Toxic TCM Supervision: Current Situations and Countermeasures
2015-05-15CAOMingchengHUANGTaikang
CAO Ming-cheng,HUANG Tai-kang
(1.Research Center of Modern Social Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China;
2.Hefei Innovative Pharmaceutical Technology Co.,Ltd.,Hefei 230088,China; 3.China Food and Drug Administration,Beijing 100810,China)
Toxic TCM Supervision: Current Situations and Countermeasures
CAO Ming-cheng1,2,HUANG Tai-kang3*
(1.Research Center of Modern Social Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China;
2.Hefei Innovative Pharmaceutical Technology Co.,Ltd.,Hefei 230088,China; 3.China Food and Drug Administration,Beijing 100810,China)
Objective To put forward suggestions for effective supervision of toxic traditional Chinese medicine (TCM) in China. Methods Classification and supervision of toxic TCM were analyzed and suggestions for the supervision of toxic TCM were given. Results and Conclusion Current situations of the supervision of toxic TCM are studied and countermeasures are raised.
toxic TCM; supervision; countermeasure
The theory of using toxic traditional Chinese medicine (TCM) and general medicine together to treat diseases gradually formed based on repeated verification and practice by TCM practitioners. With the research and development of toxic TCM,they have very good effects on the treatment of rare diseases in recent years. But at the same time,toxic TCM also has the adverse reactions. So the supervision of the toxic TCM should be strengthened.
1 The significance and purposes of the research on toxic TCM
TCM is one of the most precious treasures in China. And the toxic TCM is an important part of TCM,which is effective to treat some rare diseases. The efficacy of toxic TCM is good,while the safety is limited. If the toxic TCM is used properly,the curative effect will be remarkable; if not,the toxic side effect is high and it can cause death. Therefore,the effectiveness and the safety should be strengthened through the research on the toxicity of TCM. In addition,foreign customers pay more attention to the safety,effectiveness and controllability of drugs. Therefore,the study on toxic TCM has great significance to both the development of TCM industry and taking a share in the international medicine market.
The purpose of studying the toxic TCM is to ensure the safety and effectiveness of clinical medication and promote the development of TCM industry.
2 The definition of toxic TCM and the classification of toxic medicinal materials
2.1 The definition of toxic TCM
At present,the definition of toxic TCM is mainly based on “Chinese Pharmacopoeia (2010)”,national laws and regulations and the latest research findings,so the definition of toxic TCM includes the following: (1) the toxic medicinal materials published in “Chinese Pharmacopoeia”(2010),“Standard for the Ministry of Health of the People’s Republic of China” (including Tibetan,Uygur Medicine,Mongolian Medicine) and the standards for different provinces and municipalities (including the decoction pieces); (2) twenty-eight kinds of toxic Chinese herbal medicines issued by the State Administration of Traditional Chinese Medicine in 1992; (3) other toxic materials.[1]
2.2 The classification of toxic medicinal materials
According to “Chinese Pharmacopoeia”,the toxicity of medicinal materials is divided into three categories,including strong toxicity,middle toxicity and mild toxicity. In addition,the medicinal materials with clinical adverse reactions also should be included. As shown in Table 1.

Table 1 The intensity of China’s toxic traditional Chinese medicinal (TCM)
3 The current supervision of toxic TCM in China
The supervision of toxic TCM is implemented according to China’s drug supervision law and it included the following points:
3.1 The supervision of the listed TCM
There are 28 kinds of toxic TCM in the “Measures for the Management of Toxic Drugs for Medical Use” issued by the Ministry of Health. Ten kinds of medicinal materials and decoction pieces with strong toxicity,42 kinds with middle toxicity and 31 kinds with mild toxicity were written in“Chinese Pharmacopoeia” (2010).
3.2 Laws and regulations on the supervision of toxic TCM
The management of toxic TCM is mainly based on“The Drug Administration Law of the PRC” and “Measures for the Management of Toxic Drugs for Medical Use”. These regulations contain provisions for toxic drugs in the production,supply,use and other related legal liabilities,but there are few provisions on supervision of toxic TCM.
