WHO Prequalification of Medicines Program: Technical Assistance Effect
2015-05-15HUANGBaobinFOERGWIMMERChristinaSMIDMilanWUZhiangWUChunfu
HUANG Bao-bin,FOERG-WIMMER Christina,SMID Milan,WU Zhi-ang,WU Chun-fu*
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China; 2.Division of General Management,National Institutes for Food and Drug Control,Beijing 100050,China; 3.Team of Health System Development,WHO Representative Office in China,Beijing 100600,China; 4.Department of Essential Medicines and Health Product,WHO Headquarters,Geneva 1211,Switzerland)
WHO Prequalification of Medicines Program: Technical Assistance Effect
HUANG Bao-bin1,2,FOERG-WIMMER Christina3,SMID Milan4,WU Zhi-ang1,WU Chun-fu1*
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China; 2.Division of General Management,National Institutes for Food and Drug Control,Beijing 100050,China; 3.Team of Health System Development,WHO Representative Office in China,Beijing 100600,China; 4.Department of Essential Medicines and Health Product,WHO Headquarters,Geneva 1211,Switzerland)
Objective To evaluate the effect of technical assistance organized by WHO in order to provide effective technical assistance to China’s pharmaceutical manufacturers for reaching the standards of WHO prequalification. Methods Assessment tools and questionnaires for interview were designed,and the effect of technical assistances conducted in 4 domestic firms producing antituberculosis medicines in China was evaluated. Results and Conclusion The compliance with standards of WHO prequalification was upgraded at different scales among 4 firms accepting technical assistance. In production,it increased from an average of 53% to 80% for quality management system,and 70% to 80% for good manufacturing practices; in regulatory dossier,it increased from 28% to 67% for quality part,and from zero to 75% for efficacy and safety part. Interviews also showed difficulties faced by firms in terms of market share,regulation policies and cooperation of key partners in medicines development chain,etc. Technical assistances organized by WHO were effective in improving the compliance with the standards of WHO prequalification. However,the arrangement could be further improved and sustainability should be emphasized.
WHO prequalification; pharmaceutical manufacturer; assessment tool; technical assistance
Established in 2001,the World Health Organization (WHO) Medicines Prequalification (PQ) program provides a benchmark to screen quality assured essential medicines with competent manufacturers[1]. WHO PQ program contributes greatly to public health by ensuring quality of life-saving medicines used by millions of people in developing countries[2]. China,with a great number of pharmaceutical manufacturers and a large-size pharmaceutical industry,has the great potential to contribute to global health agenda via WHO PQ mechanism. Therefore,China’s pharmaceutical manufacturers have drawn high attention of international organizations,including WHO. However,as of now,China has only 15 medicines prequalified for anti-HIV/AIDs and anti-Malarial,without anti-TB medicines[3].
Usually,China’s pharmaceutical manufacturers are not familiar with the operation mechanism of WHO PQ program and need technical assistance provided by WHO. In order to have more China’s pharmaceutical manufacturers prequalified by WHO,WHO has taken actions to carry out technical assistances on Chinese firms. Meanwhile,WHO intended to explore some effective technical assistance suitable for firms and accumulate experiences for the conduction of similar work in other nations and areas.
Between 2009 and 2011,the Bill and Melinda Gates Foundation (BMGF) initiated a cooperative project with Chinese Ministry of Health to improve tuberculosis (TB) control,taking quality assured anti-TB fixed dose combination (FDC) as one of tools. In order to have quality assured anti-TB FDC available in China for project implementation,WHO was requested to assist 4 selected anti-TB FDC manufacturers in China to achieve the standards of WHO PQ.
This study aimed at evaluating the effect of technical assistance organized by WHO from the perspective of compliance with WHO PQ standards and the feedbackof firms. The outcome of the study could direct the improvement of the organization of technical assistance.
1 Subject
Up to now,there are 10 registered firms producing anti-TB FDC medicines in China[4]. A total of 4 anti-TB medicines manufacturers were selected for targeted assistances according to the predetermined criteria. The criteria were as follows: continuous production over past three years; the dosage and form were eligible for both WHO-PQ program and China national TB treatment guideline; not received technical assistance of WHO-PQ program yet. Table 1 showed basic information of 4 firms. From 2010 to 2011,WHO carried out a set of technical assistances to improve medicine quality and quality management system,thus to help 4 firms achieve the standards of WHO PQ.
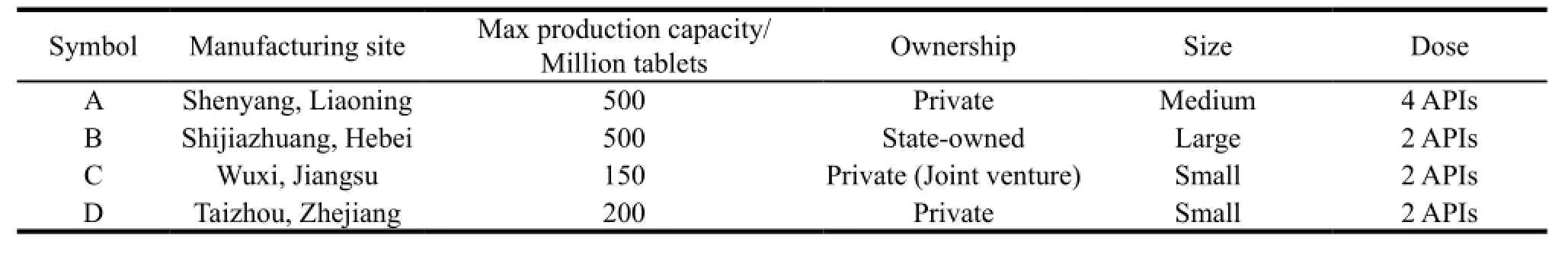
Table 1 Basic information of four firms
2 Organization of technical assistance
2.1 Signing technical assistance agreement
At first,a technical agreement was signed between WHO and each manufacturer. Each firm was requested to make commitment to implement recommendations given by WHO experts and provide sufficient fund and qualified staff as well.
