CO2 capture by carbonated carbide slag seriflux after drying in calcium looping cycles
2015-05-08HeZiruiLiYingjieLiuChangtian
He Zirui Li Yingjie Liu Changtian
(School of Energy and Power Engineering, Shandong University, Jinan 250061, China)
CO2capture by carbonated carbide slag seriflux after drying in calcium looping cycles
He Zirui Li Yingjie Liu Changtian
(School of Energy and Power Engineering, Shandong University, Jinan 250061, China)
A new carbide slag (CS) seriflux utilization was proposed. The flue gas from a coal-fired plant was first bubbled into CS seriflux for CO2capture. The obtained carbonated carbide slag seriflux (CCSS) was dried and utilized as a CO2sorbent in the calcium looping cycles. The CO2capture behavior of the dried CCSS and the raw CS was investigated in a dual fixed-bed reactor and a thermo-gravimetric analyzer. The effects of carbonation time, calcination temperature and carbonation temperature on CO2capture performance of CCSS in the multiple carbonation/calcination cycles were studied. The results show that the CO2capture capacity of CCSS was higher than that of CS. Calcined at 950 ℃, CCSS shows better carbonation reactivity than CS, which benefits CO2capture under severe calcination conditions. In the range of 700 to 725 ℃ for the carbonation, CCSS shows the optimal CO2capture performance. The calcined CCSS shows better porous microstructure than the calcined CS. The calcined CCSS exhibits a larger surface area and pore volume in the cycles, which favors a higher CO2capture capacity in the multiple cycles.
calcium looping; carbide slag; CO2capture
It is widely accepted that the increasing CO2concentration in the atmosphere is a significant cause of climate change. In order to mitigate the risk of global warming, a number of technologies have been studied such as solvent scrubbing, oxy-fuel combustion, chemical looping and calcium looping[1-3]. Calcium looping is based on the reversible reaction between CaO and CO2to remove CO2from the flue gas of power plants. Ca-based sorbent reacts with CO2in a circulating fluidized bed carbonator operated at 600 to 700 ℃. The reacted sorbent is then regenerated in another fluidized bed (named calciner), operated at a temperature above 900 ℃[4]. The heat for the regeneration is supplied by oxy-fuel combustion in order to avoid the dilution of the CO2stream when fuel is burned under air atmosphere[5]. Calcium looping is regarded as a promising technology for CO2capture owing to its numerous advantages: the use of cheap and non-toxic sorbents; the relatively small energy penalty imposed on power plants; promising deployment in conjunction with other technologies such as large-scale circulating fluidized beds and cement manufacture[6-7]. Hence, the calcium looping technology has attracted a great deal of attention[8-11].
Carbide slag (CS) is a calcium-rich industrial waste, which is the by-product of the hydrolysis reaction of calcium carbide for acetylene gas production. In acetylene gas factories, CS is discharged as seriflux which contains about 92% water. Generally, CS seriflux undergoes a solid-liquid separation process. Then the sediment, which is mostly calcium hydroxide, is utilized in construction and chemical production or buried[12]. The previous works of our group have proved that the CS can be utilized as a CO2sorbent in the calcium looping cycles and achieve a higher CO2capture capacity than the limestone[13]. However, the CO2capture capacity of the CS decreases with the number of calcination/carbonation cycles like the natural limestone. Many methods have been proposed to enhance the cyclic CO2capture capacity of the calcium-based sorbents. Kierzkowska et al.[14]synthesized a calcium-based sorbent using Na2CO3, (NH4)2CO3and aqueous solution of NH3as a precipitation agent added into the Ca(NO3)2(or Ca(CH3COO)2) and Al(NO3)3solution. They found that the CO2capture capacity of the synthetic sorbent was 1.8 times as high as that of the limestone after 30 cycles. Florin et al.[15]prepared a synthetic Ca-based sorbent by bubbling CO2through an aqueous solution containing Ca(OH)2and Al(NO3)3, and its CO2capture capacity achieved 2.5 times as high as that of limestone. Gupta et al.[16]found that a precipitated carbonate calcium prepared by bubbling CO2through a slurry of Ca(OH)2exhibited a relatively high CO2capture capacity in the calcium looping cycles.