In the process of production,the provisions require that the processing of TCM pieces should be strictly in accordance with “Chinese Pharmacopoeia” (2010) or the“Processing Standard” issued by the health administrative departments of different provinces,autonomous regions and municipalities. Only the decoction pieces that are in accordance with the “Processing Standard” can be used for the production of Chinese medicine.[2]
3.3 Other regulations and normative documents for the supervision of toxic TCM
There are 3 key projects and 2 general projects specified for toxic medicinal materials in good manufacturing practice (GMP) certification of Chinese decoction pieces from the SFDA[3]. With the steady development of Chinese decoction pieces,many negative effects also arose with quality problems. Due to the frequent occurrence of quality problems,many enterprises’GMP certificates were retracted. CFDA retracted 50 pharmaceutical companies’ GMP certificate,including 20 companies of Chinese decoction pieces,accounting for 40% in 2014. And 21 pharmaceutical companies’ GMP certificate were retracted,17 Chinese decoction pieces,accounting for 80% in the first quarter of 2015.[4]
In order to control Chinese drugs preparation strictly,the SFDA issued 5 technical standards for the approval of Chinese medicine in June 2008,which included “The Treatment Principle for the Related Problems of External Preparation of TCM”,“The Treatment Principle for the Related Problems of TCM Technology”,“The Treatment Principle for the Related Problems of Quality Control of TCM”,“The Technical Requirements for the Rational Choice of the Varieties of TCM” and “The TreatmentPrinciple for the Toxic Medicinal Materials and Other TCM with Safety Issues”[5].
The SFDA issued the “Notice of Amending the Instructions of the Varieties of TCM Decoction Pieces with Toxicity”,which indicated that the SFDA would regulate TCM decoction pieces more strictly. The notice specified that the relevant pharmaceutical production enterprises should mark the name of toxic TCM decoction pieces on the instructions,and put the warnings in the corresponding position for any prescriptions containing 28 kinds of toxic TCM decoction pieces based on the “Measures for the Management of Toxic Drugs for Medical Use”[6].
4 Problems in the supervision of toxic TCM
4.1 Poor supervision caused by the disorder of medicine source
Due to the influence of history,geography and culture,there is a phenomenon of homonym or synonym for TCM and it leads to the disorder of the decoction pieces source. One Chinese medicine is from several families with more than a dozen of the origins. The varieties of Chinese herbal medicine have different chemical composition and content after being made into the decoction pieces,which can affect the quality of the pieces. There are 37 kinds of decoction pieces which can cause confusion in drug circulation.
4.2 Lack of supervision for clinical use of toxic TCM
The varieties of toxic TCM are used in disorder without clear sources and the medicines with different levels of toxicity are often mixed which result in medicine safety issues. For example,the Wu Chia Pee can be divided into two categories; one belongs to the Araliaceae,which has no toxicity while the other belong to the Asclepiadaceae with toxicity[7]. Improper compatibility of different TCM may lead to the increase of the toxicity of medicines. The effective amount,dose and lethal dose of the active ingredient of toxic TCM are close which have to be evaluated by experienced doctors due to the lack of proper supervision. Taking these medicines for a long period can result in the accumulation of the toxic ingredients in the body and it is bound to cause damage to health. For instance,taking the small doses of Aristolochia manshuriens containing aristolochic acid for a long time can lead to chronic aristolochic acid nephropathy,and it may also lead to cancer,especially the cancer of the urinary system[8]. Because the aristolochic acid is a mutagenic agent and it can cause chromosome damage.
In addition,lots of adverse reactions are caused by ignoring contraindications in clinical use of toxic TCM.
4.3 The inadequate supervision of TCM processing
There is a series of physical and chemical changes in Chinese herbal medicines after processing,the toxicity of many TCM is reduced or eliminated through proper processing. For example,the monkshood has strong toxicity while can be reduced after processing,and it can be applied for clinical use[9]. So processing and the way for processing have an important effect on toxicity. However,the processing specification is not unified nationally,which brings a negative impact on the supervision.
4.4 The overall supervision has many difficulties
Toxic TCM has many varieties and the factors that affect quality are complicated,which leads to the difficulty in the supervision. The standards of medicinal materials are not unified because they include “Chinese Pharmacopoeia”,the ministry standard and the local standards from various provinces and municipality. Therefore,the intensity of the nation-wide supervision is low. A case in point is that many companies produce TCM without licenses. What is more,the management order also has some problems which cause the frequent events of TCM,especially the toxic TCM.
In addition,the lack of professionals of TCM leads to the deficiency of clinical medication guidance and it reduces the efficiency of the supervision of toxic medicines. The current regulatory means and methods also affect the enthusiasm of the drug administration personnel for the supervision of toxic TCM.
5 Countermeasures for strengthening the supervision of toxic TCM
5.1 Promoting good agricultural practice (GAP) certification system comprehensively and integrating industrial chains
The governance of source should be focused on in the supervision of toxic TCM. Promoting GAP rapidly is an important way to solve the quality of TCM. The process of implementing GAP is to reshuffle the varieties and sources of Chinese herbal materials which can ensure the authenticity and controllability of Chinese herbal medicine[10].
