT-PCR system (20 μl) was prepared SYBRH Premix Ex TaqTMII Reagent Kit (No. DRR081A, TaKaRa BIO) and the primer sequences used for real-time PCR is shown in table1.e reaction condition was: 95°C last 10 min, 95°C 5 min, 58~60°C last 30 s to 40~45 cycles; and then to solubility curve ( BIORAD PCR circulation,iQ5,USA). Relative OBRb mRNA expression was normalized to GAPDH transcripts and calculated as a fold change compared to control. The results were expressed as gene copy number/GAPDH mRNA ratio 2-ddC
Data analysis
All data are shown as the mean±SD. Analysis was performed by SPSS17.0 software. Unpaired, two tailed t-test and Anova comparison test were used.e dif f erences between mean values were considered statistically signif i cantly with the probability value ofP<0.05.
Results
Quinine preference aTher CTP learning
Comparison of the ration of quinine preference of control and CTP groups
There were no significant differences of quinine preference between CTP and control groups on the 1stday and 2ndday. However, by the 3rdday, compared with control group, the quinine preference increased in CTP rats (P<0.05, Fig. 1), and the differences persisted to the 5thday.
The change of learning quinine preference of CTP rats
In CTP rats, there were no signif i cant dif f erences of learning quinine preference (Compared with that of day 0,P>0.05) on the 1stday and 2ndday. However, from the 3rdday, the learning quinine preference increased day by day (Compared with that of the previous day,P<0.05, Tab. 2).
In the process of CTP, there were no significant differences of learning quinine preference of CTP rats on the 1stday and 2ndday compared with that of day 0. However, from the 3rdday, the learning quinine preference increased day by day compared with that of the previous day. The data are presented as mean± SD (n=16). ⋆P<0.05 compared with that of the previous day.
The serum leptin level aTher CTP
Compared with control group, the serum leptin level of CTP rats increased signif i cantly (4.58 ± 0.52vs1.67 ± 0.25 μg/l,P<0.01, Fig. 2).
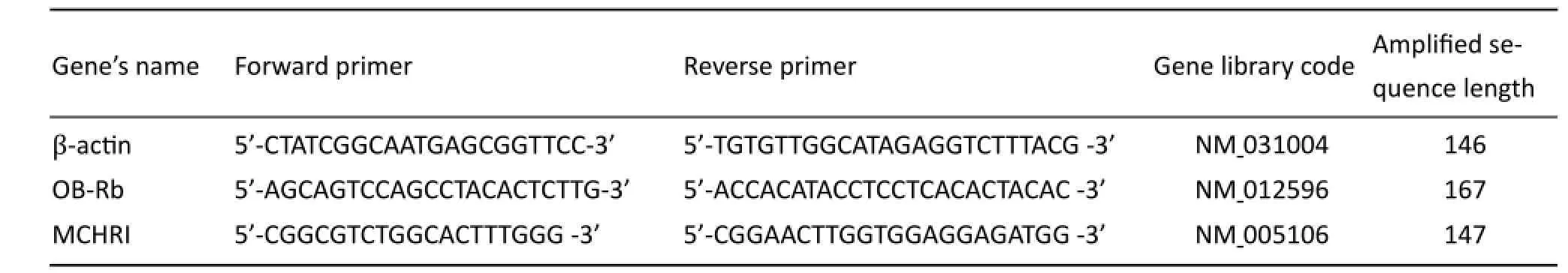
Tab. 1 Primer sequences used for real-time PCR.