2.2 Setting up working group
A working group consisted of relevant stakeholders was set up. Group members included: staff of WHO Geneva office and China office,general manager of each firm,managers in charge of quality assurance,production and dossier preparation and GMP inspectors from both national and provincial institutions as well. An expert team was formed with two international experts and one domestic expert. The international experts were experienced in GMP and pharmaceutical research and development and the domestic expert had rich experience in drug regulation policies and drug production practices.
2.3 Baseline assessment
From March to April of 2010,an assessment was conducted to understand baseline status in compliance with WHO GMP standards and WHO PQ requirements in dossier. In survey research,experts responded to inquires of staff of each firm on site,and delivered international experiences and practices. After survey,expert team drafted investigation report and each firm prepared an individual action for improvement based on identified technical gaps.
2.4 Conducting technical assistance
Common technical gaps were sorted out based on findings from survey. In response,training program was customized to cover technical issues in terms of GMP implementation,dossier preparation and conduction of bioequivalence (BE) study. A series of training were conducted between April and December of 2010. In addition,expert team carried out other technical assistances as needed. Two manufacturers producing common active pharmaceutical ingredients (APIs) were audited to evaluate WHO GMP compliance and completeness of dossier. In order to recommend qualified sites for conducting Phase I clinical trial,quality management system and the capability of conducting BE studies on anti-TB medicines in 5 sites were assessed. In vitro dissolution testing was subcontracted to drug quality control laboratory and firms could select crystal form of active pharmaceutical ingredients under the guidance of expert team.
2.5 Follow-up assessment
After 9 months of technical assistances,WHO carried out follow-up assessment on 4 firms and provided technical guidance on sites.
3 Assessment of technical assistance
3.1 Conduction in assessment tool
In order to document the changes of technical items and assess the effect of technical assistances,an assessment tool should be designed. As WHO PQ consists of two essential technical parts,WHO GMP and dossier,WHO designed two assessment tools for GMP implementation and dossier preparation respectively.