GAP can standardize and regulate varieties,place of origin,cultivation,harvest and the processing of Chinese herbal materials so that it will encourage companies with solid foundation to set up factories for toxic TCM processing,or to integrate the industrial chains to build the operation system with production,sales and clinical use of toxic TCM.
5.2 Strengthening the literature research of toxic TCM further and establishing databases
We can sort out the modern cases of toxic TCM with good therapeutic effects and classify them,and then we can make the comparison between the ancient and modern dose and the way of taking medicines,including the ancient validation and clinical experience information. At the same time,we should also collect the failed cases with death or disability and classify them as well. Establishing the toxic TCM databases can help the medical researchers to guide clinical drug use so that a scientific basis can be provided for the supervision of toxic TCM.
5.3 Strengthening the study on the safety evaluation of toxic TCM and monitoring adverse reaction
As to the safety issues of toxic TCM,Chinese scholars have started the study from several aspects,such as the study on pharmacodynamics of TCM,the toxic kinetics of TCM,the metabonomics of TCM and the biotechnology of Chinese medicine. But there is still a long way to go. They can draw lessons from the foreign method of evaluating drug safety so that they can improve the reporting system of the adverse reaction of toxic TCM. Establishing the databases of the adverse reaction of toxic TCM will further strengthen the study on the epidemiology and the toxicology of toxic TCM.
The adverse reaction of toxic TCM has been reported in some documents for a long time. Retrieving the medical literature,we could find that there were 2,747 cases of adverse reactions of toxic TCM from 1980 to 1999. Each of the 34 kinds of toxic TCM had more than 10 adverse reactions. As shown in Table 2 and Table 3.[11]
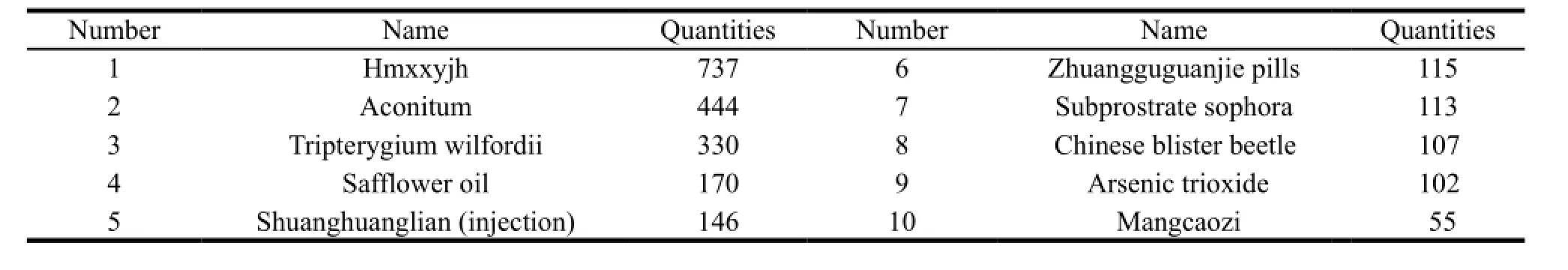
Table 2 Top 10 toxic TCM with adverse reaction
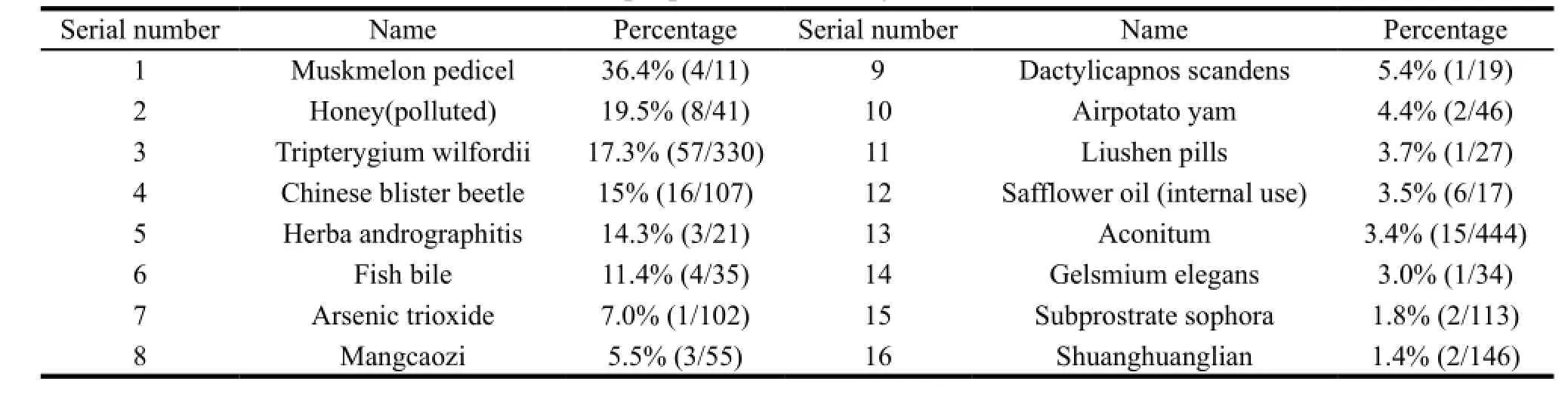
Table 3 Death proportion caused by adverse reactions of TCM
5.4 Carrying out the “3G certification” system strictly
The establishment of the production bases of medicinal materials can guarantee the stable production of Chinese herbal medicines with good quality. The quality of GAP planting should be regulated effectively; a huge investment in the advanced production equipment and processing technology must be increased as well. Technical level and testing process should be improved. Besides,GSP management should be implemented in the planning,procurement,inspection,storage and sales. At the same time,some technology must be applied to the moisture proof,mould proof and moth proof for toxic TCM ifnecessarily,and the warehouse storage conditions should be improved to control the humidity and temperature. Some necessary storage facilities must be added to prevent the medicinal materials from deterioration.
