CO2 capture using dry TiO2-doped Na2CO3/Al2O3 sorbents in a fluidized-bed reactor
2015-05-08DongWeiChenXiaopingYuFan
Dong Wei Chen Xiaoping Yu Fan
(Key Laboratory of Energy Thermal Conversion and Control, Ministry of Education, Southeast University, Nanjing 210096, China)
CO2capture using dry TiO2-doped Na2CO3/Al2O3sorbents in a fluidized-bed reactor
Dong Wei Chen Xiaoping Yu Fan
(Key Laboratory of Energy Thermal Conversion and Control, Ministry of Education, Southeast University, Nanjing 210096, China)
In order to improve the reactivity of Na2CO3/Al2O3sorbent with CO2, a new sorbent showing high reactivity was developed by doping Na2CO3/Al2O3with TiO2using impregnation. Fourteen multi-cycle carbonation/regeneration tests of the sorbent were carried out in a fluidized-bed reactor and the sorbent was characterized by X-ray diffraction and nitrogen adsorption. It is confirmed that TiO2shows a positive effect on the adsorption process of Na2CO3and the reaction rate is observed to increase significantly, especially in the first 10 min. Moreover, TiO2is stable within the temperature range of the process and no other Ti-compounds are detected. The carbonation products are NaHCO3and Na5H3(CO3)4. The surface area and the pore volume of the sorbent keep stable after 14 cycles. The Fourier transform infrared spectroscopy and the X-ray photoelectron spectroscopy are used to analyze the effect mechanism of TiO2on CO2adsorption process of Na2CO3/Al2O3.
CO2capture; Na2CO3/Al2O3; TiO2; fluidized-bed test
Applying alkali metal-based solid sorbents for CO2capture from flue gas has been investigated as an innovative concept in these years. K2CO3or Na2CO3can be used as the regenerable solid sorbent based on the chemical absorption process at the low temperature. Most studies around the world focus on the potassium-based sorbents because potassium carbonate is generally superior to sodium carbonate in terms of both CO2capacity and kinetics[1-5]. Nevertheless, the primary advantage of using sodium carbonate over potassium carbonate is due to its low price, easy accessibility, and low CO2capture cost. If the reaction characteristics of the sodium-based sorbents can be improved for high reactivity, a high conversion rate, and a short reaction time, this technology will have great market value and broad application prospects.
Researchers have also tried to develop alkali metal-based sorbents supported on various supports, such as activated carbon, Al2O3, SiO2, MgO, ZrO2, CaO, and zeolites[2-3, 5]. Activated Al2O3is confirmed to be an outstanding support for CO2capture attributed to its well-developed microstructure and excellent abrasion resistance[3-4]. A previous study[6]also demonstrated that sorbent Na2CO3/Al2O3(Na2CO3impregnated on Al2O3) shows a significant performance for CO2capture at a low temperature in the presence of vapor. Whereas the CO2absorption rate of the sorbent still needs to be increased.
Recently, increasing attention on the use of doped calcium-based CO2sorbents has been paid. Chen et al.[7]doped limestone with attapulgite and demonstrated that the doped sorbent shows better CO2capture performance than the natural limestone under the same condition. Sun et al.[8]investigated manganese salts, including Mn(NO3)2and MnCO3, doped calcium-based sorbents and reported that the cyclic CO2capture capacity of CaCO3was significantly improved. Al-Jeboori et al.[9]reported that MgCl2, CaCl2, and Grignard reagents as dopants can improve the carrying capacity of Havelock limestone in repeated cycles of carbonation and calcination in a fluidized bed reactor. Accordingly, doping is promising for developing advanced alkali metal-based solid sorbents to remove CO2more effectively. Whereas little related work has been reported so far.
The principal goal of the present research is to investigate methods to improve the CO2uptake rate in such a process by doping TiO2in Na2CO3/Al2O3. The reaction mechanism of Na2CO3/Al2O3and TiO2doped Na2CO3/Al2O3are studied and analyzed. Their carbonation/regeneration characteristics, microstructure and physical properties are investigated in 14-cycle tests using a fluidized-bed reactor.
1 Experimental Section
1.1 Samples preparation
Na2CO3and TiO2were provided as analytical reagents, andγ-Al2O3were supplied by the Research Institute of Nanjing Chemical Industry Group. Two sorbents were prepared in this study. Na2CO3/Al2O3was named Sorb 1 and TiO2doped Na2CO3/Al2O3was named Sorb 2. The sorbents were prepared by the impregnation method. Appropriate amounts of sorbent constituents were mixed and impregnated in deionized water, then dehydrated at 378 K, and calcined at 573 K. The sorbents were ground to appropriate particle sizes (0.18 to 0.315 mm) for fluidized-bed tests. Detailed information about the process can be found in previous work[10]. Loadings of Na2CO3were maintained around 25% (mass fraction) in the two sorbents. The designed loading of TiO2in Sorb 2 was 1% (mass fraction). The rest of the sorbents were Al2O3used as supports. X-ray fluorescence (XRF) was used to determine the actual loadings of Na2CO3and TiO2. The results are listed in Tab.1.

Tab.1 Sorbent components content in mass fraction %
1.2 Apparatus and procedure
A bubbling fluidized-bed reactor was operated for multiple-cycle tests as shown in Fig.1. The inner diameter of the reactor is 0.05 m and height is 1.0 m. Detailed information was reported in Ref.[3]. The sorbents mounted was about 250 g. The simulated flue gas containing 10%CO2and 12%H2O with a balance of 78% N2(volume fraction) was used for carbonate reaction. The flow rate was 750 L/h. The carbonation temperature was maintained at 333 K. After the completion of carbonate reaction, the flue gas was switched to 100%N2at a flow rate of 750 L/h as a purge gas. After the purge of CO2, the temperature of the reactor was raised to 473 K for regeneration. After regeneration, another carbonate reaction was performed by cooling the temperature of the reactor to 333 K. In this way, 14-cycle tests were carried out.