GMP implementation was divided into two subcomponents,one was quality management system and the other was manufacturing practices. Dossier preparation was divided into two subcomponents,quality part and safety and efficacy part. In further detail,quality management system consists of 15 technical items,including quality management,quality commitment and quality policies,etc. Manufacturing practices consists of 15 technical items,including premises,equipment and documentation system,etc. Dossier quality consists of 2 sections,APIs and finished product preparations (FPP) and 15 technical items. APIs section contains 7 technical items,including production,characterizations and quality control,etc. FPP section contains 8 technical items,including production,characterizations and quality control,etc. Dossier safety and efficacy consists of 4 items,design of BE study,selection comparator,selection of clinical trial site and selection of crystal form of APIs. The outcome of assessment was classified into two categories,one complied with requirements was numbered as 0 and the other that did not comply was numbered as 1.
For both baseline and follow-up assessment,two rounds of visits were conducted for each firm. One was to evaluate the status of GMP implementation by recording status and progress,and the other was to assess the completeness of dossier.
3.2 Conducting investigation with questionnaires
To understand firms’ willingness to apply for WHO PQ,the motivating factors to achieve WHO PQ standards and the technical gaps,expert team interviewed staff from administrative and technical departments in questionnaire. The questionnaire,designed in the principle of rational,universal,logical and definitive,included the following items: (1) willingness to have a market share abroad,planning to upgrade quality standards; (2) difficulties in complying with international standards: GMP implementation and dossier preparation; (3) incentives for upgrading quality to meet international standards. Table 2 showed the structure of questionnaire. According to the requirements of questionnaire,the working group and expert team communicated with firms’ staff in the process of baseline assessment,conduction of assistances and followup assessment.
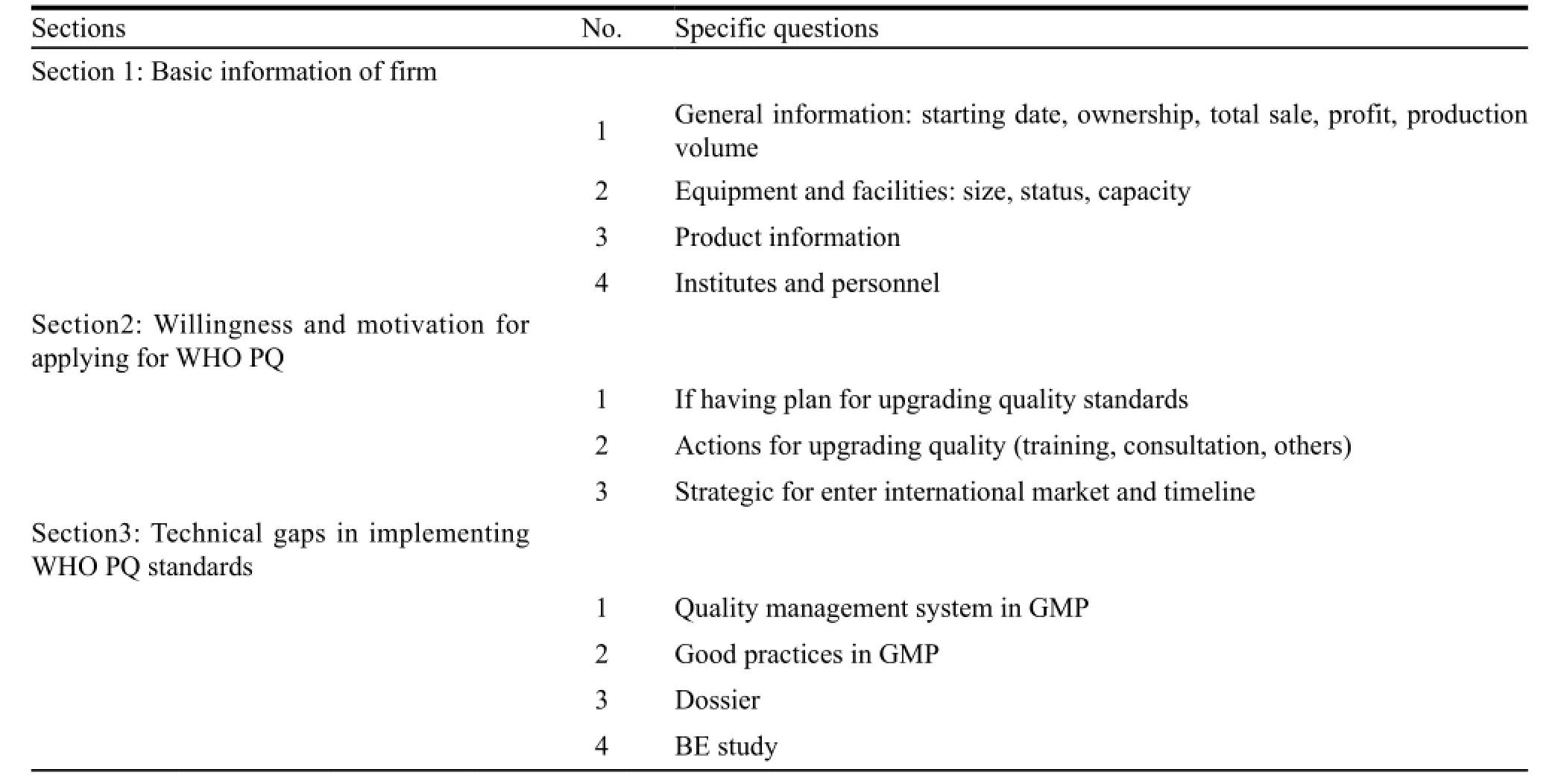
Table 2 Structure of questionnaire
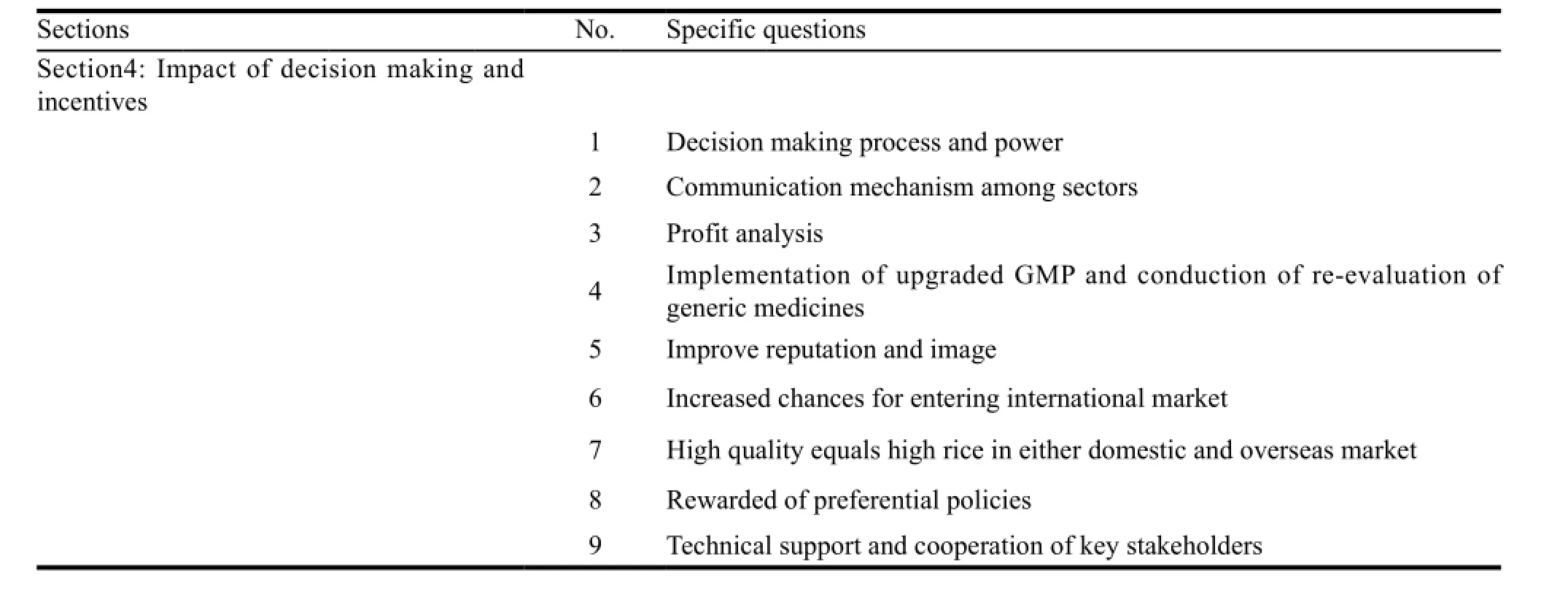
Continued Table 2
4 Results
4.1 Improvement of production operation
On average the compliance in quality management system and manufacturing practices had both reached to 80%,and each had 27% and 10% of improvement respectively. The improvements included strengthening quality assurance,conducting validation and qualification activities,improving manufacturing batch records and introducing equipment logbooks etc. Manufacturer B and C had the experience in hosting site inspections from foreign regulatory authorities,so they had higher percentage of compliance. Manufacturer D needed to construct a new plant which could not be completed when the assessment was done,so its compliance in production has only reached to 53%,below the average level. Table 3,4 and 5 showed the detail.
