Experimental studies on gas-phase mercury oxidation removal and denitration of coal combustion with NH4Br addition
2015-05-08ZhaoShilinDuanYufengZhouQiangZhuChunSheMinJianhong
Zhao Shilin Duan Yufeng Zhou Qiang Zhu Chun She Min Lü Jianhong
(Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University, Nanjing 210096, China)(School of Energy and Environment, Southeast University, Nanjing 210096, China)
Experimental studies on gas-phase mercury oxidation removal and denitration of coal combustion with NH4Br addition
Zhao Shilin Duan Yufeng Zhou Qiang Zhu Chun She Min Lü Jianhong
(Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University, Nanjing 210096, China)(School of Energy and Environment, Southeast University, Nanjing 210096, China)
In order to remove gas-phase mercury and NOxfrom flue gas, experimental studies on flue gas mercury oxidation removal and denitration of Guizhou anthracite combustion with NH4Br addition were carried out. The influence of NH4Br addition on the ignition temperature and combustion characteristics was studied using a thermogravimetric analyzer. The effects of the NH4Br addition amount on gas-phase mercury oxidation and removal were investigated in a bench scale of 6 kW fluidized bed combustor (FBC). Mercury concentrations in flue gas were determined by the Ontario hydro method (OHM) and the mercury mass balance was obtained. Results show that the NH4Br addition has little influence on the ignition temperature of Guizhou anthracite. With the mercury mass balance of 95.47%, the proportion of particulate mercury Hgp, gaseous mercury Hg0and Hg2+are 75.28%, 11.60% and 13.12%, respectively, as raw coal combustion. The high particulate mercury Hgpin flue gas is caused by the high unburned carbon content in fly ash. When the NH4Br addition amount increases from 0 to 0.3%, the concentration of gaseous Hg0and Hg2+in flue gas decreases continuously, leading to the Hgpincrease accordingly. The oxidation rate of Hg0is positively correlated to the Br addition amount. It demonstrates that coal combustion with NH4Br addition can promote Hg0oxidation and removal. NOxconcentration in flue gas exhibits a descending trend with the NH4Br addition and the removal rate reaches 17.31% with the addition amount of 0.3%. Adding NH4Br to coal also plays a synergistic role in denitration.
coal additives; NH4Br; mercury oxidation; mercury removal; synergistic denitration
Mercury is one of the most harmful trace elements, which can enter the human body through breathing, skin absorption and the digestive tract, causing language obstacles, movement disorders, and renal failure, etc[1-2]. Mercury emissions from coal combustion has become one of the largest anthropogenic emission sources, with emissions accounting for 1/3 of the total amount of mercury emitted into the air[3-4]. It has caused a huge negative effect on the environment and human beings. Owing to the widespread concern about mercury pollution, the Chinese government enacted the “Thermal Power Plant Air Pollutant Emission Standards” (GB 13223—2011) in 2012, which was executed on January 1, 2015. According to the standards, the mercury emission concentration in coal-fired power plants has been limited to 0.03 mg/m3.Mercury exists in flue gas in three forms: elemental mercury Hg0, oxidized mercury Hg2+and particulate mercury Hgp. Hg2+can be captured by the wet flue gas desulfurization due to its water solubility, and Hgpcan be removed easily by dust removal equipment such as ESP or baghouse. Due to the long-term stability, water insolubility and long-distance transport in the atmosphere, it is difficult to remove the Hg0from the flue gas, and further research is required[5-7].
Adding additives to coal to achieve the transformation of Hg0to Hg2+during combustion process in the furnace is one of the most promising mercury removal technologies, which has the features of being a simple device, having reliable operation and a low cost. Studies have shown that halogen is a key element for Hg0oxidation. Gao et al.[8]carried out coal combustion with 1%, 2% and 3% CaBr2addition in a high-temperature tube furnace, and the results showed that the addition of CaBr2was beneficial in increasing both Hgpcontent and proportion. The combustion experiment of Liuzhi bituminous coal added with NaBr was carried out in a fixed quartz reactor by Ma et al[9], which aimed to study the impact of NaBr on mercury transformation during the coal combustion process. Experimental results indicate that NaBr has a positive effect on mercury oxidation, particularly at temperatures from 250 to 400 ℃. Pan et al.[10]combined numerical simulations and experimental methods to study the effects of adding NH4Cl to simulated flue gas on Hg and NO emissions. They found that with the increase in the injected amount of NH4Cl, the proportion of gaseous Hg0and Hg2+decreased, and the Hgpproportion increased correspondingly. Within a suitable temperature range, NH3was conducive to flue gas denitrification. Cao et al.[11]pointed out that spraying HBr into the coal-fired flue gas has better oxidation effect on Hg0than HCl, and the presence of Br can promote oxidation of Cl on Hg0. Moreover, the corrosion of Br on the equipment is much less than Cl. However, the mercury transformation mechanism by adding Br into coal during combustion is not entirely understood.