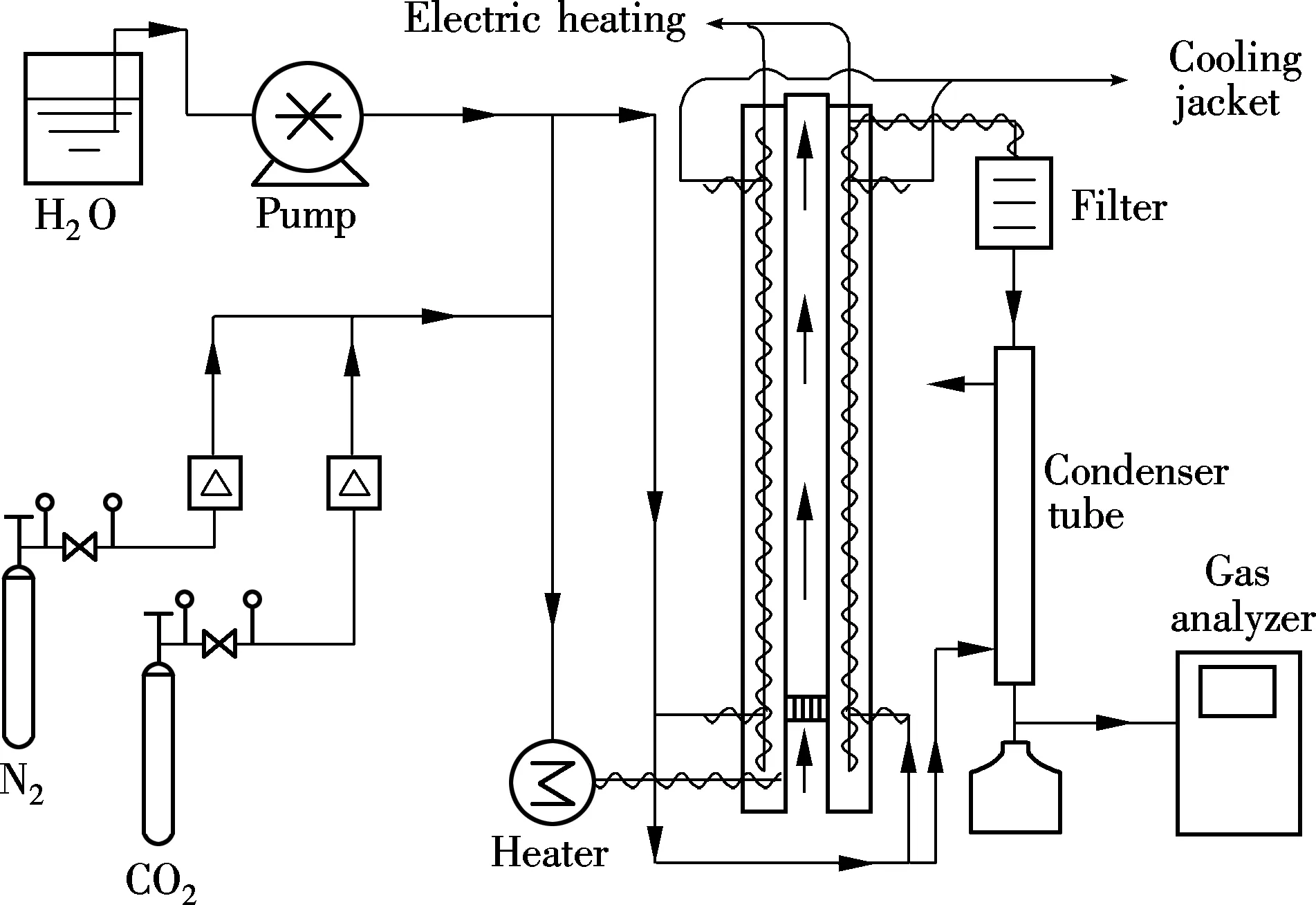
Fig.1 Schematic diagram of the experimental apparatus for fluidized-bed tests
An APHI 5 000 Versa Probe X-ray photoelectron spectroscopy (XPS) was introduced to examine the type and relative content of the surface elements. The surface spectra of TiO2were observed by a NICOLET NEXUS870 Fourier transform infrared spectrometer (FTIR). The surface areas and pore size distributions of sorbents were determined by a micropore physisorption analyzer with nitrogen adsorption-desorption.
2 Results and Discussion
2.1 Fluidized-bed tests
Fluidized-bed tests of Sorb 1 and Sorb 2 were processed successively. A typical carbonation-regeneration single cycle result of Sorb 1 is presented in Fig.2. The left part of the figure represents the carbonate reaction and the right part represents regeneration reaction.
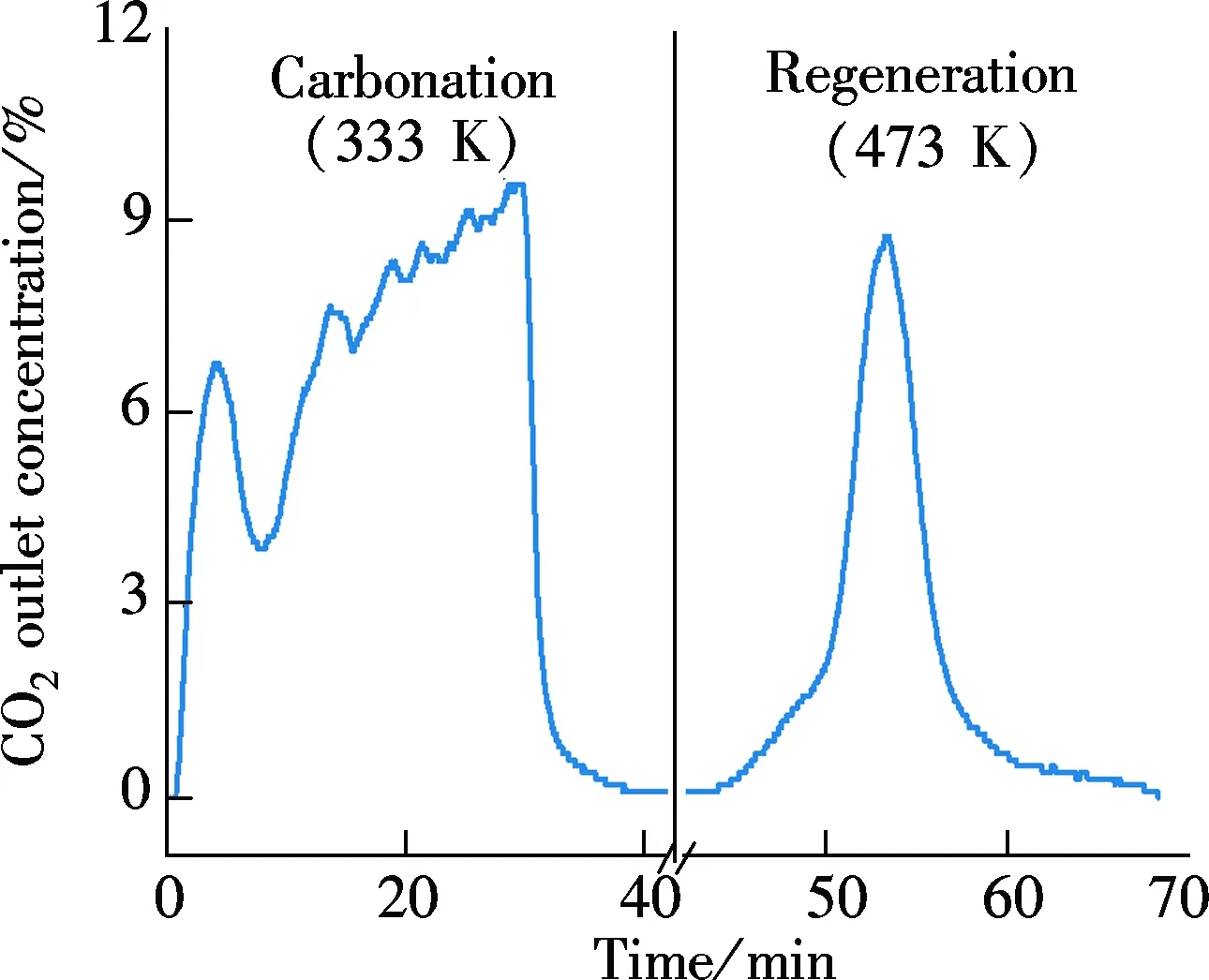
Fig.2 Fluidized-bed test result of Sorb 1 in carbonation-regeneration single cycle
XRD results of Sorb 1 and Sorb 2 before/after carbonate reaction, as shown in Fig.3, were provided in a previous work[10]. It has revealed that the main constituents of Sorb 1 before the carbonate reaction are Na2CO3and Al2O3. After the carbonate reaction, the NaHCO3phase is observed. The main constituents of Sorb 2 before the carbonate reaction are Na2CO3and TiO2, besides Al2O3. After the carbonate reaction, the NaHCO3phase and a new product known as Wegscheiderite (Na5H3(CO3)4) are observed. The relevant reactions are