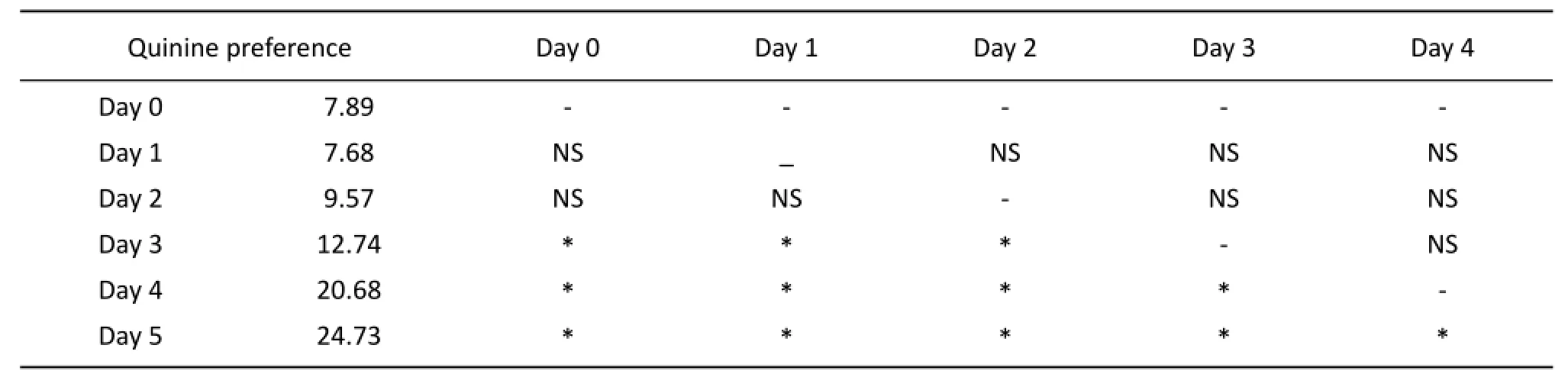
Tab. 2 The ration of Quinine preference in CTP rats.
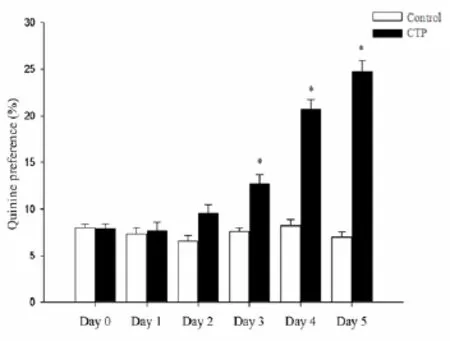
Fig. 1 Comparison the ration of Quinine preference in control and conditioned taste preference (CTP) rats. In the process of CTP, there were no significant differences of quinine preference between CTP and control groups on the 1stday and 2ndday. However, by the 3rdday, the quinine preference increased in CTP rats as compared with control group,. The data are presented as mean±SD (n=16).*P<0.05vscontrol.
The c-fos positive-immunohistochemistry strained cells were observed as brown granules spread over many regions of rat brains. Increased c-fos positive cells were observed in AH (P<0. 05), BLA (P<0. 05) and NST (P<0. 05) of CTP rats.e average numbers of positive cell were 221.75 ± 4.96vs178.50 ± 6.63 per 1 mm2in AH (P<0. 05) and 70.75 ± 6.17vs56.50 ± 3.62 per 1 mm2in BLA and 41.25 ±1.32vs32.50 ± 1.02 per 1 mm2in NST (P<0.05). But in VMH (P<0. 05), PBN (P<0. 05) and CeA (P<0. 05), c-fos positive cells were decreased in the CTP group.e average numbers of positive cell were 20.75 ± 2.73vs38.5 ± 1.54 per 1 mm2(P<0. 05) in VMH, 21.50 ± 2.24vs36.25 ± 1.49 per 1 mm2in PBN (P<0.05) and 22.25 ± 1.53vs35.50 ± 2.11 per 1 mm2in CeA (P< 0. 05, Fig. 3, Fig.4 , Fig. 5).
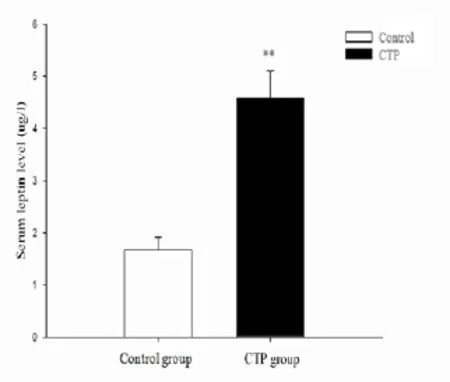
Fig. 2 The level of serum leptin in control and CTP rats. Following the CTP formation, the serum leptin level of CTP rats increased. The data are presented as mean±SD (n=16).**P<0.01vscontrol.
Compared with the control group, enhanced OB-Rb mRNA was demonstrated in PBN.e average results were 0.95 ± 0.055vs0.57 ± 0.034 in PBN (P< 0.05). However, there were no signif i cant dif f erences of OBRb mRNA expression in NST between the two groups (Fig. 6).