Based on previous research, this paper selected NH4Br as a mercury removal additive in the coal combustion process. Thermogravimetric analysis was used to study the impact of NH4Br addition on Guizhou anthracite ignition temperature and combustion characteristics. Then, the effects of adding different proportions of NH4Br to coal on flue gas mercury speciation, oxidation and removal were investigated in a bench scale of 6 kW FBC
experimental facility, which aimed to reveal the mechanism of mercury oxidation, transformation and removal with synergistic denitration.
1 Experimental
1.1 Coal analysis
The proximate and ultimate analyses of Guizhou anthracite were analyzed and the results are shown in Tab.1 and Tab.2, respectively. From Tab.2, we can see that the mercury content of coal (0.159 mg/kg) is slightly lower than the average mercury content of Chinese coal (0.22 mg/kg[12]). According to the classification scheme of chlorine content in coal (MT/5597—1996), this kind of coal belongs to special low-chlorine coal.

Tab.1 Proximate analysis of coal on as received basis %

Tab.2 Ultimate analysis of coal on as received basis
According to Ref.[13], chlorine can corrode pipes and carbonize furnace walls as well as shorten the life of equipment when its content in coal exceeds 0.3%. To enhance oxidizability resistance and weaken corrosivity, the Br addition proportion of 0.1%, 0.2% to 0.3% (mass ratio) in the form of NH4Br was added to the coal.
1.2 Thermogravimetric analysis (TGA) pre-test
To explore the effects of adding NH4Br on coal combustion characteristics, raw Guizhou coal (10 mg) and coal added with 10%NH4Br were conducted using a thermogravimetric analyzer (TGA92, a French SETARAM Company) under the conditions of air atmosphere and the heating rate of 20 ℃/min.
1.3 6 kW FBC system
The schematic diagram of a 6 kW coal-fired FBC test facility is shown in Fig.1. It consists of a circulating fluidized bed combustor, coal feeding, gas and solid sampling for mercury measurement and gas component analyses. Two levels of coal feeding hopper were installed to ensure coal feed continuity. There was an air supply system and furnace temperature control device mounted to the FBC. The average particle size of the coal was 0.5 to 1.6 mm. The coal feeding rate was 1 kg/h and the excessive air ratio was 1.4 with the furnace temperature of 900 ℃.
The mercury speciation sampling and analysis were conducted by strictly following the Ontario hydro method (OHM) recommended by US-EPA[14]. The temperature of the sampling line was kept at 140 ℃ by using the electric heating system to prevent the mercury vapor from condensing. The mercury content in coal, fly ash and bottom ash were determined by the Leeman Labs automatic mercury analyzer Hydra-C. Gaseous Hg0and Hg2+were determined by the automatic mercury analyzer Hydra AA, which was based on cold vapor atomic absorption spectrometry (CVAAS). The NOxconcentration in flue gas was determined by German Ecom J2KN multifunctional gas analyzer.
2 Results and Discussion
2.1 TGA test results
The TGA results of Guizhou raw coal and the coal mixed with 10% NH4Br are shown in Fig.2 and Fig.3. The weight loss of raw coal in Fig.2 can be divided into stages of evaporation of moisture, combustion of volatile matter and the residual coke burnout. According to the TG-DTG classification method[15], the ignition temperatureTiand burnout temperatureTbof raw coal can be determined to be about 550 and 800 ℃, respectively. Compared to Fig.2, the DTG curve in Fig.3 has a distinct peak approximately at 200 to 350 ℃. In this temperature range, the weight loss is about 1 mg, which corresponds to the amount of 10%NH4Br. According to the physical and chemical properties of NH4Br, it can be inferred that NH4Br decomposition and volatilization occur between 200 and 350 ℃. The TG and DTG curves in Figs.2 and 3 are basically the same when the temperature goes higher than 350 ℃, which indicates that NH4Br addition has no effect on the raw coal combustion characteristics.