In this paper, we propose a precipitation method on the carbide slag seriflux by directly bubbling CO2into the seriflux. Then the CCSS was obtained. The CO2capture performance of CCSS in the calcination/carbonation cycles was investigated in a thermo-gravimetric analyzer (TGA) and a dual fixed-bed reactor (DFR).
1 Experimental
1.1 Sample preparation
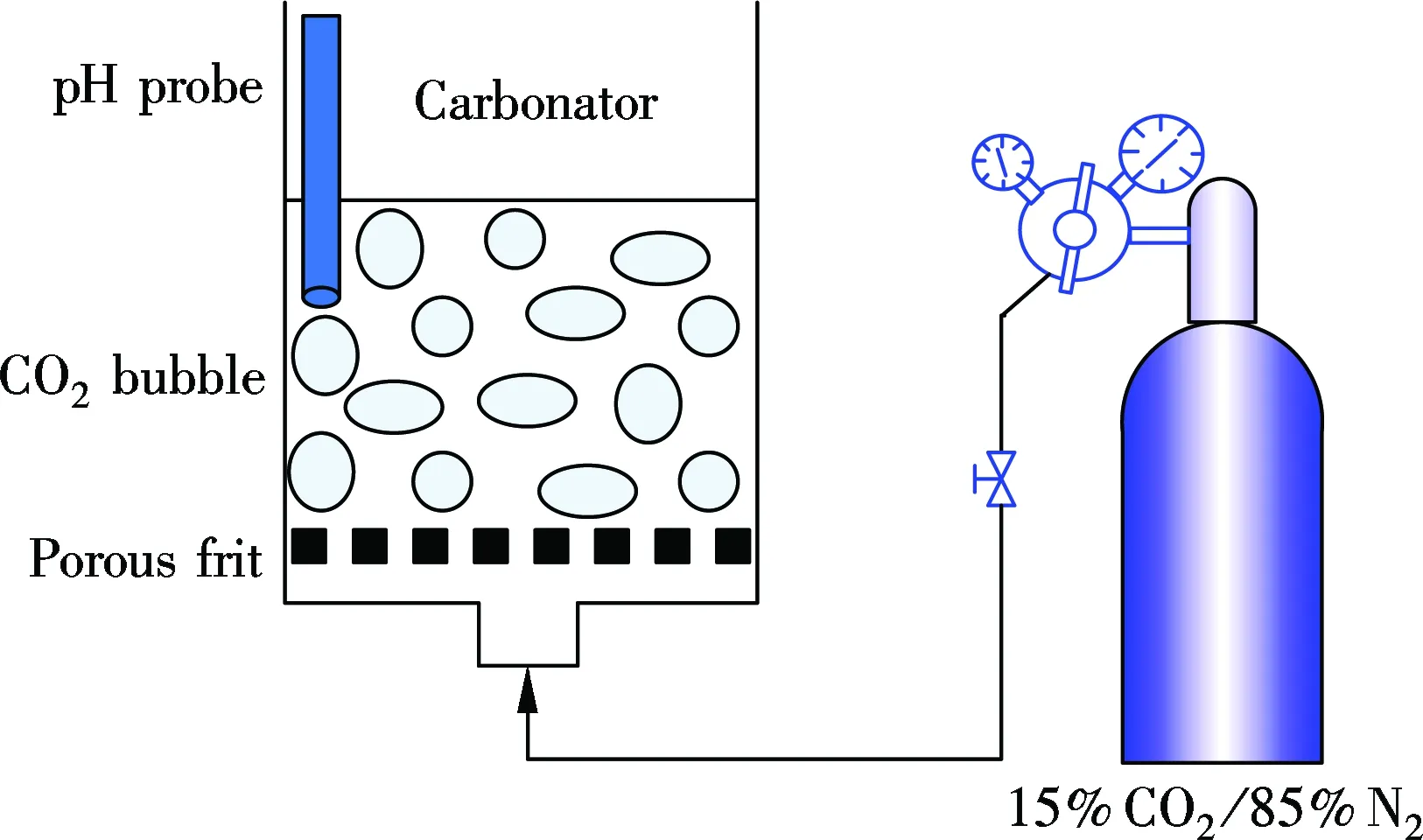
The CS was sampled from a chlor-alkali plant in Shandong Province, China. The chemical components of the CS were analyzed by X-ray fluorescence (XRF) as shown in Tab.1.A schematic diagram for the precipitation experiment of the CS seriflux is shown in Fig.1(a). The wet carbonator contains a porous frit at the bottom providing a good distribution of gas mixture through the seriflux. A
pH probe was used to monitor the variation of pH of the CS seriflux during the carbonation process. In order to model the real CS seriflux, 10 g CS was mixed with 90 g distilled water in a precipitation reactor, and the particles of the CS with the size below 125 μm were chosen. Then, a gas mixture containing 15% CO2and 85% N2was bubbled into the reactor for the wet CO2capture at room temperature until the pH value decreased from 12.6 to 6, which indicated that all the Ca(OH)2was converted into CaCO3. The precipitated product was filtrated and dried in a drying oven at 105 ℃, and named CCSS.