Fig. 3 Numbers of c-fos positive cells in the brain nuclei of control and CTP rats.Increased c-fos positive cells in AH, BLA and the nucleus of the solitary tract (NST) in CTP rats while decreased c-fos positive cells in ventromedial nucleus of the hypothalamus (VMH), parabrachial nucleus (PBN) and the central nucleus of the amygdala (CeA) by meanings of CTP learning. The data are presented as mean± SD(n=8).*P<0.05vscontrol.
Discussion
The novel findings of the present study are: i) increased serum leptin level in CTP rats; ii) increased c-fos positive cells in AH, BLA and NST in CTP rats while decreased c-fos positive cells in VMH, PBN and CeA by meanings of CTP learning; and (iii) enhanced OB-Rb mRNA in PBN followed by CTP formation.
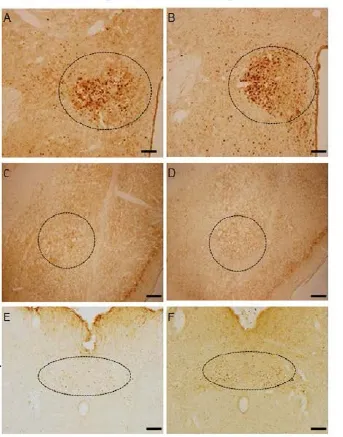
Fig. 4 The representative picture for c-fos positive cells in the anterior hypothalamus (AH, panels A and B), the basal lateral amygdala (BLA, C and D), NST (E and F) in control rats (A, C and E) and CTP rats (panels B, D and F) under the laser scanning confocal microscope (×40). Scale bar = 100 mm.
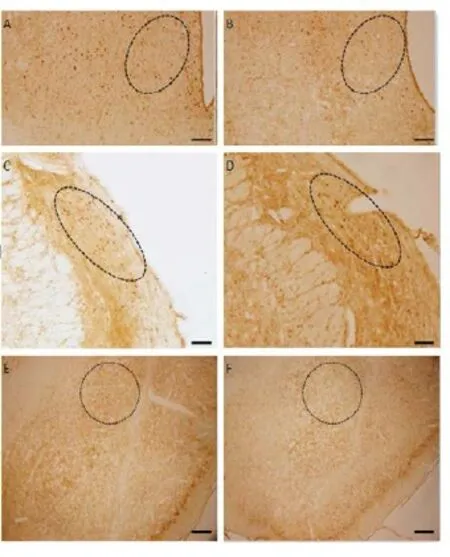
Fig. 5 The representative picture for c-fos positive cells in VMH (panels A and B), PBN (C and D), CeA (E and F) in control rats (A, C and E) and CTP rats (panels B, D and F) under the laser scanning confocal microscope (×40). Scale bar = 100 mm.
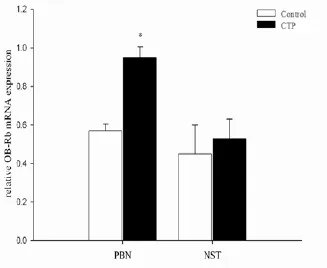
Fig. 6 RT-qPCR assay showing the relative mRNA levels of OB-Rb in the control and CTP rats. Compared with the control group, enhanced OB-Rb mRNA level was demonstrated in PBN. While there were no significant differences of OB-Rb mRNA expression in NST. The data are presented as mean ± SD(n= 8).*P<0.05vscontrol.
The formation of mammals taste preference is based on acquired taste stimuli and feeding experiences. Hypothalamus, NST, PBN, CeA and cortex have been found participate in the management and integration of taste signals [17,18]. c-fos, as an early gene, is a sensitive marker of brain neurons responding to taste stimulus[3,19,20]. When animals receive various taste stimuli, the formative taste signals would transmit to the gustatory associated brain nuclei, where c-fos expression is stimulated [16] .erefore the c-fos expression can display the stimulated brain neurons aer the taste stimulation.e neuronal response is quantif i able by count the number of c-fos positive cells. A large number of previous investigations that have used c-fos to elucidate taste-related nuclei and the mechanism involved. In addition, c-fos as a marker for neural activation has many advantages.erefore, in present study we used c-fos expression as a marker to investigate possible neural transduction pathways of CTP and possible roles of dif f erent taste nuclei in learning and maintaining of CTP. In this study, we trained the quinine CTP learning of rats with a 2-bottle preference test, the learning results was showed in Fig. 1 and Tab. 2. And then we mainly observed c-fos expression in some nuclei related to animals, intake and emotion, such as AH and VMH in the hypothalamus, BLA and CeA in the amygdala, NST, and PBN. In our study, we found that increased c-fos positive cells were observed in AH, BLA and NST in CTP rats and decreased c-fos positive cells were in VMH, PBN and CeA (Fig. 3, Fig. 4, Fig. 5).e results were consistent with the previous studies (unpublished data), which found that c-fos expression changd significantly in many brain regions such as cortex, hypothalamus, CeA, PBN, Arc and NST aer the CTP formation.e c-fos expression acts as a marker of the stimulated animals’ brain neurons’ response aer accepting CTP taste stimulus. So according to the immunohistochemical results, we can draw a conclusion that AH, BLA, NST, VMH, PBN and CeA participated in the conduction tracts of CTP. Besides, the results also can tell us that these regions are involved in taste discrimination, reward assession, and the integration of associated sensory and behavior information.