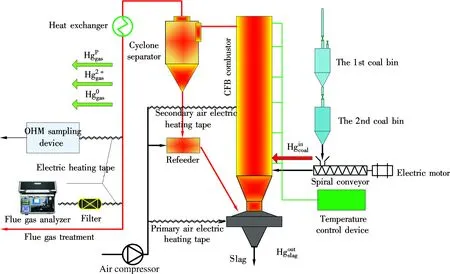
Fig.1 Schematic diagram of 6 kW coal-fired FBC test facility
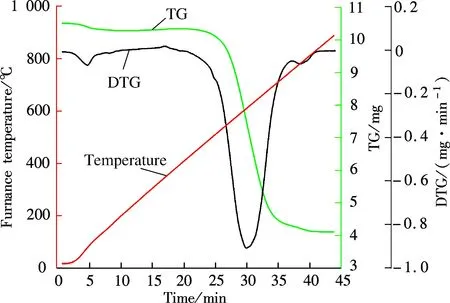
Fig.2 The TGA of raw coal
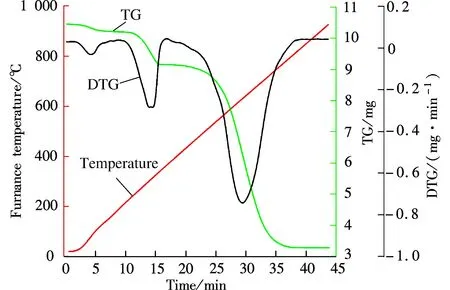
Fig.3 The TGA of coal added with 10%NH4Br
2.2 Mercury mass balance of the raw coal
Measuring mercury mass balance, which is an effective way to verify the data accuracy of the sampling, is usually defined as the ratio of output to input of mercury mass flow per unit time,
(1)
whereCHg0,gas,CHg2+,gas,CHgp,gasrepresent the concentrations of Hg0, Hg2+and Hgpin flue gas, respectively, μg/m3;Qgasis the flue gas volume flow per unit coal mass, m3/kg;mcoalis the coal feed rate, kg/h;CHg,slagis the mercury content in slag, μg/kg;mslagis the slag mass flow rate, kg/h;CHg,coalis the mercury content in coal, μg/kg.
Due to the unavoidable error factors in sampling, analysis and experimental running, the mercury mass balance rate between 70% and 130% is conventionally acceptable[16]. Fig.4 shows the mercury mass balance of 