Tab.1 Chemical components of CS %
(a)

(b)
1.2 CO2capture in calcination/carbonation cycles
The cyclic calcination/carbonation experiment of the sample for CO2capture was performed in a dual fixed-bed reactor (DFR) and a thermo-gravimetric analyzer (TGA) and operated under atmospheric pressure. The DFR contains a carbonator and a calciner as shown in Fig.1(b). The sample boat loading the sorbent (about 500 mg) can be shifted between two reactors. The calciner was operated at 850 to 950 ℃ in pure N2, and the carbonator was operated at 650 to 750 ℃ in a 15%CO2/85%N2gas mixture. Based on the preliminary experiments, the carbonation time was specified to be 20 min, and the calcination time was 10 min. The calcined sample and its re-carbonated counterpart after each cycle were picked out, stored and cooled for 2 min in a dry container under N2. Then the sample was weighted by an electronic balance with a resolution of 0.1 mg. The cyclic carbonation conversion of the sorbent is calculated as
(1)
wheretis the carbonation time, s;XNstands for the carbonation conversion of sorbent attduring theN-th cycle;bis the content of CaO in the initial sorbent, %;mcarb,N(t) represents the mass of carbonated sorbent attduring theN-th cycle, mg;mcal,Nis the mass of sorbent after complete calcination during theN-th cycle, mg;WCaOandWCO2are the molar mass of CaO and CO2, respectively, g/mol.
The carbonation kinetics of the CCSS and CS were investigated in a thermo-gravimetric analyzer (TGA). In order to study the cyclic carbonation behavior of the CCSS and CS, the original sorbents and carbonated sorbents (5±0.1) mg after 9 calcination/carbonation cycles in the DFR were chosen as the samples in the TGA. Therefore, the carbonation behaviors of sorbents were obtained as a function of reaction time during the 1st and the 10th cycles. The furnace temperature of the TGA was increased to a calcination temperature of 850 ℃ at a heating rate of 30 ℃/min, and the sample was held for 15 min under pure N2. Then, the temperature was dropped to 700 ℃ for carbonation under pure N2. At the same time, the reaction atmosphere was switched to 15% CO2/85% N2gas mixture, and the calcined sample was carbonated for 30 min. The carbonation conversions of the sample after theN-th cycle in TGA were calculated by Eq.(1).
1.3 Analysis
The crystalline structure of the sorbents was characterized by a X-ray diffraction (XRD). The calcined CCSS and the CS in the 1st cycle and the 10th cycle were analyzed by the nitrogen adsorption analyzer. The surface area was calculated by the BET method. The pore volume and pore area were computed by the BJH model.