In recent years, leptin and its receptor as appetite regulatory factors have been studied with much interest. Leptin is a hormone believed to be one of the regulatory factors of animals’ taste sensitivity; combines with its receptor in the brain to fulf i ll its function as appetite regulation [10, 19-22]. Leptin may be the medium in the integration of taste stimulus and intake.us our study used leptin and OB-Rb gene as research objects to investigate the possible role of leptin and leptin receptor in the formation and maintenance of CTP taste behaviors. PBN is closely related to the transmission and management of taste signals and the regulation of food intake [23, 24]. In the rats’ gustatory conduction pathways, the PBN receives gustatory and visceral afferent projections from the NST [6, 25]. Lesion studies demonstrated that PBN integrity was critical for developing nutrient conditioned preference [6].e changes of rats, taste preference in the process of CTP essentially ref f ects the changes of rats, rewarding cognizance of taste stimuli. Thus in the present study, we mainly discussed the OB-Rb mRNA expression in PBN and NST. OB-Rb mRNA expression was demonstrated in both CTP and control rats, and compared with the control, the OB-Rb mRNA expression in PBN in CTP rats increased signif i cantly, but there was no significant difference of OB-Rb mRNA expression in NST between the two groups (Fig. 6). According these results, we can imply that PBN is crucial in the CTP learning, and leptin with its receptors in PBN play an important role in the nerve pathway of CTP learning. Furthermore,, Yamamoto’ study has found that PBN is associated with the reward information of bitterness[26], so the results also can illustrate that rats were quinine preferred rather than averse before. In summary, the present study demonstrates that following CTP learning, the serum leptin level in CTP rats increases; and c-fos positive cells in AH, BLA and NST in CTP rats increase while that in VMH, PBN and CeA decrease; and in the hind brain, enhanced OB-Rb mRNA is observed in PBN after CTP formation. From the results, we draw a conclusion that AH, BLA, NST, VMH, PBN, and CeA are crucial parts in the circuit of CTP learning; moreover, we can conclude that leptin and its receptor in PBN may play an important role in the formation and maintenance of CTP.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (31171052).
1. Berthoud HR, Zheng H. Modulation of taste responsiveness and food preference by obesity and weight loss[J]. Physiol Behav, 2012, 107(4): 527-532.
2. Panguluri SK, Kuwabara N, Kang Y,et al. Conditioned taste aversion dependent regulation of amygdala gene expression[J]. Physiol Behav,2012, 105(4): 996-1006.
3. Golden GJ. B ehavioral and neural characterization of conditioned flavor-taste preferences[M]. Ann Arbor, Michigan, USA: ProQuest Information and Learning Company, 2007: 8-16.
4. Han J, Yan JQ. Expression of leptin receptor in the brain of conditioned taste aversion rats[J]. J Fourth Mil Med Univ, 2003, 24(1): 11-13.
5. Giza BK, Ackroff K, McCaughey SA,et al. Preference conditioning alters taste responses in the nucleus of the solitary tract of the rat[J]. Am J Physiol, 1997, 273(4 Pt 2): R1230-1240.
6. Sclafani A, Azzara AV, Touzani K,et al. Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats[J]. Behav Neurosci, 2001, 115(4): 920-933.
7. Touzani K, Sclafani A. Insular cortex lesions fail to block flavor and taste preference learning in rats[J]. Eur J Neurosci, 2007, 26(6): 1692-1700.
8. Touzani K, Sclafani A. Conditioned flavor preference and aversion: role of the lateral hypothalamus[J]. Behav Neurosci, 2001, 115(1): 84-93.
9. Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats[J]. Eur J Neurosci, 2005, 22(7): 1767-1774.
