Analysis of TCM Patented Technology Based on the IPC Classification System
2015-05-15DINGLeiWEIYingCHENYanYUANHongmei
DING Lei,WEI Ying,CHEN Yan,YUAN Hong-mei
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Analysis of TCM Patented Technology Based on the IPC Classification System
DING Lei,WEI Ying,CHEN Yan,YUAN Hong-mei*
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Objective To explore the trend of traditional Chinese medicine (TCM) R&D and patent applications on the basis of technical coverage of Chinese medicine enterprises. Methods Multidimensional data analysis was made based on 73,459 patents approved by the State Intellectual Property Office before 2013 from the aspects of the concentration of (international patent classification) IPC large groups for invention patents,the trend of patent applications for TCM and the interdisciplinary phenomenon of typical Chinese medicine enterprises. Results and Conclusion Fields of A61K35 and A61K26 patent application are the hotspots of TCM R&D from the perspective of IPC,and it is increasing year by year. Drug research for gastrointestinal or digestive diseases is another hotspot for the development of TCM from drug’s therapeutic activity aspects. In addition,it is found that the number of patents from Tasly Company is larger than that of Yiling Company,which indicates Tasly Company has a broader range of TCM patented technology.
Chinese medicine manufacturing; patented technology; IPC classification system
In recent years,the awareness of intellectual property rights (IPR) protection has deepened with the development of economic globalization and the in-depth implementation of China’s “National Intellectual Property Strategy”. Many research institutes and companies begin to seek patent protection for their researches which leads to the number of patent applications rising rapidly. In 2013,the State Intellectual Property Office (SIPO) received a total of 825,000 patents,an increase of 26.3%,and the amount of patent applications ranked first in the world for 3 consecutive years since 2011[1]. As a promising industry,pharmaceutical industry is very important for our national economy and its intellectual property rights have always been valued by the government. Due to the special nature of traditional Chinese medicine (TCM) industry,the protection of its intellectual property has become a priority. As an important means of protection of intellectual property,patents are a set of legal,technical and economic information,which not only reflects the forefront of technological development but also embodies human wisdom.
In the publication information of patents,technology fields are defined according to the international patent classification (IPC),and IPC contains a wealth of information which can be used to analyze the trend of technology in order to provide a reference for decisionmaking of the business or related organizations.[2]
1 Statistical analysis of patent of TCM manufacturing based on IPC
The IPC divides all the field of invention into 8 sections (represented by the letters A−H) which covers everything. Then each section is divided into category,subcategory,large group and small group. The scope of the technical areas also can be divided into smaller parts.[3]Through the analysis of TCM patent applications the author found that 96% of the applications were concentrated in a section (necessity for human life). C section (chemistry; metallurgy) accounted for only about 2%. Moreover,90.25% of applications concentrated in A61 (medical or veterinary science; hygiene) category and A23 involving food and C12 involving beverages accounted for 3.93% and 1.07% respectively; 58.61% of applications was concentrated in the A61K (medical,dental or toilet purposes formulations) subcategory and the subcategory of A61Prelated to a compound or pharmaceutical formulation of a particular therapeutic activity accounted for 29.88% in all of the applications. This paper makes a multidimensional data analysis of the concentration of IPC for invention patents,the trend of applications in major IPC of TCM,the core technology for major applicants and popular therapeutic areas based on large group which is more specific for TCM manufacturing.
1.1 Concentration analysis of IPC large groups
It involves a total of 1134 large groups through the statistics of Chinese medicine manufacturing patents for invention,and Table 1 shows the top 10 large groups and their respective percentage; we found that the patent applications for TCM concentrated mainly in three large groups of A61K36,A61K9 and A61K35 with the proportion of 28.39%,10.71% and 10.07% respectively.