2 Results and Discussion
2.1 Wet carbonation and crystallography of CS and CCSS
Fig.2(a) shows the XRD spectrum of the CS. It is observed from Fig.2(a) that the main composition of the CS is Ca(OH)2, and a little amount of CaCO3is also detected. The mixture of 10 g CS and 90 g water is a supersaturated solution of Ca(OH)2with the pH value of above 12. When the gas mixture of 15% CO2/85% N2is bubbled into the slurry, CO2dissolves in the water and generates CO32-.Then Ca2+is precipitated according to the following reaction[16]:
(2)

(a)

(b)
The reaction proceeds until the pH value decreases to 6, which indicates that all Ca(OH)2in the CS has converted into CaCO3. The XRD spectrum of the CCSS is shown in Fig.2(b). The main composition of the CCSS is CaCO3. It should be noted that in this experiment, the precipitated product was not removed in time. As a consequence, CaCO3was aged in the high alkaline condition of the precipitating solution.
2.2 Effect of reaction time on CO2capture performance of CCSS
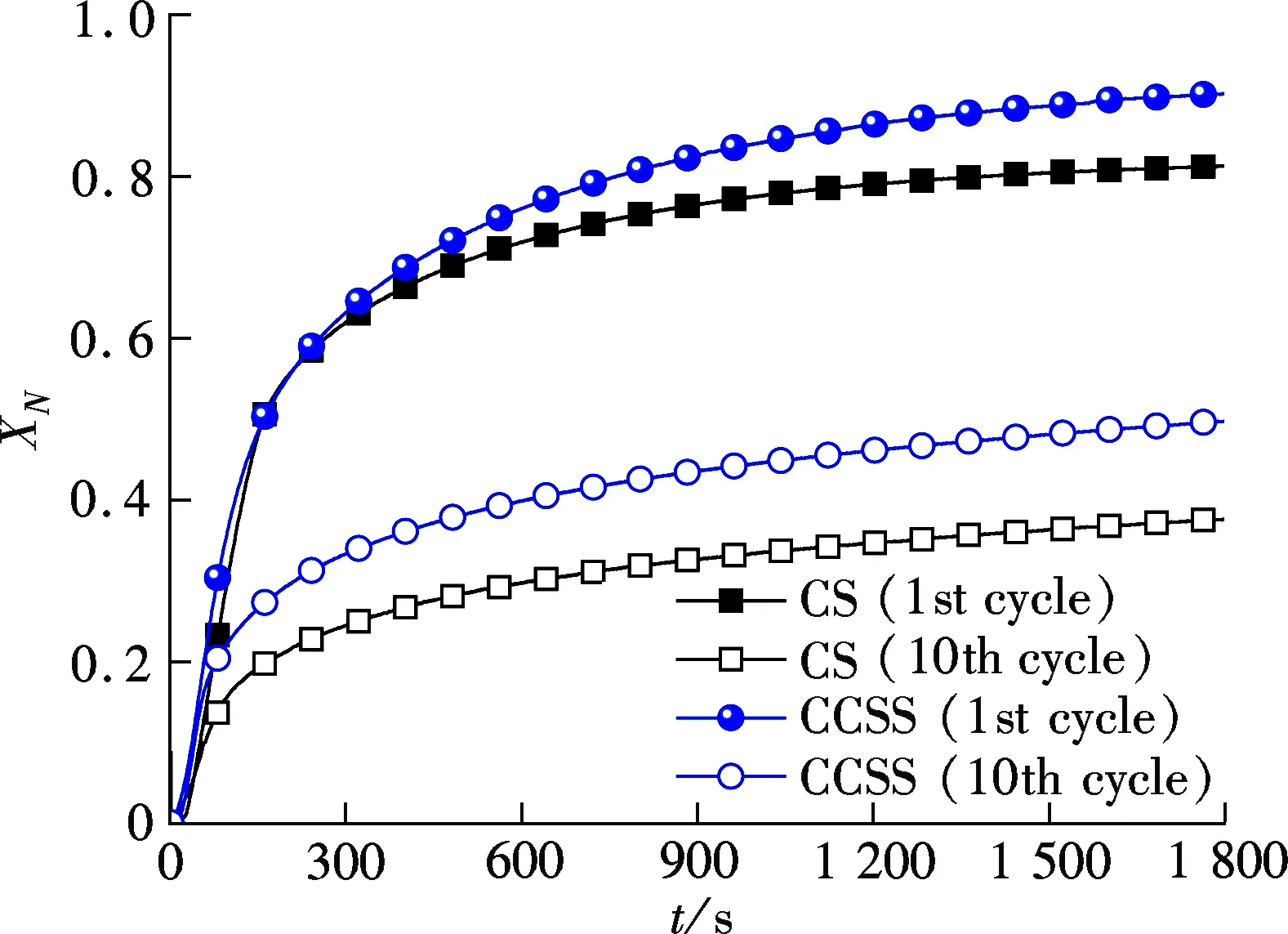
The carbonation conversions of the CCSS and CS with the carbonation time in the 1st and the 10th cycles in the TGA are depicted in Fig.3(a). It is found that the cyclic carbonation conversion of the CCSS is higher than that of the CS. In the 1st cycle, the carbonation conversions of CCSS at 1 and 30 min achieve 1.3 and 1.1 times as high as those of the CS under the same reaction conditions, respectively. In the 10th cycle, the conversions of the CCSS increase to 1.7 times in 1 min and 1.3 times in 30 min as high as those of the CS, respectively. Fig.3(b) presents the carbonation rates of the CCSS and CS in the 1st and the 10th cycles in the