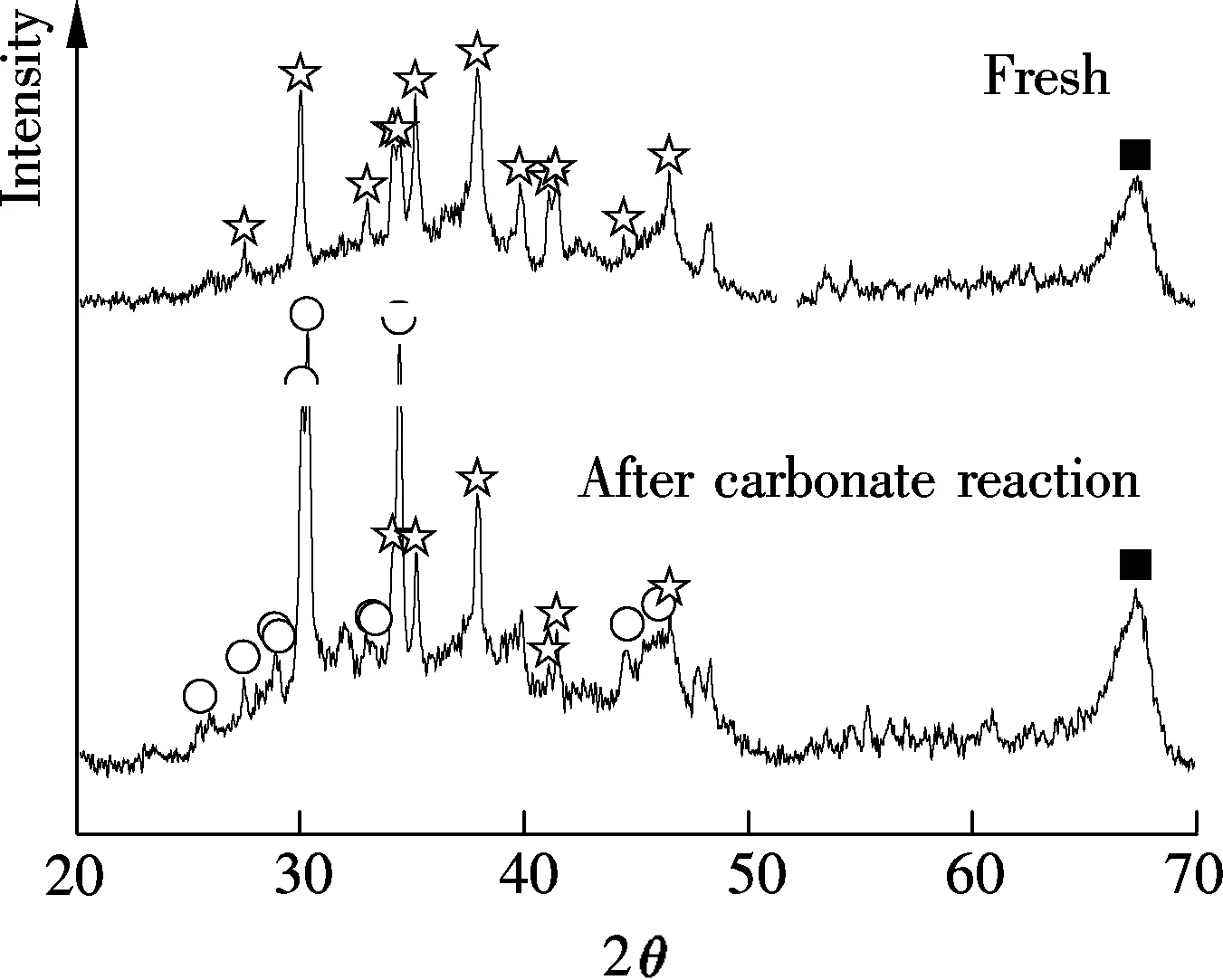
(a)
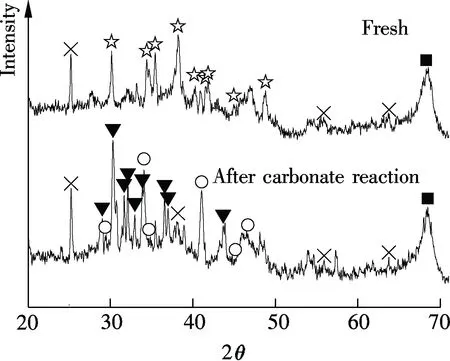
(b)☆—Na2CO3;■—Al2O3;○—NaHCO3;×—TiO2;▼—Na5H3(CO3)4
Fig.3 XRD patterns of Sorb 1 and Sorb 2 before/after carbonate reaction[10]. (a) Sorb 1; (b) Sorb 2
The two reactions are reversible. Furthermore, no Ti-containing compound is detected before/after carbonate reaction except for the TiO2phase which coincides with Lee’s study[1].
CO2absorbed/desorbed amounts were exhibited by a numerical integration of the CO2concentration curve. The amounts of CO2absorbed in the whole carbonate reactionnct, amounts of CO2absorbed in the first 10 min of carbonate reactionnciand amounts of CO2desorbed in regenerationnrof the two sorbents are listed in Tab.2, respectively. Good material balance closure was obtained, which indicates that the absorption of CO2is practically reversible on Al2O3support.

Tab.2 Amounts of CO2 absorbed and desorbed in each cycle of the two sorbents mol
The carbonation capacity of CO2Ac(milligram of CO2absorbed by per gram of sorbent) and regeneration percent conversionηrare used for expressing the carbonation and regeneration characteristics of the two sorbents. They can be calculated by
(1)
(2)
wherew(0)is the initial mass of sorbent;MCO2is the molecular mass of CO2.