95.47% for raw coal combustion. The mercury speciation distribution in flue gas of raw coal combustion is shown in Fig.5. Gaseous Hg0and Hg2+concentrations are 2.276 and 2.572 μg/m3,respectively with the corresponding proportions of 11.60% and 13.12%. However, the Hgpconcentration in fly ash is 14.766 μg/m3, which accounts for 75.28% of the total mercury. Carpi et al.[17-18]indicated that Hg0and Hg2+occupied approximately 20% to 50% and 50% to 80% of the flue gas of the coalfired power plant, respectively, of which the
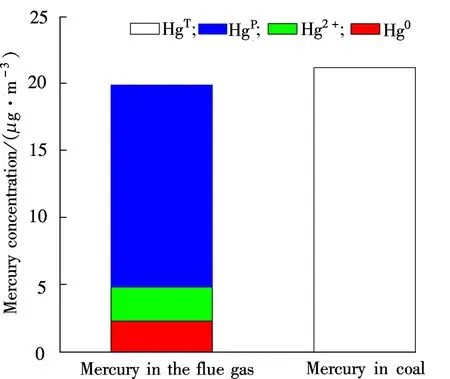
Fig.4 Mercury mass balance of raw coal
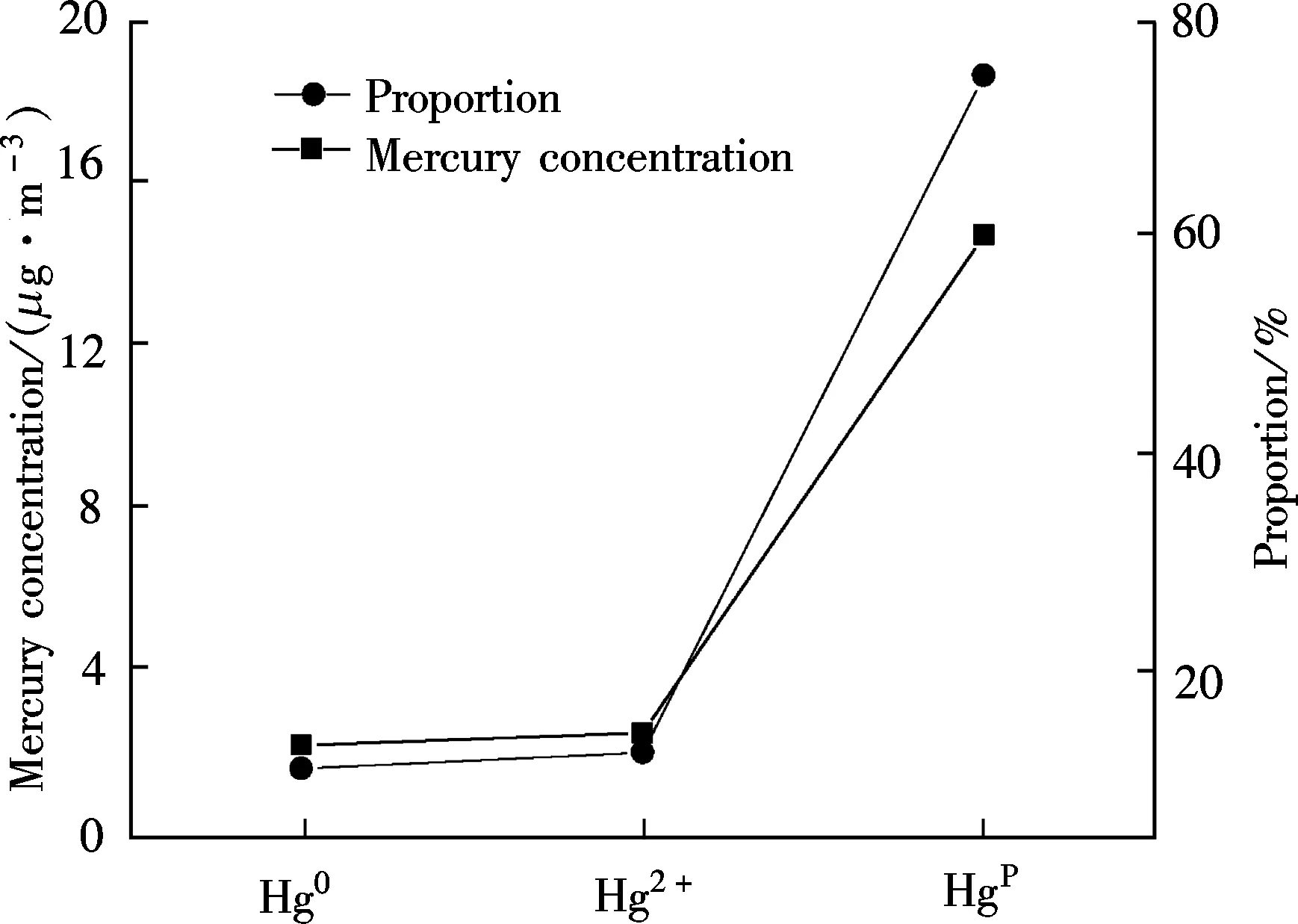
Fig.5 Mercury speciation in flue gas of raw coal
Hg2+content was significantly higher than that of Hg0. In this experiment, Hg0and Hg2+take 46.94% and 53.06% of the sum of gaseous Hg0and Hg2+, which is consistent with their results. The Hgpcontent is much higher than that in the pulverized coal furnace, because the fly ash in FBC has a higher unburned carbon content (about 15% to 20%).