TGA. It can be seen that the carbonation rate of the CCSS reaches its peak value at 50 s, while the peak value of the CS appears after 100 s. The CCSS exhibits a higher carbonation rate compared with the CS in the initial 100 s in the 1st and the 10th cycles. Although the carbonation conversions and rates of the CCSS and CS decrease with the cycle number, the effect of the number of cycles on the CCSS is less than that on the CS.
(a)

(b)
2.3 Effect of calcination temperature on CO2capture performance of CCSS
Fig.4 shows the effect of calcination temperature on the cyclic carbonation conversions of the CCSS and CS. With the calcination temperature rising, the carbonation conversions of the CCSS and CS decay significantly with the number of cycles. The CCSS achieves a higher carbonation conversion than the CS at the same calcination temperature. In addition, the CCSS shows almost the same cyclic carbonation conversion at a calcination temperature of 950 ℃ with the raw CS calcined at 850 ℃. It indicates that CCSS shows better carbonation performance at a high calcination temperature, which favors CO2capture by the CCSS under the severe calcination conditions.
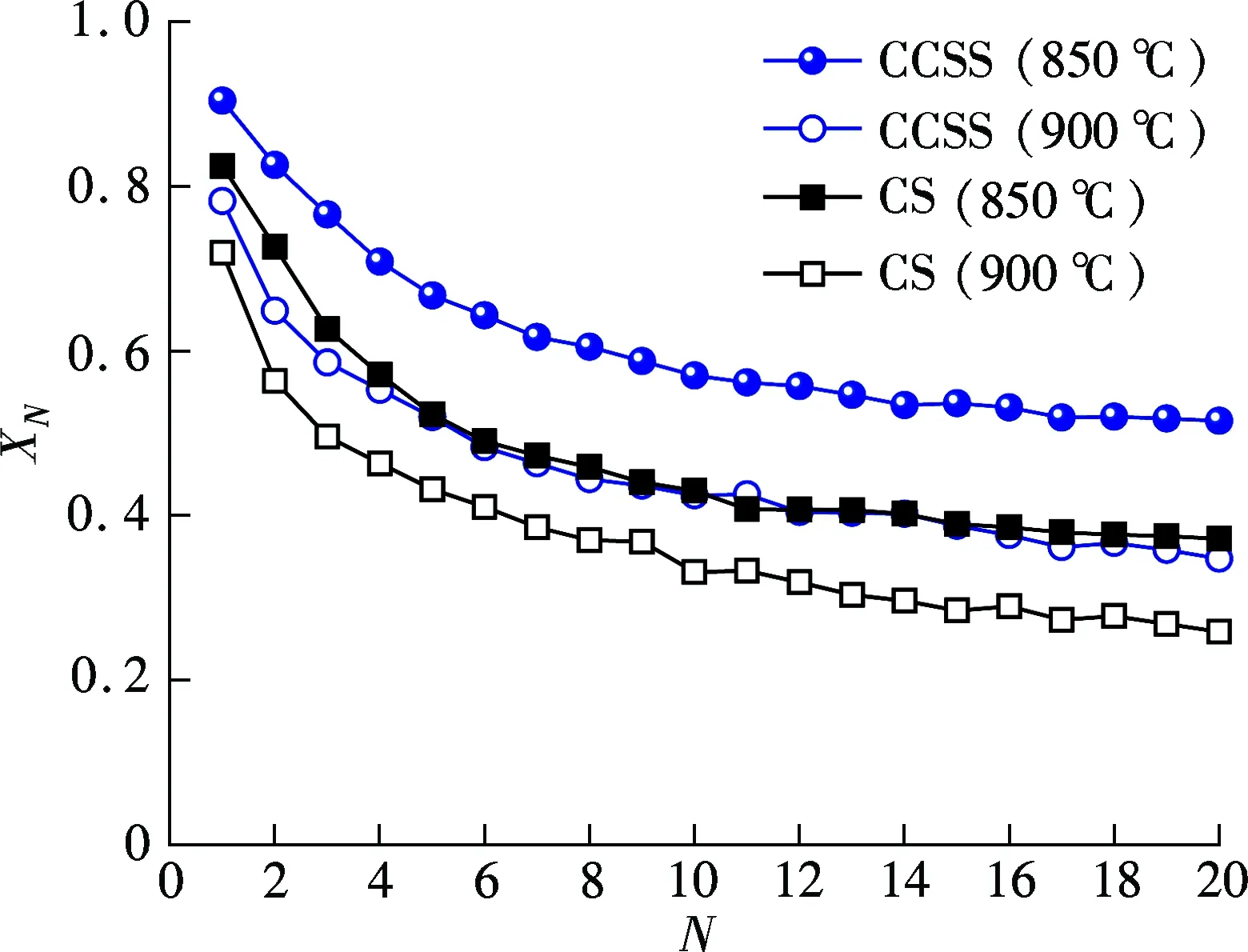
Fig.4 Effect of calcination temperature on carbonation conversion of CCSS and CS in DFR (10 min calcination in N2, 20 min carbonation at 700 ℃ in 15% CO2/85% N2)
2.4 Effect of carbonation temperature on CO2capture performance of CCSS
Fig.5 illustrates the cyclic carbonation conversions of the CCSS in the carbonation temperature range of 650 to 750 ℃. The carbonation conversion of CCSS increases with the carbonation temperature increasing from 650 to 700 ℃. As the temperature increases