4.2 Improvement of dossier preparation
The compliance on average in quality and safety had reached to 67% (an increase of 38%) and 75% (an increase of 75%) respectively. The improvements were made in terms of obtaining necessary information from two common APIs producers,identifying the suitable crystal form of API-rifampicin with low bioavailability and selecting one domestic qualified clinical research organization (CRO) for BE testing. The anti-TB FDC produced by manufacturer A had 4 APIs among which the information of two APIs were not under control,so the compliance in quality of dossier was at 53%,below the average level. Table 5,6 and 7 showed the detail.
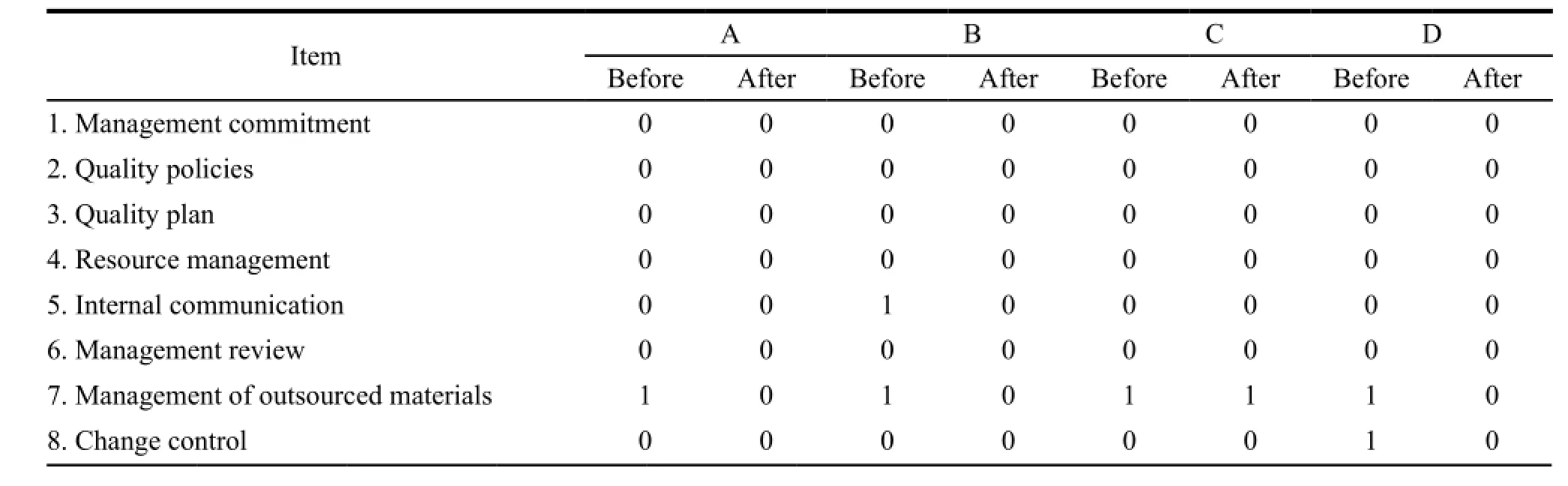
Table 3 Comparison of compliance in production before and after assistance
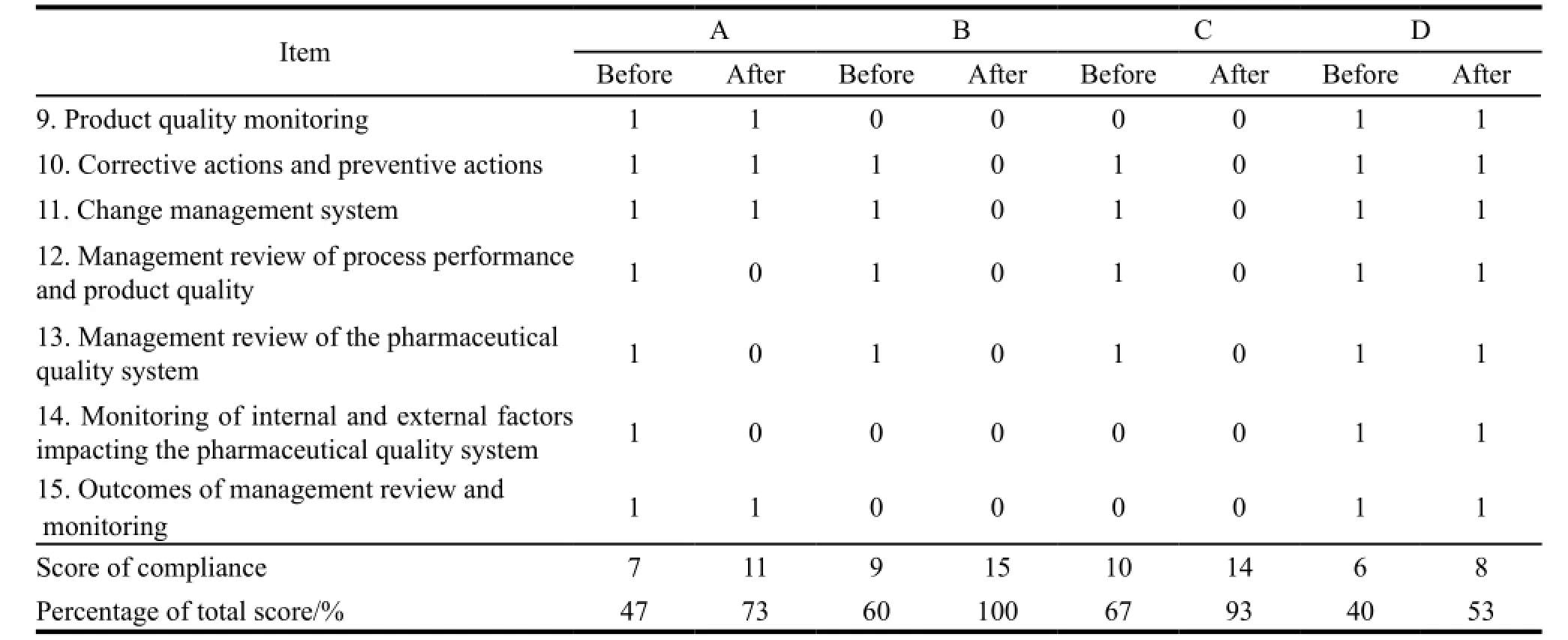
Continued Table 3
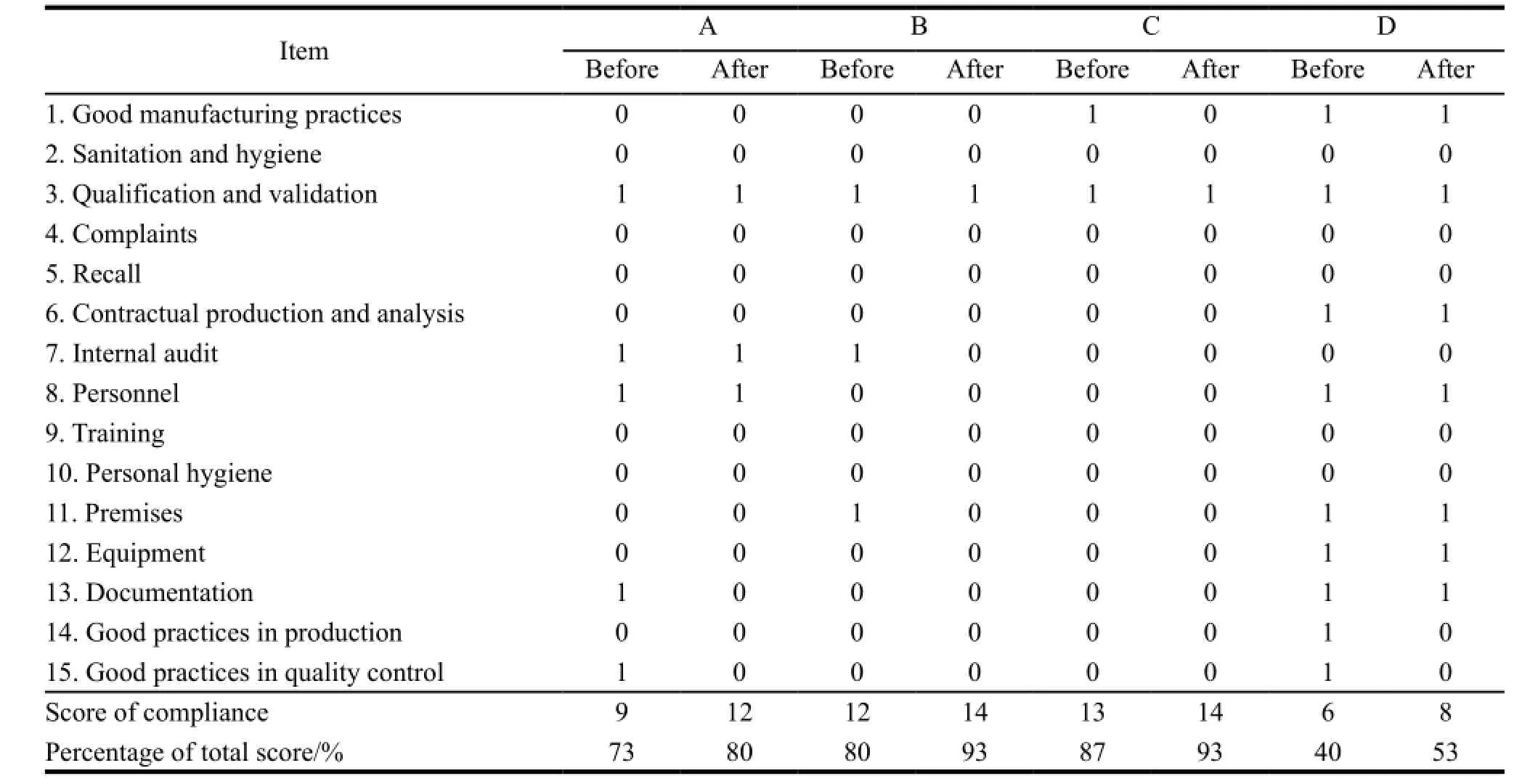
Table 4 Comparison of compliance in production management system before and after assistance
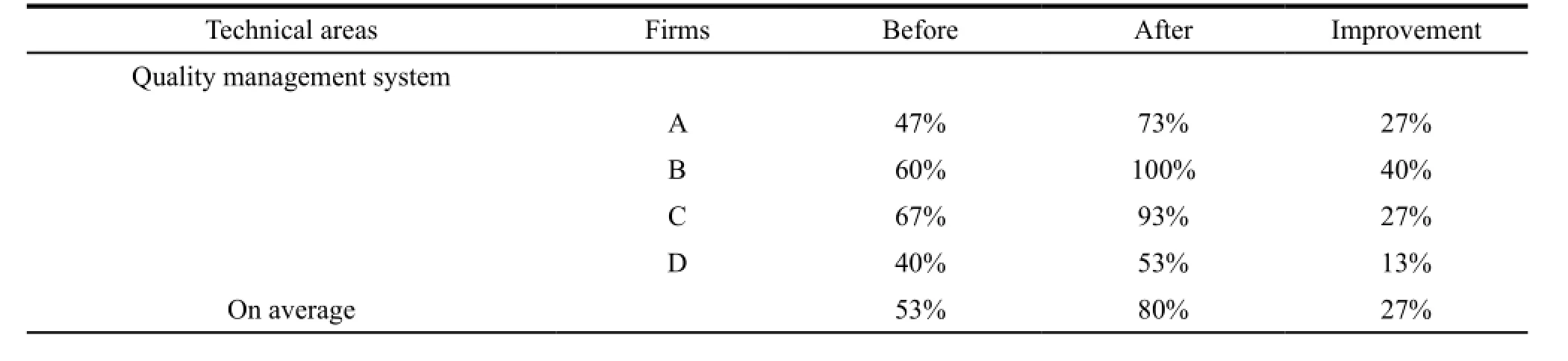
Table 5 Summary of four firms in compliance before and after assistance
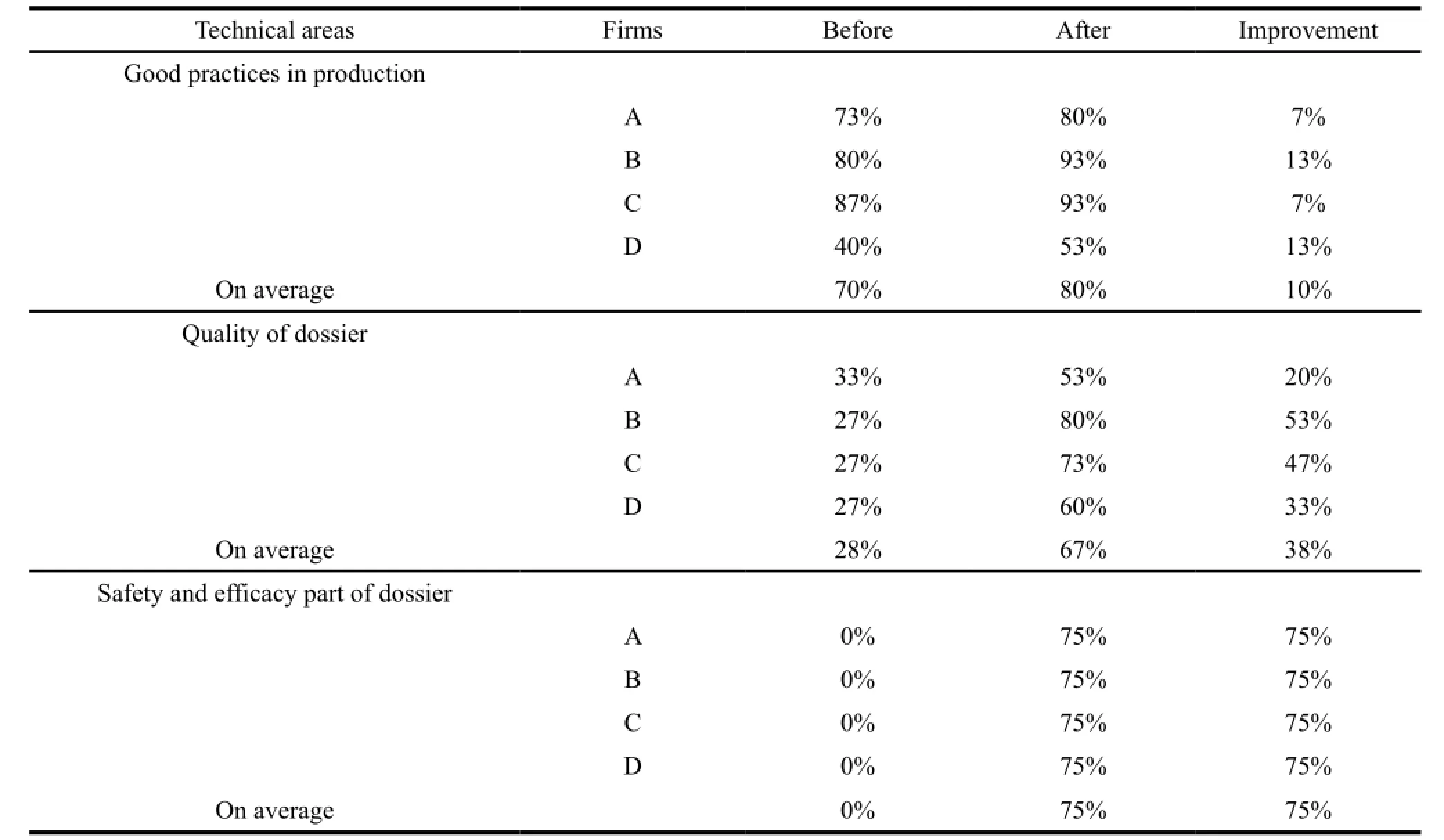
Continued Table 5
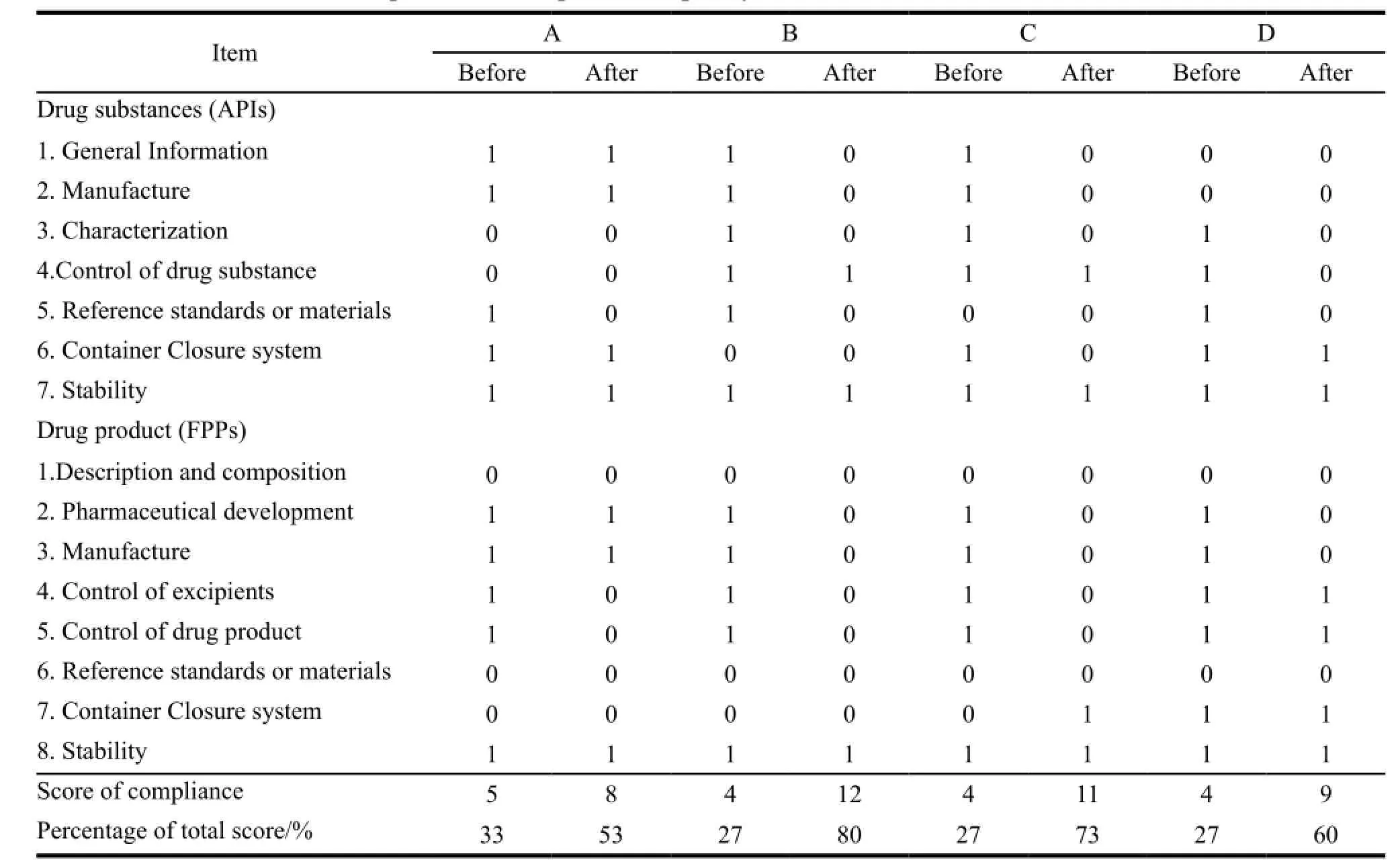
Table 6 Comparison of compliance in quality of dossier before and after assistance