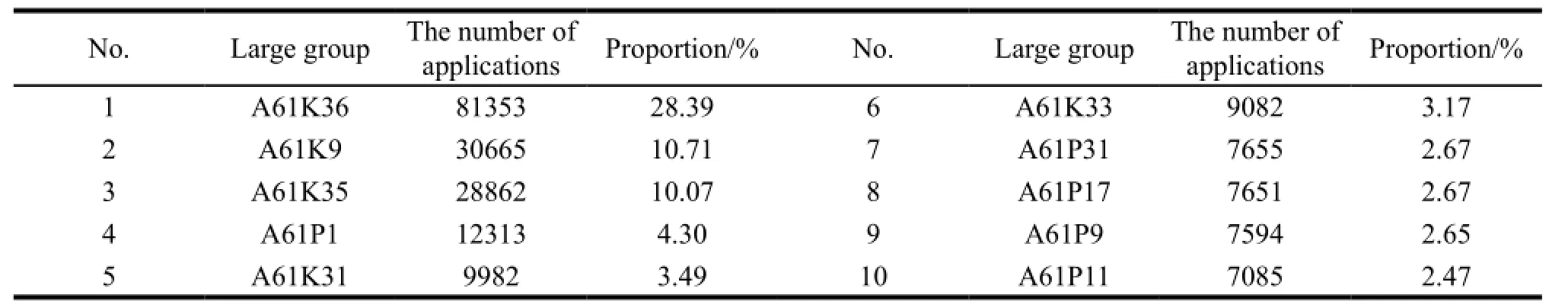
Table 1 Categories and the proportion of the top 10 large groups
In general,medicinal plants,animals and minerals are 3 main sources for TCM,and the group of A61K36 contains the relevant applications for plant origins,which involves algae,mosses,fungi,or plant derivatives,e.g. the undetermined structure for traditional herbal drug preparations. Moreover,this large group is divided into small groups of algae,ephedra,gmnospermae and angiospermae according to the phylum,class,family and genus of plants. From Table 1 we can see that the group of A61K36 has the highest proportion. It is the core of TCM manufacturing technology innovation. The main reason is that the diversity of TCM plant resources provides excellent conditions for research in this field.
The group of A61K9 refers to the relevant patent applications for pharmaceutical preparations characterized by special physical forms which mean the technical characteristics of the drug dosage and administration. In recent years,the drug dosage and administration of the TCM industry should be innovated constantly with the clinical high requirement and the improvement of preparation technology. Therefore the research on this technology covered by the group of A61K9 has become a hotspot. In addition,TCM firms should not only set up patent protection for their product formulation,but also develop new formulations with better efficacy.
The group of A61K35 refers to the patent applications for medicines with raw material which contains unknown structure or its reaction product. It involves the materials that derive from animal (e.g. A61K35/12 “the material derives from mammals or birds”,A61K35/56 “the material comes from animals other than mammals and birds”) and minerals (such as A61K35/02 “from non-living materials”). Compared with the research of plant-derived medicine,the technological innovation of Chinese medicine derived from animal and mineral is limited due to the lack of materials and the old technology. Thus the number of patent applications for such medicines is small. However,this phenomenon provides less technical resistance for TCM firms that focus on the medicines derived from animal and mineral,and it will be easier to break through the patent defense set up by their competitors and have an all-round patent layout.
1.2 Analysis of the trend of applications in major IPC groups for TCM
Firstly,the author analyzed the trend of applications in major IPC groups,as shown in Figure 1. We can see from the figure that the growth rate of applications in the group of A61K36 is very fast,but the relative volatility is big at the same time. We could get the curve fitting function y = 5.423x2.184(R2= 0.928),and as can be seen from the function that the number is creasing though it is highly volatile. In addition,the author analyzed the data in 2007 and foundout that SIPO received the most applications in the group of A61K36; the analysis results show that the applicant of Beijing Yixin Institute of Medicine filed 763 patents in this year which accounted for 12.90% of the annual total,and most of them were in the group of A61K36 which resulted in the rapid increase in the group of A61K36. It also shows that the major applicant’s technical breakthrough has significant impact on the development of technology.
Secondly,the number of patent applications in the group of A61K35 has increased significantly since 1993,and then it kept growing. The author tried to get the curve function and the result was not good because its fitting degree was too low to make predictions on the development trend of A61K35 after 2012. Moreover,the number of patent applications in the group of A61K35 increased rapidly between 2003 and 2005,but then it showed a downward trend which may be caused by “the Notice on the Suspension of Accepting Drug Registration Application for Yinxingdamo Injection and other 116 Varieties”issued by the State Food and Drug Administration. This notice published 117 varieties that already have national registration but they wanted to change the dosage form or the route of administration. This notice resulted in the retreat in the field of research in subsequent years[4].