(a)
(b)
(c)

(d)
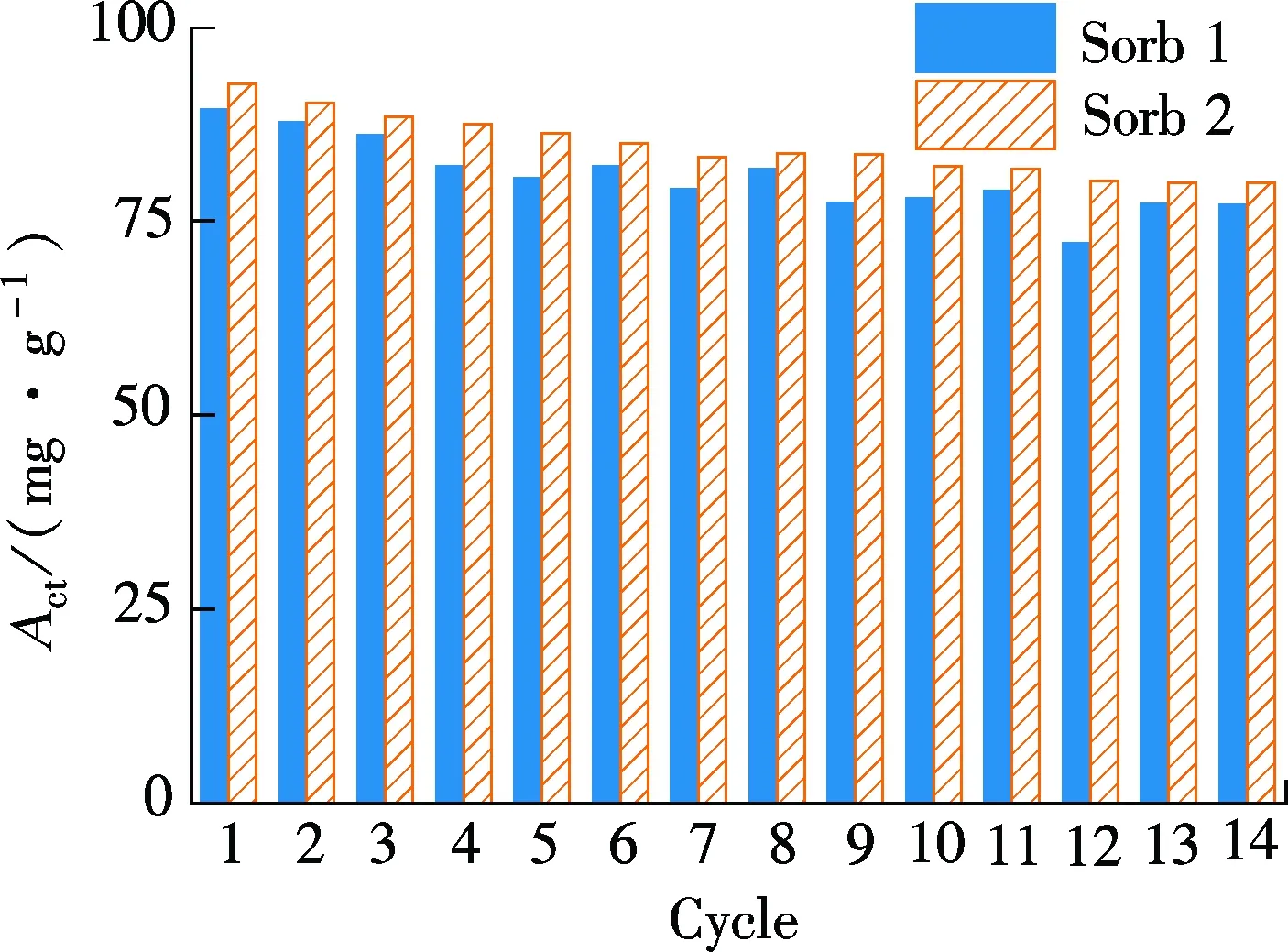
According to Eq.(1), the carbonation capacity of CO2in the whole carbonate reactionActand carbonation capacity of CO2in the first 10 min of carbonate reactionAciof the two sorbents are calculated and given in Figs.4(a) and (b). Lee et al.[2]reported their potassium-based sor-bents by impregnation K2CO3on Al2O3.Actof their sorbent with 30% loading of K2CO3is about 82 mg/g in the 1st cycle and decreases to about 50 mg/g after 5 cycles. Distinct from their claim, as can be noted from Fig.4(a), theActof Sorb 1 is between 77 and 90 mg/g in 14 cycles with a quite slow deactivation. As for Sorb 2,Actis slightly higher than that of Sorb 1 in the 14 cycles.
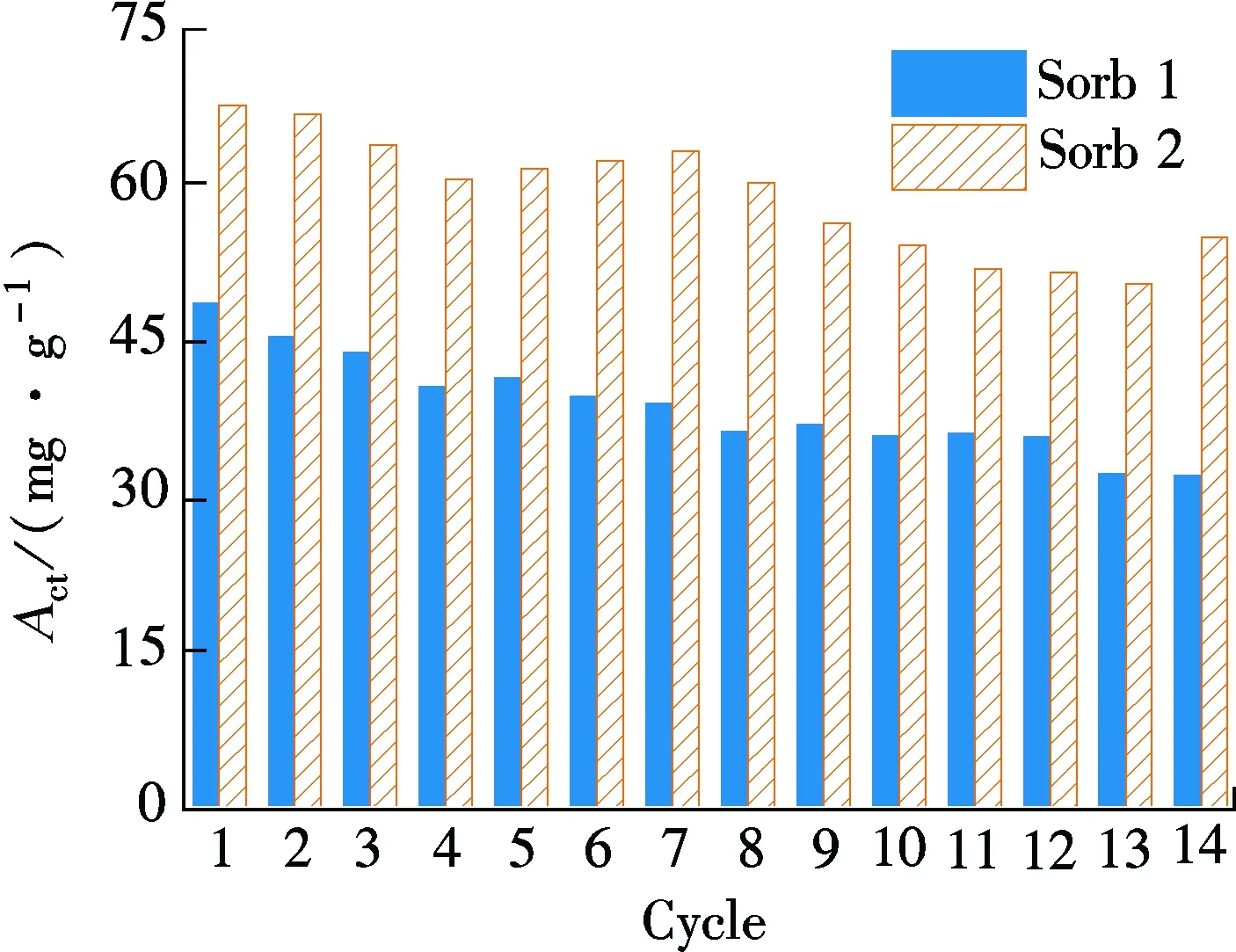
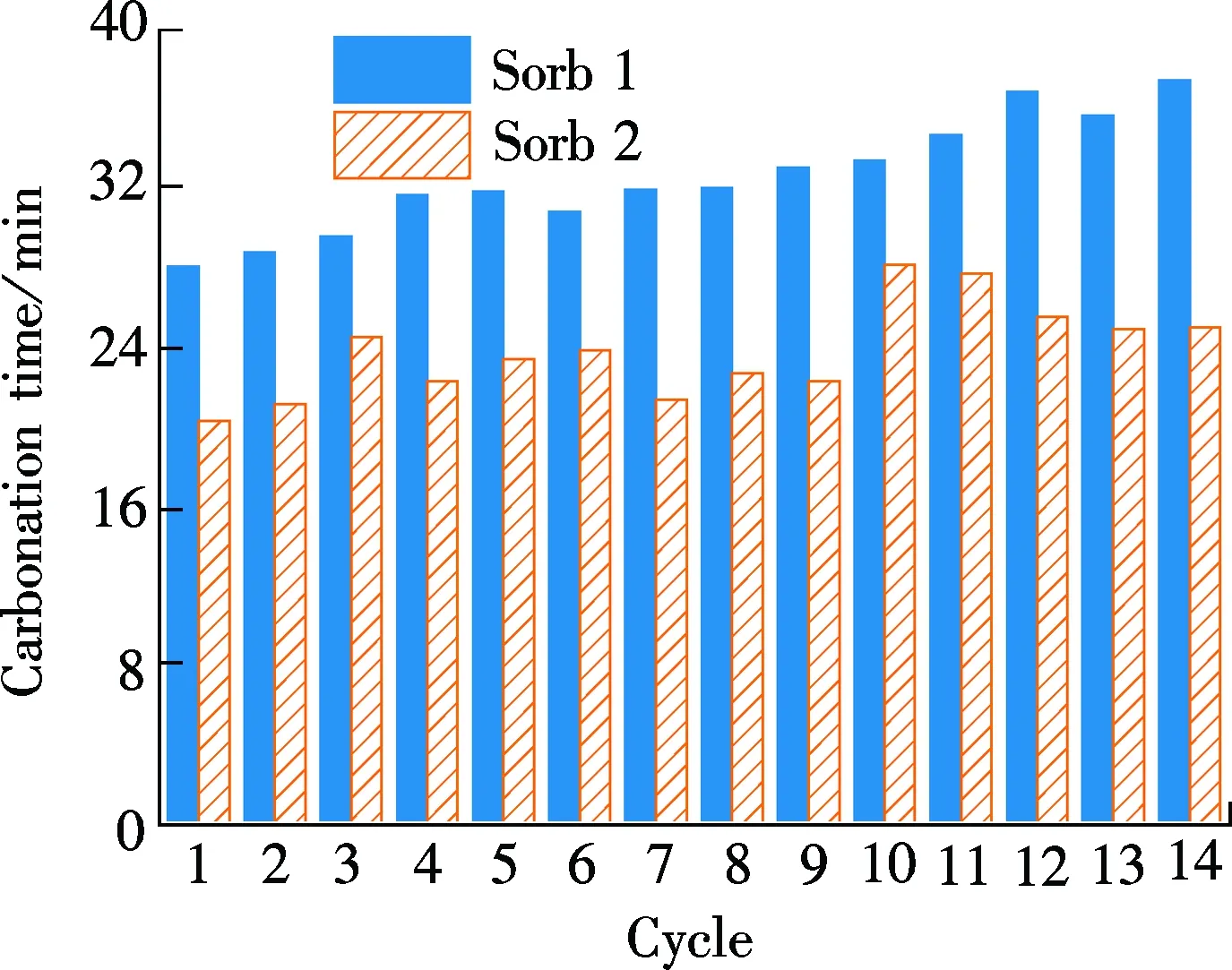
As can be noted from Fig.4(b), theAciof Sorb 1 is around 40 mg/g in each cycle, less than half of the correspondingActof Sorb 1. The carbonate reaction rate of Sorb 1 is low. TheAciof Sorb 2 in each cycle is about 0.5 times higher than that of Sorb 1 in each cycle between 55 and 68 mg/g, occupying about 70% of the correspondingActof Sorb 2. It suggests that the use of TiO2as a dopant can increase the carbonate reaction rate, particularly in the first 10 min. Previous research[11]reported that theAciof K2CO3/Al2O3is around 50 mg/g in a 4 cycles test. TheAciof our Sorb 2 is higher than that of the potassium-based sorbent. Moreover, the total carbonate reaction time is also shortened in each cycle, as shown in Fig.4(c), owing to the optimization of the carbonate reaction in the first 10 min.
Lee et al.[2]reported a novel potassium-based sorbent by impregnation K2CO3on zirconium oxide (ZrO2).Actof the sorbent with 30% loading of K2CO3is around 85 mg/g in 10 cycles, showing excellent carbonation/regeneration cycle performance. ItsActis somewhat lower than theActof our sorbents, which can be attributed to the different reactors and absorption atmospheres researched.
With respect to the regeneration section, one can observe from Fig.4(d) that theηrof the two sorbents in each cycle are all above 95%, which indicates that the decompositions of NaHCO3and Na5H3(CO3)4are almost completed within regeneration temperature range and the doping of TiO2hardly has an impact on the regeneration part.