from 700 to 725 ℃, the carbonation conversion of CCSS hardly increases. However, the carbonation conversion decreases as the carbonation temperature increases further. Therefore, the CCSS achieves high CO2capture capacity in the carbonation temperature range of 700 to 725 ℃.

Fig.5 Effect of carbonation temperature on cyclic carbonation conversion of CCSS in DFR (10 min calcination at 850 ℃ in N2, 20 min carbonation in 15% CO2/85% N2)
2.5 Microstructure analysis
The surface areas and the pore volumes of the calcined CCSS and the calcined CS in the 1st and the 10th cycles are presented in Tab.2. After the wet carbonation process, the surface area and the pore volume of the calcined CCSS is higher than those of the calcined CS. The surface area and the pore volume of the calcined CCSS after 1 cycle are 9.92 m2/g and 0.045 cm3/g which are 1.3 and 1.2 times greater than those of the calcined CS for the same number of cycles, respectively. A larger surface area and pore volume favor a higher CO2capture capacity of the sorbent. Fig.6 shows the pore volume distributions of the calcined CCSS and CS in the 1st and the 10th cycles. Compared with the calcined CS, the calcined CCSS shows a higher volume and area of pores in the pore size range of 2 to 10 nm and 30 to 100 nm. The pores in the pore size ranges of 2 to 10 nm and 30 to 100 nm are generated during the calcium ion precipitation in the CS seriflux. In addition, the calcined CCSS maintains more pores in 30 to 100 nm after 10 cycles, compared with the calcined CS. The pores in the range of 30 to 100 nm are important for CO2adsorption by the calcium-based sorbent[2]. Thus, the CCSS exhibits a higher CO2capture capacity than the CS.