5.5 Improving the professional skills of practitioners continuously
The supervision of toxic TCM should be completed by professional Chinese medicine personnel,and enhancing the cultivation of professional Chinese medicine personnel continuously plays an important role in improving the supervision of TCM. We can cultivate the practical talents through the orientation training for enterprises,the university commissioned training and establishing the service oriented postgraduate training base etc.,various types of workshop and training courses should be held occasionally to improve the supervision of toxic TCM in the procurement,inspection,maintenance,storage and other aspects.
6 Conclusions
TCM is China’s treasure,and it is good to use toxic TCM to cure human’s diseases fully and rationally. Meanwhile,we should be aware of the adverse reaction of toxic TCM so that we can carry out the supervision. Based on the theory of TCM,a scientific supervision system can be formed for toxic TCM finally.
[1]JIN Xi-zhen. Simple discussion on the reasonable application of toxic TCM [J]. Seek Medicine and Ask the Medicine,2013,11 (8): 164-165.
[2]WANG Chong. Problems and countermeasures of drug supervision and management in China [J]. Continuing Medical Education,2014 (9):121-122.
[3]HAN Wu-qi. Clinical application and management of toxic Chinese herbal medicine pieces [J]. China Food and Drug Administration,2010,12: 64-66.
[4]Analysis of the current situation and development trend of Chinese herbal medicine industry in 2015 [EB/OL]. http://chengdu.zyczs. gov.cn,2015-04-28.
[5]The notice of 5 technical standards for the approval of related Chinese medicine as treatment principle for the related problems of TCM technology issued by SFDA [EB/OL]. http://law. pharmnet.com.cn/laws,2008-06-12.
[6]The notice of amending the instructions of the varieties of proprietary Chinese medicine that contains toxic TCM decoction pieces issued by the SFDA [EB/OL]. http://www.sda.gov.cn/WS01/CL0844/94123.html,2013-11-04.
[7]BAI Xiao-ju,ZHAO Yan. Discussion on rational use of poisonous Chinese herbal drugs [J]. Chinese Journal of Pharmacovigilance,2009,6 (9): 526-529.
[8]YUAN He-yong. Discussion on toxic side effect on kidney from TCM caused by aristolochic acid [J]. Guangdong Pharmaceutical Journal,2003,13 (3): 59.
[9]YE Ding-jiang. Science of processing Chinese material medicine [M]. Shanghai: Shanghai Science-Technology and Publication,1996.
[10]SHENG Ming-zhi. The quality of Chinese herbal medicine should be monitored from the origin [J]. Journal of Modern Medicine & Health,2006,22 (19): 3003-3004.
[11]TANG Jing-bo,WU Peng. A survey of adverse reactions of TCM reported in the last 20 years [J]. West China Journal of Pharmaceutical Sciences,1999,14 (5): 377.
* Corresponding author: HUANG Tai-kang,professor. Major research area: social pharmacy. Tel: 024-23986543,E-mail: iwenwen@sina.com
杂志排行
亚洲社会药学杂志的其它文章
- Drug Evaluation Programs in China: Problems and Countermeasures
- Improving the Development of Online Pharmacies in China
- An Empirical Study on Model of Consumers’ Initial Trust in Online Pharmacies
- A Study on the Contract Research Organization
- Detection and Analysis on Outlier of the Average Medical Expense in China
- Analysis of TCM Patented Technology Based on the IPC Classification System