2.3 Effects of additives on flue gas mercury oxidation and removal
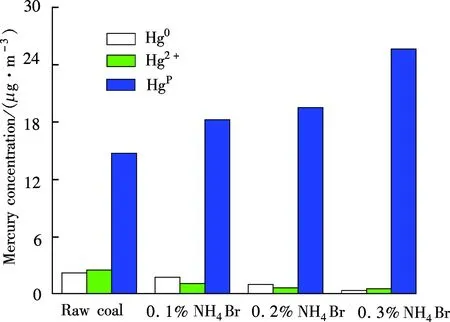
Mercury species in flue gas varying with the addition amount of NH4Br is shown in Fig.6. It illustrates that the
(a)
(b)
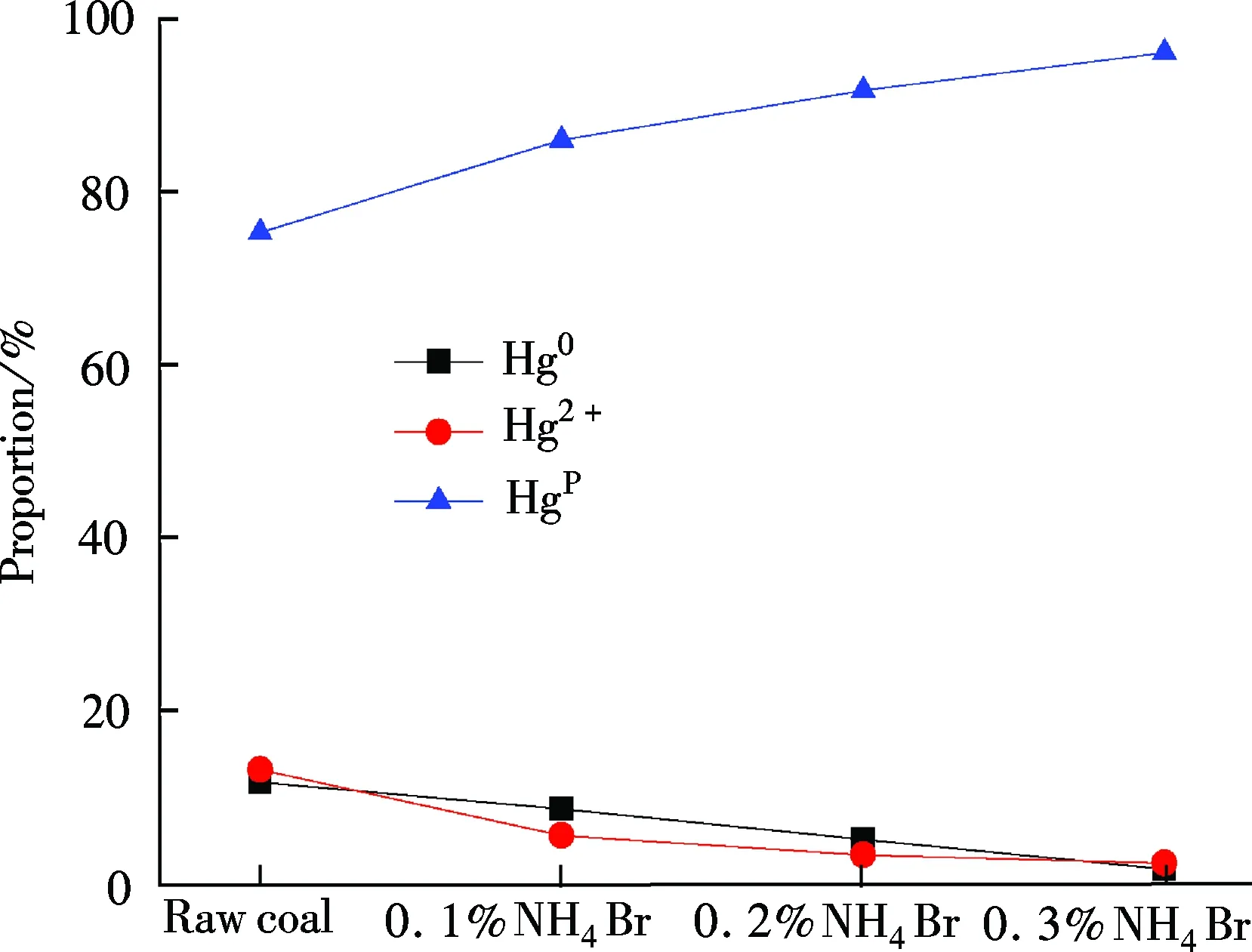
Hg0concentrations are 2.276, 1.808, 1.069 and 0.424 μg/m3,respectively, showing a decreasing trend with the NH4Br addition amount increasing from 0.0% to 0.3%. The corresponding proportions are 11.60%, 8.51%, 5.02% and 1.59%, respectively. Hg2+concentrations are 2.572, 1.162, 0.682 and 0.604 μg/m3, which also shows a reducing tendency with the proportions of 13.11%, 5.47%, 3.21% and 2.27% in total flue gas mercury. However, the Hgpconcentrations are 14.766, 18.273, 19.524 and 25.629 μg/m3, sharing a portion of 75.28%, 86.02%, 91.77% and 96.14%. It exhibits a considerable increasing tendency. Therefore, with the increase in the content of NH4Br loaded in coal, both Hg0and Hg2+concentrations decrease, while Hgpconcentrations increase accordingly.