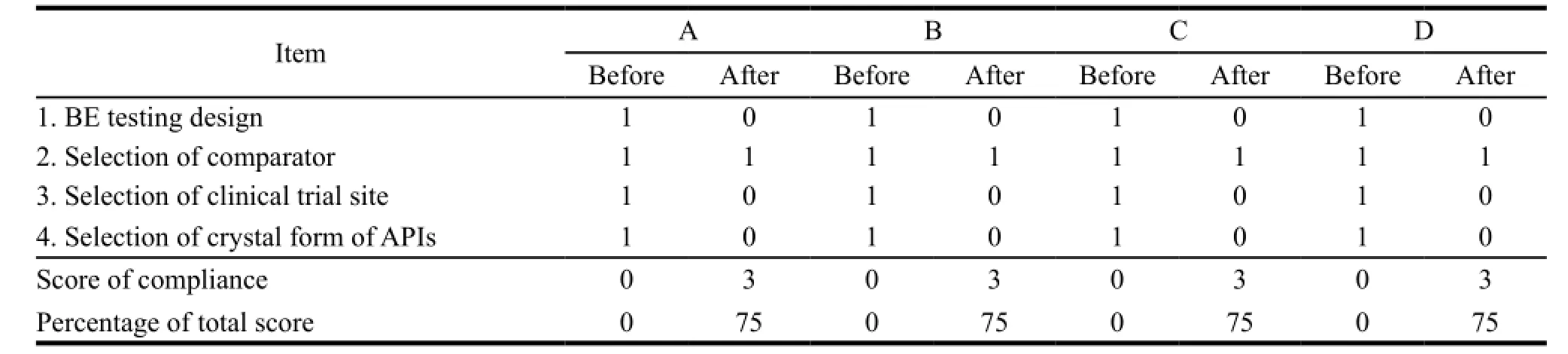
Table 7 Comparison of compliance in safety and efficacy part of dossier before and after assistance
However,because of the existing gaps such as the implementation of risk management,validation and qualification,correcting the deviation in GMP and conduction of BE studies,manufacturers could not achieve full compliance in production and dossier at the time of follow-up assessment.
4.3 Willingness,changes and difficulties
All 4 firms were willing to achieve WHO PQ standards by accepting WHO’s technical assistance. The motivation could be classified into two categories: direct one and indirect one. Directly,manufacturers expected to have an international market share once producing prequalified anti-TB FDC medicines. Indirectly,manufacturers wanted to have preferential treatment in domestic procurement regarding the prequalified medicines or even other medicines in house. In this case,manufacturers knew that achieving the standards of WHO PQ was an approach to improve their image. Apart from market motivation,the upgrading of national drug regulatory requirements in GMP and dossier preparation was perceived as another motivation to improve quality standards.