Finally,the number of groups for patent applications is increasing as well. There are seven groups including A61P1 (treatment of digestive tract,or drugs for digestive system),A61K31 (pharmaceutical products containing organic active ingredients),A61K33 (medicinal preparation containing inorganic active ingredients),A61P31 (anti-infective drugs,antibiotics,antibacterial agent,chemotherapy agent),A61P17 (drugs for skin disease),A61P9 (drug for cardiovascular system) and A61P11 (drugs for respiratory diseases). The number of patent applications in these seven groups is rising steadily as well.
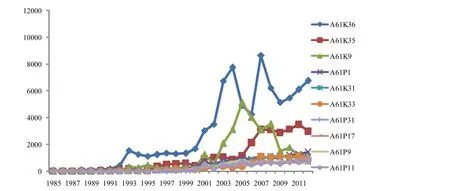
Figure 1 Applications trend chart of international patent classification (IPC) large groups
1.3 Analysis of IPC technology scope in TCM enterprises
The technology scope refers to the scope covered by a patent. Lerner (1994) was the first person who proposed that the technology scope could be calculated by using the first four numbers of IPC[5]. Lerner also found that the first four numbers of IPC were in proportion to patent quality,and 173 private US biotechnology company’s market value increased along with the increase of the scope of patent protection. The reason for this phenomenon may be that the more IPC of a patent was assigned to,the larger technology scope and the higher degree of innovation would be. This view was agreed by scholars Klemperer P (1990)[6],Gilbert R and Shapiro C (1990)[7]later.
We selected 2 TCM firms to analyze their technology scope. One was Tasly Pharmaceutical Group Co.,Ltd. and the other was Hebei Yiling Pharmaceutical Institute Co. Ltd. We used the following formula:
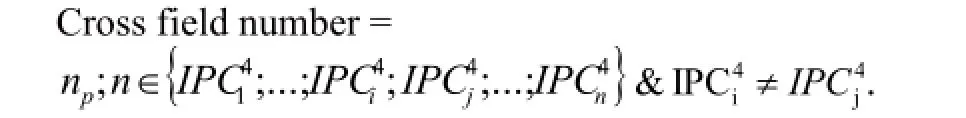
In the formula,nprepresents the categories of thefirst four numbers (i.e.,subclass) that listed in a patent. The results were shown in Table 3 and we could find that the 2 applicants had the same average cross field number. What’s more,we analyzed the cross field number in large groups and obtained np’. The result showed that the average cross field number from Tasly was higher than Yiling which indicated that the patents of Tasly covered more scope and they owned higher potential value in technology and market. In order to show the situation of cross field of the 2 applicants clearly,the author selected the samples of np=3 to analyze their technology scope. We can see from Table 2 that the number of cross field for Tasly Company is greater than Yiling,and they included G01N (to determine or analyze material by means of measurements that used in chemical or physical properties of materials),C07B (general methods of organic chemistry; the use of apparatus),C07H (saccharides; and derivatives thereof; nucleosides; nucleotides; nucleic acid),C07J (steroids),B01D (separation) and C08J (processing; general process ingredients;). However,Yiling focused on A23L (food,beverage or non-alcoholic drinks; its preparation or processing) and G01N. At this point,the results of the technical analysis were consistent with the calculated result of cross field number. In a word,Tasly Pharmaceutical Group Co.,Ltd. involved in all aspects of drug research and development and materials testing,compounds,synthesis,preparation and separation,which could provide a full range of three-dimensional patent protection for its products. While Hebei Yiling Pharmaceutical Institute Co. Ltd. just focused on the development of TCM,it was also committed to innovative technology of food and beverage based on the theory of TCM.