(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
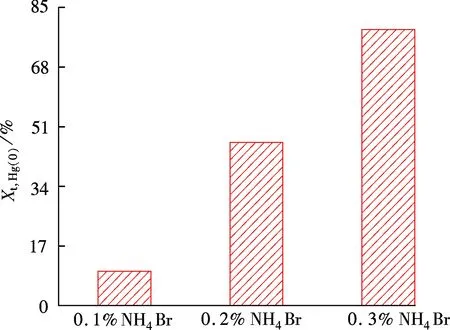
Fig.7 Hg0 oxidation rate varied with the added amount of NH4Br
Combining the TGA analytical results, the HBr generated by NH4Br decomposition in the combustion process can increase active Br content in the flue gas, which then promotes the reactions (2) to (10) and strengthens Hg0oxidation according to the chemical reaction kinetics. Unburned carbon in fly ash plays an important role in directly adsorbing gaseous mercury in flue gas, and Hg2+can be easily adsorbed to form Hgpthan Hg0. The presence of Br can promote the generation of active sites on the fly ash surface, which enhances chemisorption. Therefore, the proportion of Hgpincreases with NH4Br addition. In this paper, the Hg0oxidation rate increases while the Hg2+content decreases, because the presence of Br makes Hg2+adsorption capacity for fly ash stronger than its oxidation on Hg0.
2.4 Effect of additives on NOxemission
NOxin flue gas varying with the additional amount of NH4Br is plotted in Fig.8. It was reported that when the temperature was higher than 800 ℃, NH3decomposed from NH4Br can react with NOxand reduce it to N2[20],
(11)
(12)
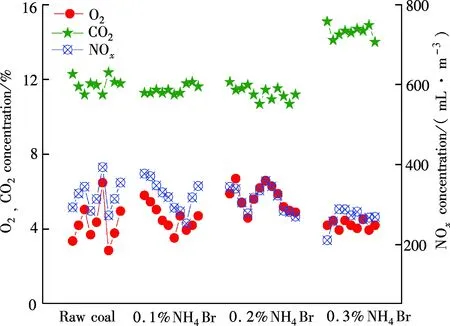
Fig.8 NOx variation with the added amount of NH4Br
With the added amount of NH4Br increasing from 0.1%, 0.2%, to 0.3%, the corresponding NH3concentrations are 33.5, 75.2 and 101.0 mL/m3, respectively, which are the equivalent amounts of NH3injected into the furnace. Fig.8 shows that when the amount of NH4Br increases, the NOxin flue gas presents a tendency to decrease on the whole. Hence, the NOxremoval rate tends to increase. With the added amount of 0.3%, the NOxremoval rate reaches the maximum of 17.31%. This is because the increase of NH3concentration in the flue gas promotes the reductive reaction of NOx.
3 Conclusions
The influence of adding NH4Br into coal on the ignition temperature and combustion characteristics of Guizhou anthracite was studied in a thermogravimetric analyzer. The effects of different adding proportions of NH4Br to coal on flue gas mercury oxidation and removal were investigated in a bench scale of 6 kW FBC experimental facility. The OHM method was used to determine the mercury speciation in flue gas and calculate the mercury mass balance rate. The mechanism of the impacts on flue gas mercury speciation and NOxemission by adding NH4Br into the coal was analyzed and discussed. The main conclusions are listed as follows:
1) The TGA results indicate that when the Guizhou anthracite ignition temperature is about 550 ℃ and the burnout temperature is about 800 ℃, the NH4Br addition has no effect on the coal combustion characteristics.