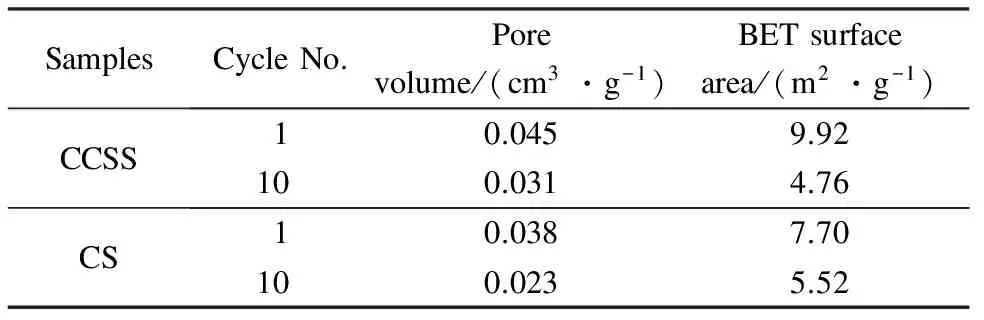
Tab.2 BET surface areas and pore volumes of calcined sorbents in the 1st and 10th cycles

Fig.6 Pore volume distributions of calcined CCSS and CS in the 1st and 10th cycles (10 min calcination at 850 ℃ in N2, 20 min carbonation at 700 ℃ in 15% CO2/85% N2)
3 Conclusion
A wet carbonation process was employed on the CS seriflux, an industrial waste which is discharged as slurry and contains 90% water, by bubbling the gas mixture into it. Then it was dried and utilized as a CO2sorbent in the calcium looping. The carbonation behavior of the CCSS in the calcination/carbonation cycles was investigated. Compared with the CS, the CCSS shows a higher carbonation conversion and rate. The favorable carbonation temperature range of the CCSS is 700 to 725 ℃. The CCSS possesses better sintering resistance performance than the CS at the high calcination temperature of 950 ℃. The wet carbonation process contributes to the better pore structure of the CCSS in the pore size range of 2 to 10 nm and 30 to 100 nm. The calcined CCSS maintains more pores in 30 to 100 nm than the calcined CS, which is the reason why CCSS exhibits a better CO2capture reactivity.
[1]Boot-Handford M E, Abanades J C, Anthony E J, et al. Carbon capture and storage update [J].Energy&EnvironmentalScience, 2014, 7(1): 130-189.
[2]Li Y J, Sun R Y, Liu C T, et al. CO2capture by carbide slag from chlor-alkali plant in calcination/carbonation cycles [J].InternationalJournalofGreenhouseGasControl, 2012, 9: 117-123.
[3]Dean C C, Blamey J, Florin N H, et al. The calcium looping cycle for CO2capture from power generation, cement manufacture and hydrogen production [J].ChemicalEngineeringResearchandDesign, 2011, 89(6): 836-855.
[4]Shimizu T, Hirama T, Hosoda H, et al. A twin fluid-bed reactor for removal of CO2from combustion processes [J].ChemicalEngineeringResearchandDesign, 1999, 77(1): 62-68.
[5]Donat F, Florin N H, Anthony E J, et al. Influence of high-temperature steam on the reactivity of CaO sorbent for CO2capture [J].EnvironmentalScience&Technology, 2012, 46(2): 1262-1269.
[6]Charitos A, Hawthorne C, Bidwe A R, et al. Parametric investigation of the calcium looping process for CO2capture in a 10kWth dual fluidized bed [J].InternationalJournalofGreenhouseGasControl, 2010, 4(5): 776-784.
[7]Manovic V, Anthony E J. Lime-based sorbents for high-temperature CO2capture—a review of sorbent modification methods [J].IntJEnvironResPublicHealth, 2010, 7(8): 3129-3140.
[8]Abanades J C, Anthony E J, Wang J, et al. Fluidized Bed Combustion Systems Integrating CO2Capture with CaO [J].EnvironmentalScience&Technology, 2005, 39(8): 2861-2866.