All participated firms acknowledged that they benefited from WHO’s technical assistance. In one hand,improvement was seen in staff’s awareness in drug quality. Manager of quality assurance department of firm A said that WHO’s technical assistance expedited its compliance with upgraded Chinese GMP standards through training and technical guidance. Manager of quality department of firm D said,with WHO’s technical assistance,staff had a deep understanding of quality and realized that quality assurance should be the first priority from medicine development to distribution. In another hand,firms also exposed the difficulties of improving quality they encountered in the process of upgrading. They were as follows: lack of resolution for long-term investment on finance and personnel due to uncertainty of potential market share; regulatory barriers in improving quality standards,such as time-consuming and repeated review and approval for BE studies; cooperation of two key stakeholders,one was sustainable supply of APIs with sufficient information as needed,and another was qualified clinical trial site.
5 Conclusion
Measurable changes in quality standards occurred among firms committed and motivated to participate in international procurement,particularly in strengthening regulatory dossier for quality and safety. This showed the effectiveness of WHO’s assistances in pulling manufacturers towards the standards of WHO PQ. This study had some limitations in that only 4 firms were assessed,and they were unlikely to represent the whole Chinese pharmaceutical industry with thousands of pharmaceutical manufacturers. However,as 4 firms were representative in terms of locations,size and ownership,this study could provide certain insight on whole pharmaceutical industry in China.
Other factors which contributed to the effect of WHO’s technical assistances should be taken into consideration. Questionnaire investigation showed that implementing upgraded national drug regulatory standards was perceived as one of incentives. In follow-up assessment,all firms had achieved higher percentage of compliance with international standards in production than in dossier. One reason behind it was that as upgraded Chinese GMP standard was to be released,and there was no critical difference between WHO GMP standard and upgraded Chinese GMP standard[5]. Apart from WHO’s assistance,it was probably because of pushing force of upgrading regulation in Chinese GMP standard. The pushing role of upgraded regulatory standard had also been recognized by WHO. The 67th World Health Assembly had recently approved a resolution on strengthening regulatorysystem and the new development of WHO PQ was to progressively transit WHO PQ to strengthen regulatory capacities[6]. Besides,the effect of preferential policies should be considered,such as making a plan for upgrading pharmaceutical industry[7]and emphasizing on quality in bidding and procurement of essential medicines[8].
Because WHO’s assistance could not last forever,the sustainability of assistance needs to be ensured. Firstly,it is the long-lasting commitment of top management of each manufacturer to improving quality standards. Manufacturers were willing to participate because they knew that achieving the standards of WHO PQ could help them in participating in international markets. However,the commitment to invest in the production of essential medicines for international procurement requires manufacturers to have up-to-date information on procurement demands. Secondly,two key partners should be closely involved APIs suppliers and CROs. Four anti-TB FDC manufacturers involved in this study outsourced APIs and the service of CRO. The information needed in dossier should be obtained from them,which were beyond the control of anti-TB medicines manufacturers
6 Discussion
The study evaluated the effect of WHO’s technical assistance on 4 firms for upgrading quality and providing suggestions for improving the design and arrangement of assistances. The outcome of the study could be used as a reference for organizing technical assistances to pharmaceutical manufacturers of other medicines in China and in other countries. Currently,China’s contribution on the quality assured reproductive health products and second line anti-TB medicines are the interest of international society[9,10]. The common technical gaps faced by 4 manufacturers are similar to the gaps faced by the reproductive health products produced in China and other countries in applying for WHO PQ. The organization of proper assistances to these products could be learnt from the support to the 4 anti-TB FDC manufacturers in China. Besides,the organization of WHO’s assistances to medicines manufacturers in other developing countries could benefit from this study.
[1]Editorial.The important world of drug prequalification [J]. Lancet,2004: 364-1830.
[2]Hoen EFM’,Hogerzeil HV,Quick JD,et al. A quiet revolution in global public health: the World Health Organization’s prequalification of medicines programme [J]. Journal of Public Health Policy,2014,35 (2): 137-61.
[3]WHO list of prequalified medicinal products [EB/OL]. http://apps.who.int/prequel,2015-01-28.
[4]SFDA database on registered medicines in China [EB/OL]. http://app1.sfda.gov.cn/datasearch/face3/base.jsp,2014-11-22.
[5]The website of China Food and Drug Administration [EB/OL]. http://www.sda.gov.cn/WS01/CL0001/,2014-10-28.
[6]World Health Assembly closes [EB/OL]. http://www.who. int/mediacentre/news/releases/2014/WHA-20140524/en/,2014-05-24.
[7]The 12th 5-year plan on upgrading Chinese pharmaceutical industry [EB/OL]. http://www.miit.gov.cn/n11293472/n11293832/n11293907/n11368223/14439892.html,2010-07-25.
[8]YAN Jian-zhou,ZHANG He-na,SHAO Rong. Study on quality evaluation indicator system for essential medicines [J]. China Pharmaceutical Affairs,2012,26 (5): 427-431.
[9]Global Fund to Fight AIDS,Tuberculosis and Malaria. Proposal for reducing the morbidity and mortality of tuberculosis in China [EB/OL]. http://portfolio.theglobalfund.org/en/Grant/Index/CHNS10-G14-T,2010-07-25.
[10]Hall P,Oehler J,Woo P,et al. A study of the capability of manufacturers of generic hormonal contraceptive in lower-and middle-income countries [J]. Contraception,2007,75: 311-317.
This study was supported by Bill and Melinda Gates Foundation [Global Health Grant Number OPPGH5258].
* Corresponding author: WU Chun-fu,professor. Major research areas: pharmacology and drug regulatory administration. Tel: 024-23986339,E-mail: wucf@syphu.edu.cn
杂志排行
亚洲社会药学杂志的其它文章
- Toxic TCM Supervision: Current Situations and Countermeasures
- Drug Registration and Approval System in China: Current Status,Controversies and Future Perspectives
- Analysis of TCM Patented Technology Based on the IPC Classification System
- Detection and Analysis on Outlier of the Average Medical Expense in China
- A Study on the Contract Research Organization
- An Empirical Study on Model of Consumers’ Initial Trust in Online Pharmacies