2.2 Microstructure change of the two sorbents with cycle numbers
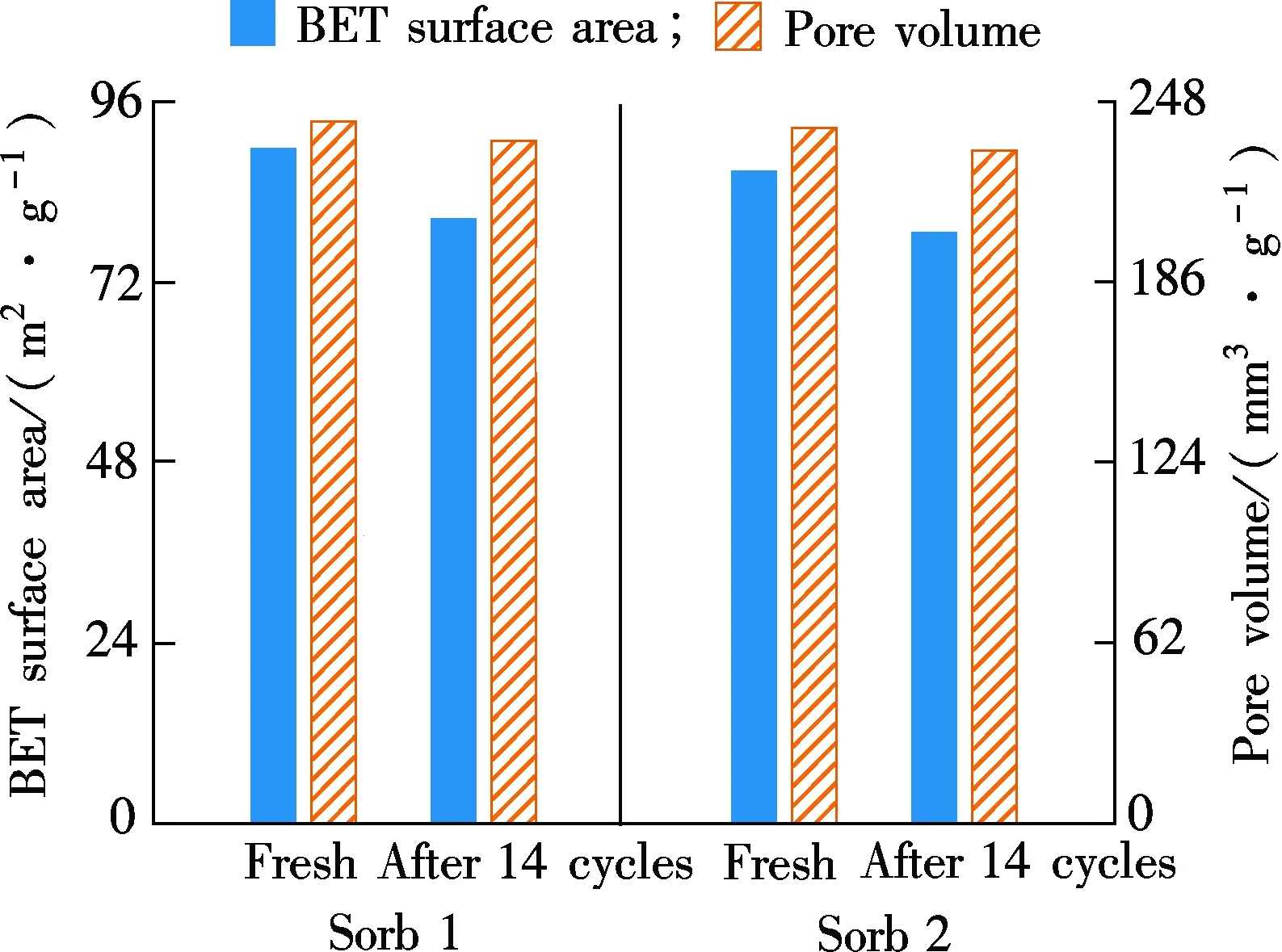
In the fluidized-bed reactor, due to the intense movements of sorbents and chemistry reactions, the microstructure of the two sorbents can be changed after the 14-cycle test. In Fig. 5, the change in the surface area, pore volume and pore size distribution of Sorb 1 and Sorb 2 before/after multi-cycle tests are presented.
As shown in Fig.5(a), after 14 carbonation/regeneration cycles, the surface area of the two sorbents decreases from 89.72 and 86.75 m2/g to 80.45 and 78.56 m2/g, respectively. The pore volume of the two sorbents decreases from 241.2 and 239.0 mm3/g to 234.5 and 231.1 mm3/g, respectively. The decrements are quite insignificant. In Fig.5(b), the pore size distributions are consistent during the 14 cycles and the change is small. The excellent microstructure performance is attributed to the usage of Al2O3as support which is consistent with Ref.[3].
(a)
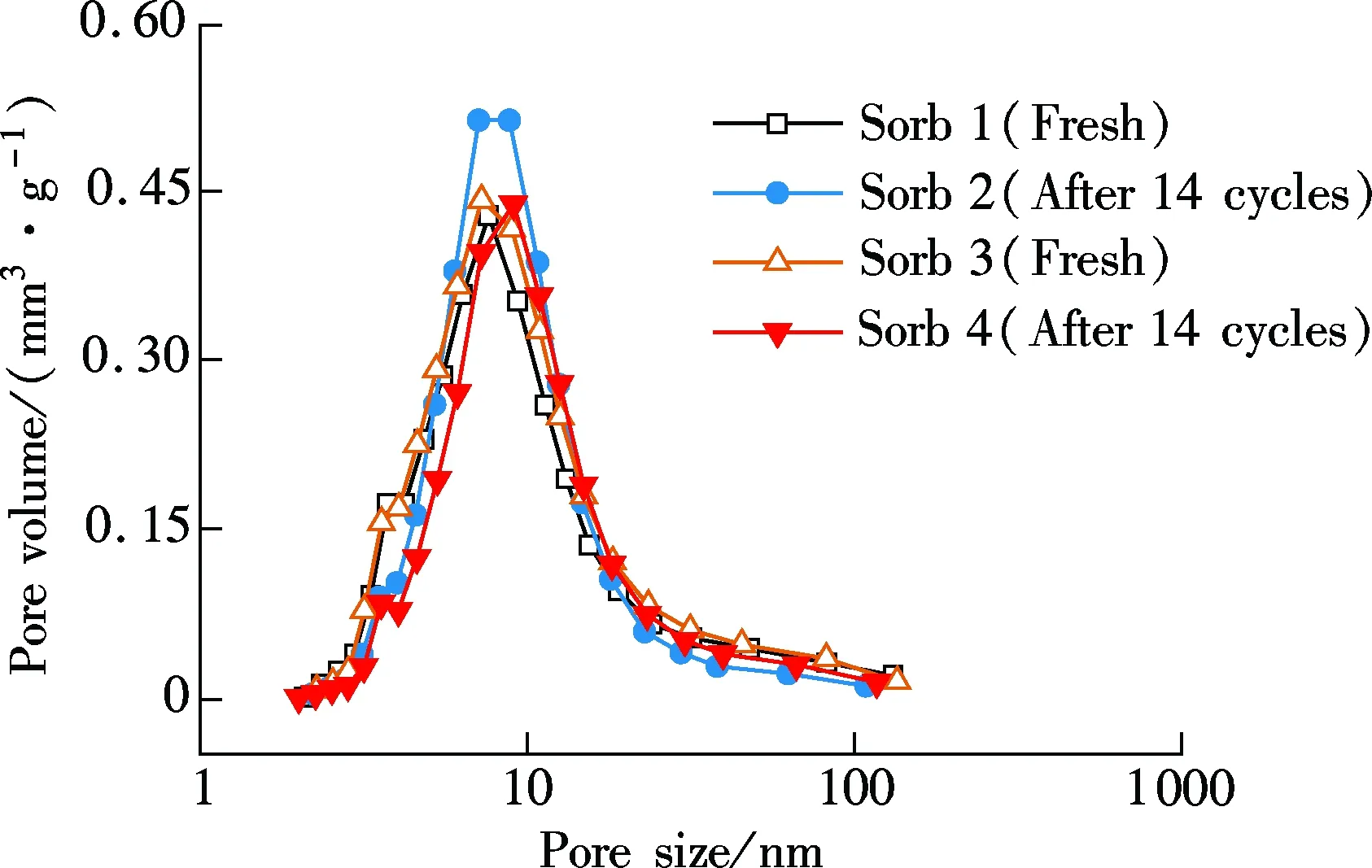
(b)
2.3 XPS test
In order to reveal the promotion mechanism of TiO2on CO2absorption, the XPS test of TiO2was introduced. As shown in Fig.6, the O1sphotoelectron peak of the TiO2surface displayed a broad shoulder which was resolved into two peaks after a numerical fit (dotted lines in Fig.6). The main peak at 529.4 eV can be associated with the titanate oxygen. The second oxygen peak at 531.0 eV can be assigned to OH groups that likely are incorporated on TiO2surfaces when they are exposed to H2O. These results are in good agreement with the XPS measurements of Nagarkar et al[12].