2) Based on the mercury mass balance of 95.47% conducted in the 6 kW FBC furnace, it is demonstrated that particulate mercury Hgp, gaseous mercury Hg0and Hg2+account for 75.28%, 11.60%, 13.12%, respectively, for the raw coal combustion. The high Hgpproportion is caused by the high carbon content in fly ash.
3) With the added amount of NH4Br loaded into coal increasing from 0.1%to 0.3%, the concentrations of gaseous Hg0and Hg2+in flue gas decrease and Hgpincreases accordingly. The oxidation rate of Hg0is positively correlated to the amount of NH4Br added to coal. The presence of Br makes fly ash adsorption for Hg2+stronger than its oxidation on Hg0.
4) NOxemission decreases with the increasing amount of NH4Br added to coal, which indicates that NH4Br plays a synergistic role of denitration.
[1]Swaine D J, Goodarzi F.Environmentalaspectsoftraceelementsincoal[M]. Dordrecht: Kluwer Academic Publishers, 1995:24-50.
[2]Yu M, Dong Y, Wang P, et al. Progress of effects of chloride on mercury removal for coal-fired flue gas [J].ChemicalIndustryandEngineeringProgress, 2012, 31(7):1610-1614. (in Chinese)
[3]Wu C, Cao Y, Dong Z, et al. Evaluation of mercury speciation and removal through air pollution control devices of a 190 MW boiler [J].JournalofEnvironmentalSciences, 2010, 22(2):277-282. (in Chinese)
[4]Wu C, Cao Y, Dong Z, et al. Mercury speciation and removal across full scale wet FGD systems at coal-fired power plants [J].JournalofCoalScienceandEngineering, 2010, 16(1):82-87.
[5]Park K S, Seo Y C, Lee S J, et a1. Emission and speciation of mercury from various combustion sources [J].PowderTechnology, 2008, 180(1/2): 151-156.
[6]Zhong L P, Cao Y, Li W Y, et a1. Effect of the existing air pollutant control devices on mercury emission in coal-fired power plants [J].JournalofFuelChemistryandTechnology, 2010, 38(6):641-646.
[7]Wang J, Wang W, Xu W, et a1. Mercury removals by existing pollutants control devices of four coal-fired power plants in China [J].JournalofEnvironmentalSciences, 2011, 23(11):1839-1844.
[8]Gao Z Y, Yin L B, Zhou L M, et al. Formation characteristics of Hg particulates during combustion of different coals [J].JournalofFuelChemistryandTechnology, 2012, 40(9):1135-1141. (in Chinese)
[9]Ma J J, Yao H, Luo X Q, et al. Effect of sodium bromide on nitric oxide reduction and mercury oxidation during coal combustion [J].JournalofEngineeringThermophysics, 2010, 31(8):1407-1410. (in Chinese)
[10]Pan W G, Wu J, Wang W H, et al. Study on the effect of NH4Cl addition on Hg and NO produced by coal combustion [J].ProceedingsoftheCSEE, 2009, 29(29):41-46. (in Chinese)
[11]Cao Y, Wang Q, Chen C, et al. Investigation of mercury transformation by HBr addition in a slipstream facility with real flue gas atmospheres of bituminous coal and Powder River Basin coal [J].Energy&Fuels, 2007, 21(5):2719-2730.
[12]Kuang M, Yang G H, Hu W J, et al. Analysis and prospect of technology for removing mercury from flue gas [J].EnvironmentalScience&Technology, 2008, 31(5):66-70. (in Chinese)
[13]Zhao Y, Liu X M, Li M, et al. Progress of study on release characteristics and removal technology of chlorine in coal during coal combustion and pyrolysis [J].JournalofShandongUniversityofScienceandTechnology:NaturalScience, 2004, 2(2):108-111. (in Chinese)
[14]US EPA. Standard test mercury from coal-fired stationary sources (Ontario hydro method) [R]. Washington, DC: US Government Printing Office, 1999.
[15]Li Q Z, Zhao C S. Investigation on characteristics of pulverized coal combustion in O2/CO2mixtures [J].ProceedingsoftheCSEE, 2007, 27(35):39-43. (in Chinese)[16]Yokoyama T, Asakura K, Matsuda H, et al. Mercury emissions from a coal-fired power plant in Japan [J].ScienceofTotalEnvironment, 2000, 259(1/2/3):97-103.