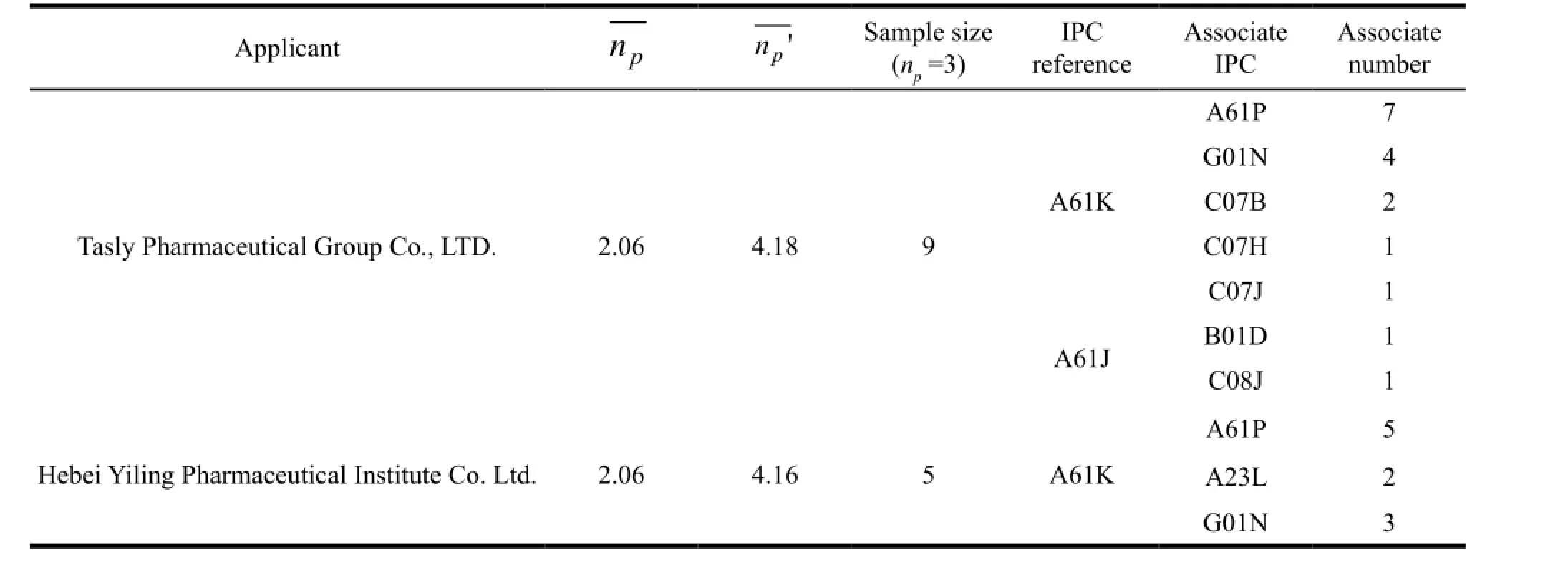
Table 2 Analysis of IPC technology scope from famous traditional Chinese medicine firms
1.4 Analysis of TCM patents in therapeutic areas
The patent applications for TCM industry have continued to increase with the vigorous development of China’s intellectual property rights. Therefore,the method of assigning IPC for TCM patents according to the sources in the sixth edition of IPC was not applicable any more. The category of A61P was added to the seventh edition of the IPC to solve the issue of literature excess in small group of A61K35/78,and making it possible to further subdivide TCM patents based on different therapeutic areas[8]. The group of A61P can not only be used to retrieve the analysis of the patented medicine,but also provides a way to analyze the core technology of diseases from the competitors of TCM firms. The author made further statistical analysis based on the patent database that contained the subclass of A61P,and as shown in Figure 2,the applications that related to “drug for gastrointestinal or digestive diseases”(A61P1) accounted for the largest proportion and became the hot research field for TCM; in addition,the applications that contain the large group of A61P31,A61P17,A61P9,A61P11,A61P19 (Drugs for bone disease),A61P15,A61P29 (Non-central analgesic,antipyretic or antiinflammatory agents,such as anti-rheumatic drugs; nonsteroidal anti-inflammatory drug),A61P25 (Drugs for nervous system diseases) and A61P3 also covers a large proportion.