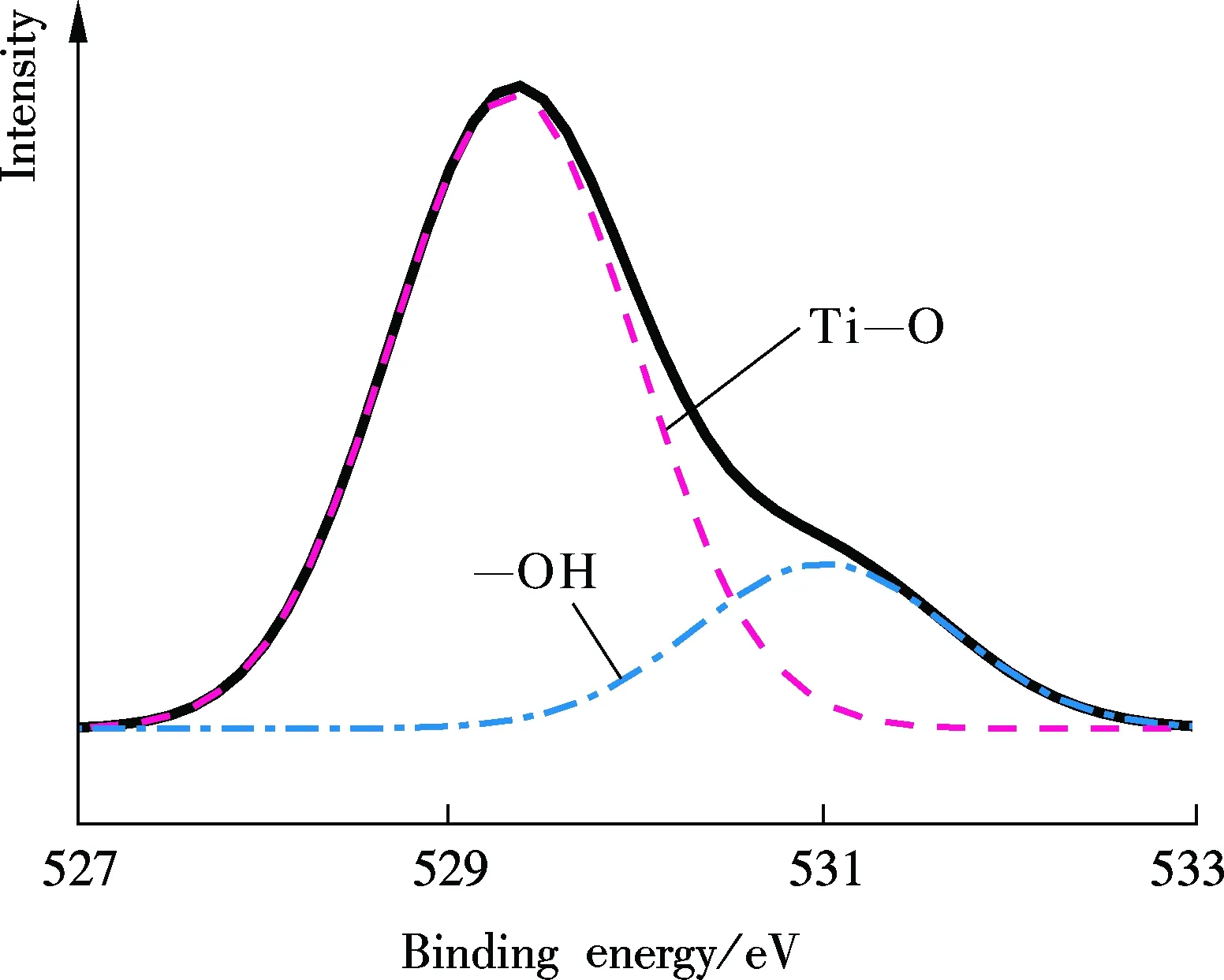
Fig.6 The XPS spectra of O1s of TiO2
2.4 FTIR tests
Fig.7 shows the changes in the FTIR spectra of TiO2progressive heated from an ambient temperature to 473 K in the oven. It can be noted that, a large, broad band centered around 3 300 cm-1is assigned to the vibration of isolated surface OH groups and absorbed water on TiO2. A shoulder around 1 620 cm-1assigned to the vibration of surface OH groups. As the temperature rises, the broad band around 3 300 cm-1reduces in intensity.
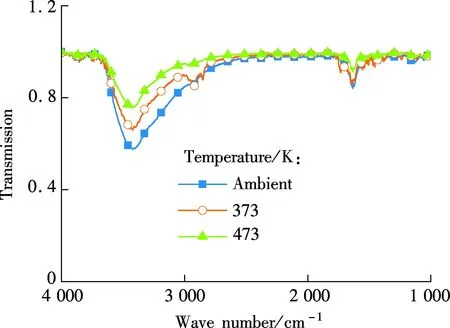
Fig.7 The FTIR spectra of TiO2 before and after heat treatment
3 Mechanism Analysis of the TiO2-doped Na2CO3/Al2O3 Sorbent
The surface property of TiO2is decisive for its catalytic application. The reaction activity and selectivity of NaCO3itself depend directly on the type and concentration of the different active sites.
Zhao et al.[4]verified that the H2O adsorption is regarded as the rate-controlling step of carbonation. As the H2O concentration increases, the diffusion and adsorption capacities of H2O in the sorbent are improved. Therefore, the total carbonation conversion increases when the H2O concentration increases.
Takeuchi et al.[13]pointed out that, as shown in Fig.8, the H2O molecules, which directly interact with the solid surface of TiO2such as surface cations or surface hydroxyls, form a monolayer as chemisorbed H2O molecules and then the hydrogen-bonded H2O molecules form multilayers as physisorbed H2O molecules. Finally, H2O molecules without active hydrogen bonds cover the polymeric chained H2O molecules to form the outermost shell of the H2O cluster. These polymeric chained H2O molecules are called “hydrogen-bonded water”, while on the other hand, the hydrogen-bond-free H2O molecules in the outermost shell are called “free water”.