[17]Carpi A. Mercury from combustion sources: a review of the chemical species emitted and their transport in the atmosphere [J].WaterAirandSoilPollution, 1997, 98(3/4):241-254.
[18]Sliger R N, Kramlich J C, Marinov N M. Towards the development of a chemical kinetic model of homogeneous oxidation of mercury by chlorine species [J].FuelProcessTechnology, 2000, 65/66:423-438.
[19]Vosteen B W, Kanefke R, Koser H. Bromine-enhanced mercury abatement from combustion flue gase-recent industrial applications and laboratory research [J].VGBPowerTechInternationalJournalforElectricityandHeatGeneration, 2006, 86(3):70-75.
[20]Cao Qingxi. Effects of gaseous additives for selective non-catalytic reduction of NOx[D]. Harbin: School of Energy Science and Engineering, Harbin Institute of Technology, 2009. (in Chinese)
煤中添加NH4Br对气相汞氧化脱除及脱硝的实验研究
赵士林 段钰锋 周 强 朱 纯 佘 敏 吕剑虹
(东南大学能源热转换及其过程测控教育部重点实验室,南京210096)
(东南大学能源与环境学院, 南京 210096)
为脱除烟气中的气相汞和NOx,进行了贵州无烟煤中添加NH4Br对烟气汞氧化脱除协同脱硝的实验研究.在热重分析仪上研究添加NH4Br对着火温度和燃烧特性的影响.在6 kW流化床燃烧(FBC)试验装置上研究了在煤中不同NH4Br添加量对气相汞氧化脱除的影响.采用安大略法(OHM)对烟气中汞形态浓度进行了测定并得出汞质量平衡.结果表明,添加NH4Br对贵州无烟煤的着火温度没有影响.原煤燃烧汞质量平衡率为95.47%,颗粒汞Hgp、气态Hg0和Hg2+比例分别为75.28%, 11.60%, 13.12%.由于飞灰中含有较高的未燃尽碳, 烟气中Hgp浓度较高.随着NH4Br添加量从0增加至0.3%,烟气中气态Hg0和Hg2+减少,Hgp相应增加.Hg0的氧化率与煤中Br的添加量成正相关.这表明煤燃烧中添加NH4Br可促进Hg0的氧化和脱除.烟气中NOx随着NH4Br的添加呈减小趋势,其脱除率在0.3%添加量时达最大(17.31%).添加NH4Br同时具有协同脱硝的作用.
煤添加剂;溴化铵;汞氧化;汞脱除;协同脱硝
TK16
10.3969/j.issn.1003-7985.2015.02.012
Foundation items:The National Natural Science Foundation of China (No.51376046, 51076030), the National Key Technology R&D Program of China during the 12th Five-Year Plan Period (No.2012BAA02B01), the United Creative Foundation of Jiangsu Province (No.BY2013073-10), the Fundamental Research Funds for the Central Universities, the Scientific Innovation Research of College Graduates in Jiangsu Province (CXZZ13_0093, KYLX_0115, KYLX_0184).
:Zhao Shilin, Duan Yufeng, Zhou Qiang, et al. Experimental studies on gas-phase mercury oxidation removal and denitration of coal combustion with NH4Br addition[J].Journal of Southeast University (English Edition),2015,31(2):226-231.
10.3969/j.issn.1003-7985.2015.02.012
Received 2015-01-09.
Biographies:Zhao Shilin (1992—),male, graduate; Duan Yufeng (corresponding author), male, doctor, professor, yfduan@seu.edu.cn.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Construction of crash prediction model of freeway basic segment based on interactive influence of explanatory variables
- CO2 capture using dry TiO2-doped Na2CO3/Al2O3 sorbents in a fluidized-bed reactor
- Comparative study on SO2 release and removal under air and oxy-fuel combustion in a fluidized bed combustor
- Effect of sulfation during carbonation on CO2 capture in calcium looping cycle
- Synthesis of highly reactive sorbent from industrial wastes and its CO2 capture capacity
- CO2 capture by carbonated carbide slag seriflux after drying in calcium looping cycles