[9]Abanades J C, Grasa G, Alonso M, et al. Cost structure of a postcombustion CO2capture system using CaO [J].EnvironmentalScience&Technology, 2007, 41(15): 5523-5527.
[10]Alonso M, Rodríguez N, Gonzlez B, et al. Carbon dioxide capture from combustion flue gases with a calcium oxide chemical loop. Experimental results and process development [J].InternationalJournalofGreenhouseGasControl, 2010, 4(2): 167-173.
[11]Romeo L M, Lara Y, Lisbona P, et al. Economical assessment of competitive enhanced limestones for CO2capture cycles in power plants [J].FuelProcessingTechnology, 2009, 90(6): 803-811.
[12]Liu C T, Li Y J, Sun R Y, et al. Cyclic CO2capture of carbide slag modified by pyroligneous acid in calcium looping cycles [J].Asia-PacificJournalofChemicalEngineering, 2014, 9(5): 678-685.
[13]Sun R Y, Li Y J, Zhao J L, et al. CO2capture using carbide slag modified by propionic acid in calcium looping process for hydrogen production [J].InternationalJournalofHydrogenEnergy, 2013, 38(31): 13655-13663.
[14]Kierzkowska A M, Poulikakos L V, Broda M, et al. Synthesis of calcium-based, Al2O3-stabilized sorbents for CO2capture using a co-precipitation technique [J].InternationalJournalofGreenhouseGasControl, 2013, 15: 48-54.
[15]Florin N H, Blamey J, Fennell P S. Synthetic CaO-based sorbent for CO2capture from large-point sources [J].Energy&Fuels, 2010, 24(8): 4598-4604.
[16]Gupta H, Fan L. Carbonationcalcination cycle using high reactivity calcium oxide for carbon dioxide separation from flue gas [J].Industrial&EngineeringChemistryResearch, 2002, 41(16): 4035-4042.
湿法碳酸化电石渣干燥后在钙循环中的CO2捕集
何梓睿 李英杰 刘长天
(山东大学能源与动力工程学院,济南250061)
提出一种电石渣资源化利用的新方法.首先,将燃煤电站烟气通入电石渣浆液捕集CO2.碳酸化后的电石渣浆液(CCSS)干燥后在钙循环中作为吸收剂捕集CO2.在双固定床反应器和热重仪上研究了CCSS和电石渣的CO2捕集特性,包括碳酸化时间、煅烧温度和碳酸化温度对CCSS循环碳酸化特性的影响.结果表明CCSS的CO2捕集性能和碳酸化速率均高于电石渣.煅烧温度为950 ℃ 时,CCSS比电石渣具有更好反应活性,这有利于在恶劣煅烧条件下捕集CO2.在700~725 ℃,CCSS表现出了最佳的碳酸化性能.煅烧CCSS比电石渣孔隙结构更好,具有更大比表面积和比孔容,这有利于循环捕集CO2.
钙循环;电石渣;CO2捕集
TK123
Foundation item:The National Natural Science Foundation of China (No.51376003).
:He Zirui, Li Yingjie, Liu Changtian. CO2capture by carbonated carbide slag seriflux after drying in calcium looping cycles[J].Journal of Southeast University (English Edition),2015,31(2):204-208.
10.3969/j.issn.1003-7985.2015.02.008
10.3969/j.issn.1003-7985.2015.02.008
Received 2015-01-02.
Biographies:He Zirui (1990—), male, graduate; Li Yingjie(corresponding author), male, doctor, associate professor, liyj@sdu.edu.cn.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Construction of crash prediction model of freeway basic segment based on interactive influence of explanatory variables
- Experimental studies on gas-phase mercury oxidation removal and denitration of coal combustion with NH4Br addition
- CO2 capture using dry TiO2-doped Na2CO3/Al2O3 sorbents in a fluidized-bed reactor
- Comparative study on SO2 release and removal under air and oxy-fuel combustion in a fluidized bed combustor
- Effect of sulfation during carbonation on CO2 capture in calcium looping cycle
- Synthesis of highly reactive sorbent from industrial wastes and its CO2 capture capacity