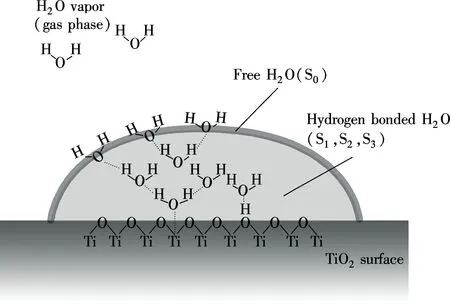
Fig.8 Structural model of H2O cluster absorbed on solid surface of TiO2[13]
By doping TiO2on Na2CO3/Al2O3, the multilayers of H2O can be adsorbed on the surface of sorbent. The mul-tilayers of H2O contain plenty of ions forming new active sites for CO2absorption. The reaction rate is improved, and hence the CO2uptake in the first 10 min of carbonation is increased. After that, the reaction rate is controlled by the diffusion of CO2in the sorbent. So the rate is close to that of Sorb 1.
4 Conclusion
CO2capture/desorption behaviors of Na2CO3/Al2O3doped with TiO2were researched in a bubbling fluidized-bed reactor. The effect of TiO2on carbonation is significant. Na2CO3/Al2O3with around 1% TiO2loading exhibits a high CO2capture rate, particularly in the first 10 min. It is also indicated that TiO2is stable within the temperature range of the process. The sorbent structures of Sorb 1 and Sorb 2, including the surface area, the pore volume, and the pore size distribution, keep stable in 14 cycles. Furthermore, the impacts of flue gas constituents on TiO2doped Na2CO3/Al2O3sorbents should be investigated in the future. We believe that this interesting research is promising for the use of dry sodium-based sorbents for CO2removal.
[1]Lee S C, Choi B Y, Lee T J, et al. CO2absorption and regeneration of alkali metal-based solid sorbents [J].CatalysisToday, 2006, 111(3/4): 385-390.
[2]Lee S C, Chae H J, Lee S J, et al. Novel regenerable potassium-based dry sorbents for CO2capture at low temperatures [J].JournalofMolecularCatalysisB:Enzymatic, 2009, 56(2/3): 179-184.
[3]Zhao C, Chen X, Zhao C, et al. K2CO3/Al2O3for capturing CO2in flue gas from power plants. Part 3: CO2capture behaviors of K2CO3/Al2O3in a bubbling fluidized-bed reactor [J].Energy&Fuels, 2012, 26(5): 3062-3068.
[4]Zhao C, Chen X, Zhao C. K2CO3/Al2O3for capturing CO2in flue gas from power plants. Part 1: Carbonation behaviors of K2CO3/Al2O3[J].Energy&Fuels, 2012, 26(2): 1401-1405.
[5]Lee S C, Kim J C. Dry potassium-based sorbents for CO2capture [J].CatalysisSurveysfromAsia, 2007, 11(4): 171-185.
[6]Dong W, Chen X, Wu Y, et al. Carbonation characteristics of dry sodium-based sorbents for CO2capture [J].Energy&Fuels, 2012, 26(9): 6040-6046.
[7]Chen H, Zhao C, Yu W. Calcium-based sorbent doped with attapulgite for CO2capture [J].AppliedEnergy, 2013, 112: 67-74.
[8]Sun R, Li Y, Liu H, et al. CO2capture performance of calcium-based sorbent doped with manganese salts during calcium looping cycle [J].AppliedEnergy, 2012, 89(1): 368-373.
[9]Al-Jeboori M J, Fennell P S, Nguyen M, et al. Effects of different dopants and doping procedures on the reactivity of CaO-based sorbents for CO2capture [J].Energy&Fuels, 2012, 26(11): 6584-6594.
[10]Dong W, Chen X, Wu Y. Effect of TiO2dopant on CO2capture performance of Na2CO3/Al2O3[J].CIESCJournal, 2014, 65(9): 3618-3625. (in Chinese)
[11]Dong W, Chen X, Wu Y. TiO2-doped K2CO3/Al2O3sorbents for CO2capture [J].Energy&Fuels, 2014, 28(5): 3310-3316.
[12]Nagarkar P V, Searson P C, Gealy F D. Effect of surface treatment on SrTiO3: an X-ray photoelectron spectroscopic study [J].JournalofAppliedPhysics, 1991, 69(1): 459-462.
[13]Takeuchi M, Martra G, Coluccia S, et al. Investigations of the structure of H2O clusters adsorbed on TiO2surfaces by near-infrared absorption spectroscopy [J].TheJournalofPhysicalChemistryB, 2005, 109(15): 7387-7391.
TiO2掺杂Na2CO3/Al2O3吸收剂在流化床中的CO2捕集特性
董 伟 陈晓平 余 帆
(东南大学能源热转换及其过程测控教育部重点实验室,南京 210096)
由于Na2CO3/Al2O3吸收剂的活性成分Na2CO3与CO2反应活性较低,选用TiO2作为掺杂剂,采用浸渍法将其添加到Na2CO3/Al2O3吸收剂中进行改性,研制一种新型具有高反应活性的钠基固体吸收剂.利用小型流化床反应器进行了14次循环脱碳/再生试验,并对吸收剂进行了XRD和氮吸附表征.结果表明:掺杂TiO2后,吸收剂与CO2的反应速率加快,特别是在碳酸化反应的前10 min内;反应前后除TiO2外无其他含Ti化合物生成;碳酸化反应产物为NaHCO3和Na5H3(CO3)4;14次循环反应后吸收剂仍保持稳定的微观结构.采用XPS和FTIR分析了TiO2对Na2CO3/Al2O3吸收剂脱碳特性的改性机理.
CO2捕集; Na2CO3/Al2O3; TiO2; 流化床试验
TK09
Biographies:Dong Wei (1986—), male, graduate; Chen Xiaoping (corresponding author), male, doctor, professor, xpchen@seu.edu.cn.Foundation items:The National Natural Science Foundation of China (No.51476030), the Specialized Research Fund for the Doctoral Program of Higher Education (No.20130092110006).
:Dong Wei, Chen Xiaoping, Yu Fan. CO2capture using dry TiO2-doped Na2CO3/Al2O3sorbents in a fluidized-bed reactor[J].Journal of Southeast University (English Edition),2015,31(2):220-225.
10.3969/j.issn.1003-7985.2015.02.011
10.3969/j.issn.1003-7985.2015.02.011
Received 2015-01-02.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Construction of crash prediction model of freeway basic segment based on interactive influence of explanatory variables
- Experimental studies on gas-phase mercury oxidation removal and denitration of coal combustion with NH4Br addition
- Comparative study on SO2 release and removal under air and oxy-fuel combustion in a fluidized bed combustor
- Effect of sulfation during carbonation on CO2 capture in calcium looping cycle
- Synthesis of highly reactive sorbent from industrial wastes and its CO2 capture capacity
- CO2 capture by carbonated carbide slag seriflux after drying in calcium looping cycles