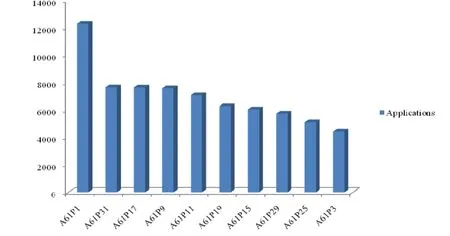
Figure 2 Analysis of the Top 10 large groups categories on therapeutic areas
2 Summary and suggestions
The increasingly international competition has promoted the development of China’s intellectual property since China joined the WTO,and the government is also introducing new policies constantly to encourage inventions,thus enhancing China’s comprehensive competitiveness,patent applications in TCM manufacturing field have also experienced a process from a small amount to rapid growth. As the increasing complexity and diversity of TCM,judging the technologies in the industry accurately and analyzing the variation trend play an important role in making policies. This paper herein makes a multidimensional data analysis of patents of TCM from the aspects of IPC and finds out the groups of A61K36 and A61K9 have the largest proportion and fluctuation. As to applicants,different applicants have their own technological advantages. Secondly,by analyzing IPC interdisciplinary distribution of typical TCM enterprises,we found that the interdisciplinary was different for these enterprises with the same number. For example,although related to many domains of IPC large groups,Tasly Company is mainly active in pharmaceutical industry. As for Yiling Company,it sets foot in food and drinks industry on the basis of TCM. Finally,as to the therapeutic field we could conclude that developing TCM treating digestive system diseases is the key technology innovation for TCM manufacturing industry.
Through the above multidimensional analysis of IPC of patents in TCM manufacturing industry,we make the following suggestions based on the above analysis. Firstly,patent protection for ingredients and new dosage forms should be conducted simultaneously. TCM enterprises should not only attach importance to the research for ingredients but also select the most effective and convenient dosage forms by comparison. If possible,the protection of products should include patents from the composition,extraction and separation to the determination of effective components,thus building an all-around patent fence for the core products to improve their core competence. Secondly,TCM is not only used for medical treatment but also in food,drinks,health care products and many other fields. Therefore,combined with features of the patentconcentrated fields,TCM enterprises can learn experience from those successful enterprises to cross fields and make a reasonable patent layout for realizing their goals. Finally,TCM enterprises can not only screen the same or similar technologies with their focused fields to conduct a secondary development or re-innovation but also get to know the latest research trends of their competitors so that they can adjust their research direction or market strategy properly. As to local government and the IPR Management Departments,they can make developing policies based on a multidimensional analysis of the patents of IPC in their jurisdictions and they can promote the fields with high innovation degree continuously,at the same time they can give some policy support to the fields with low innovation degree in order to have a balanced and healthy development for the whole TCM industry.
[1]China’s invention patents and related conditions in 2013 [EB/OL]. http://www.sipo.gov.cn/zcfg/tjxw/201410/t20141023_1024513. html,2014-03-04.
[2]Squicciarini M,Dernis H,Criscuolo C. Measuring patent quality: indicators of technological and economic value [EB/OL]. http://dx.doi.org/10.1787/5k4522wkw1r8-en,2013-06-06.
[3]ZHANG Yang,SHI Yi. Discussion on the distribution of traditional Chinese medicine technology in the international patent classification [J]. World Science and Technology- Modernization of Traditional Chinese Medicine,2012,14 (3): 1728-1731.
[4]The Drug Safety Note. No. 52 notice about the suspension of accepting yinxingdamo injection and other 117 varieties with national standards for drug registration application and the related matters [EB/OL]. http://www.sda.gov.cn/WS01/CL0055/10358. html,2005-02-07.
[5]Lerner J. An empirical analysis of the importance of patent scope [J]. RAND Journal of Economics,1994,25 (2): 319-333.
[6]Klemperer P. How broad should the scope of patent protection be [J]. RAND Journal of Economics,1990,21 (1): 113-130.
[7]Gilbert R,Shapiro C. Optimal patent length and breadth [J]. Rand Journal of Economics,1990,21 (1): 106-112.
[8]ZHANG Wei-bo. The development of tools of international patent classification for TCM literature—information about ipc 30th expert meeting organized by WIPO [EB/OL]. http://www.doc88. com/p-2572953234305.html,2013-03-04.
* Corresponding author: YUAN Hong-mei,professor. Major research area: drug intellectual property. Tel: 13604027062,E-mail: yuanhm612@163.com
杂志排行
亚洲社会药学杂志的其它文章
- Toxic TCM Supervision: Current Situations and Countermeasures
- WHO Prequalification of Medicines Program: Technical Assistance Effect
- Drug Registration and Approval System in China: Current Status,Controversies and Future Perspectives
- Detection and Analysis on Outlier of the Average Medical Expense in China
- A Study on the Contract Research Organization
- An Empirical Study on Model of Consumers’ Initial Trust in Online Pharmacies