10. Jiang ES, Yan JQ. Taste stimulus and c-fos protooncogene expression[J]. Foreign Med Sci, 2001, 21(6): 440-442.
11. Han Z, Yan JQ, Luo GG,et al. Leptin receptor expression in the basolateral nucleus of amygdala of conditioned taste aversion rats[J]. World J Gastroenterol, 2003, 9(5): 1034-1037.
12. Kawai K, Sugimoto K, Nakashima K,et al. Leptin as a modulator of sweet taste sensitivities in mice [J]. Proc Natl Acad Sci U S A, 2000, 97(20): 11044-11049.
13. Heshka JT, Jones PJ. A role for dietary fat in leptin receptor, OB-Rb, function [J]. Life Sci, 2001, 69(9): 987-1003.
14. Guo QY, Gao Y, Cong L,et al. Free fatty acids on gene and protein expression and tyrosine phosphorylation of the leptin-receptor in rat skeletal muscle cells [J]. J Fourth Mil Med Univ, 2001, 22(14 ): 1307-1309.
15. Burguera B, Couce ME, Long J,et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain[J]. Neuroendocrinology, 2000, 71(3): 187-195.
16. Li Z, Ceccarini G, Eisenstein M,et al. Phenotypic effects of an induced mutation of the ObRa isoform of the leptin receptor[J]. Mol Metab, 2013, 2(4): 364-375.
17. Thorsell A, Caberlotto L, Rimondini R,et al. Leptin suppression of hypothalamic NPY expression and feeding, but not amygdala NPY expression and experimental anxiety [J]. Pharmacol Biochem Behav, 2002, 71(3): 425-430.
18. Wang YY, Wu SX, Li YQ. Localization of 5-HT5A receptor subtype in the rat nervous system [J]. J Fourth Mil Med Univ, 2001, 22(23): 2175-2178.
19. Jiang ES, Yan JQ, Song XA. The effects of gustatory and visceral stimulation on c-Fos expression in parabrachial nucleus in rats[J]. J Xi’an Med Univ, 2003, 1: 007.
20. Leifheit-Nestler M, Wagner NM, Gogiraju R,et al. Imporance of leptin signaling and signal transducer and activator of transcription-3 activation in mediating the cardiac hypertrophy associated with obesity[J]. J Transl Med, 2013, 11: 170.
21. Kohno D, Nakata M, Maekawa F,et al. Leptin suppresses ghrelin induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase and phosphodiesterase 3-mediated pathway [J]. Endocrinology, 2007, 148(5): 2251-2263.
22. Wu N, Tan HR. Progress of ob gene receptor molecular biological investigation[J]. J Dalian Med Univ, 2004, 26(2): 2175-2178.
23. Lei Q, Yan JQ, Shi JH. Inhibitory responses of parabrachial neurons evoked by taste stimuli in rat[J]. Acta Physiol Sinica, 2007, 59(3): 260-266.
24. Huang T, Yan JQ. The physiological characterisitics of taste neurons in the parabrachial neurons of rats[J]. Chin J Neurol, 2002, 18(2): 518-521.
25. Huang T, Yan JQ. Parabrachial nucleus and taste[J]. Foreign Medical Science · Section Pathophysiol Clinical Med, 2002, 22(2): 135-138.
26. Geng DD, Wang CQ. Effects of NaCl stimulus of c-fos expression in parabrachial nucleus in sodium def i cient rat[J]. Chin J Anat, 2007, 30(6): 763-765.
Jian-qun YAN, MD, Department of Physiology and Pathophysiology, Xi’an Jiaotong University, School of Basic Medical Science, 76 West Yanta Road, Xi’an 710061, China. Tel: 86-029-82655199; E-mail: jqyan@xjtu.edu. cn
Received 2015-06-08; accepted 2015-09-10
杂志排行
中国应用生理学杂志的其它文章
- A mini review: Tau transgenic mouse models and olfactory dysfunction in Alzheimer’s Disease
- Ethical inspection about laboratory animals
- Better parameters of ventilation-CO2output relationship predict death in CHF patients
- Association between endothelial nitric oxide synthase (ENOS) G894T polymorphism and high altitude (HA) adaptation: a meta-analysis
- Flow cytometric analysis of circulating microvesicles derived from myocardial ischemic preconditioning and cardioprotection of ischemia/reperfusion injury in rats
- Synergisms of cardiovascular effects between iptakalim and amlodipine, hydrochlorothiazide or propranolol in anesthetized rats
