新型二唑类4-氨基喹唑啉衍生物的合成及其抗肿瘤活性
2015-04-23李文举徐海丽欧阳贵平周黔兰
李文举,陈 琨,徐海丽,欧阳贵平,周黔兰
(1.贵州省环境监测中心站,贵州贵阳 550081;2.贵州师范学院化学与生命科学学院,贵州贵阳 550018;3.贵州大学精细化工研究开发中心教育部绿色农药与农业生物工程重点实验室,贵州贵阳 550025)
4-氨基喹唑啉类化合物是一类具有广泛生物活性的含氮杂环类化合物[1-4],在抗癌、抗菌、抗炎、抗HIV以及治疗糖尿病等方面显出优良的活性,成为目前药物化学研究的热点之一[5-23]。
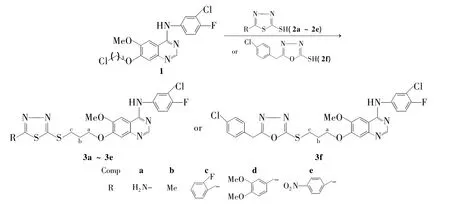
Scheme 1
在本课题组研究的基础上[24-26],本文采用活性拼接原来,以N-(3-氯-4-氟苯胺基)-7-(3-氯丙氧基)-6-甲氧基喹唑啉-4-胺(1)为起始原料,与取代1,3,4-噻二唑(2a ~ 2f)或对氯苯甲基-1,3,4-噁二唑(2f)经取代反应合成了6个新型的二唑类4-氨基喹唑啉衍生物(3a~3f,Scheme 1),其结构经1H NMR,13C NMR,IR及元素分析表征。并用MTT法初步测定了3a~3f对前列腺癌细胞(PC-3)和人乳癌细胞(Bcap-37)的抑制率。
1 实验部分
1.1 仪器与试剂
X-4型显微熔点仪(温度未校正);ZF-Ⅰ型三用紫外分析仪;ECX-500型核磁共振仪(CDCl3为溶剂,TMS为内标);Shimadzu IR Prestige-21型红外光谱仪(KBr压片);VarioⅢ型元素分析仪。
1按文献[1]方法制备;其余所用试剂均为化学纯或分析纯。
1.2 3a~3f的合成(以3a为例)
在三口瓶中依次加入1 0.5 g(1.7 mmol),2-巯基-5-氨基-1,3,4-噻二唑(2a)0.5 g(4.2 mmol),DMF 15 mL及 K2CO32 g,搅拌下于85℃反应4 h~7 h(TLC跟踪)。冷却至室温,倒入水中,静置析晶;过滤,滤饼经硅胶柱层析(洗脱剂:乙酸乙酯)纯化得3a。
用类似的方法合成3b~3e。用2f替代2a,用类似的方法合成3f。
N-(3-氯-4-氟苯基)-7-[3-(5-氨基-1,3,4-噻二唑-2-巯基)丙氧基]-6-甲氧基喹唑啉-4-胺(3a):黄色固体,产率68.8%,m.p.281 ℃ ~286℃;1H NMR δ:2.26 ~2.28(m,2H,b-H),3.97(s,3H,OCH3),4.21 ~4.23(t,J=6.3 Hz,2H,a-H),4.26 ~ 4.29(t,J=6.9 Hz,2H,c-H),7.17(s,1H,ArH),7.30(s,2H,NH2),7.43 ~7.47(d,J=9.15 Hz,1H,ArH)7.79 ~7.81(m,2H,ArH),8.12 ~8.14(m,1H,ArH),8.50(s,1H,d-H),9.57(s,1H,NH);13C NMR δ:27.61,47.61,56.80,66.44,102.32,108.36,109.30,116.99,117.16,119.23,119.38,122.81,123.98,137.34,147.43,149.63,153.15,154.08,156.54,158.97;IR ν:3 475,3 361,2 954,1 653,1 622,1 506,1 423,1 219 cm-1;Anal.calcd for C20H18N6O2S2FCl(492.9):C 48.73,H 3.68,N 17.05;found C 48.62,H 4.12,N 19.92。
N-(3-氯-4-氟苯基)-6-甲氧基-7-[3-(5-甲基-1,3,4-噻二唑-2-巯基)丙氧基]喹唑啉-4-胺(3b):白色固体,产率56%,m.p.168℃ ~170℃;1H NMR δ:2.03(s,3H,CH3),2.10 ~2.15(m,2H,b-H),3.97(s,3H,CH3),4.18 ~4.21(t,2H,a-H),4.21 ~ 4.23(t,2H,c-H),7.21(s,1H,ArH),7.43 ~7.47(t,1H,ArH),7.77 ~7.78(m,2H,ArH),8.12 ~8.14(m,1H,ArH),8.50(s,1H,ArH),9.57(s,1H,NH);13C NMR δ:21.24,28.35,56.80,61.37,65.79,102.40,108.42,109.32,116.12,116.98,117.15,122.81,123.97,134.75,147.47,149.57,150.49,153.18,154.03,156.55,171.02;IR ν:3 585,2 943,1 718,1 633,1 498,1 460,1 244,1 022,848,690,553 cm-1;Anal.calcd for C21H19N5O2S2FCl(491.9):C 52.27,H 3.89,N 14.23;found C 52.22,H 4.18,N 14.20。
N-(3-氯-4-氟苯基)-7-{3-[5-(2-氟苯基)-1,3,4-噻二唑-2-巯基]丙氧基}-6-甲氧基喹唑啉-4-胺(3c):黄色固体,产率72.4%,m.p.98 ℃ ~103 ℃;1H NMR δ:2.30 ~2.35(m,2H,b-H),3.54 ~3.57(t,J=7.45 Hz,2H,a-H),3.97(s,3H,OCH3),4.28 ~ 4.30(t,J=6.25 Hz,2H,c-H),7.19(s,1H,ArH),7.37 ~ 7.40(t,J=7.45 Hz,1H,ArH),7.43 ~7.48(m,2H,ArH),7.59~7.63(m,1H,ArH),7.78~7.82(m,2H,ArH),8.11 ~ 8.16(m,2H,ArH),8.48(s,1H,d-H),9.52(s,1H,NH);13C NMR δ:28.95,31.38,56.78,67.10,102.33,108.47,109.35,116.93,117.13,119.21,119.36,122.78,123.91,125.97,128.74,113.83,137.32,147.42,149.54,152.67,153.11,156.45,158.15,160.13,160.40,167.28;IR ν:3 564,3 421,2 941,1 627,1 577,1 527,1 498,1 215 cm-1;Anal.calcd for C26H20N5O2S2F2Cl(572.0):C 54.59,H 3.52,N 12.24;found C 54.16,H 3.79,N 12.58。
N-(3-氯-4-氟苯基)-7-{3-[5-(3,4-二甲氧基苯基)-1,3,4-噻二唑-2-巯基]丙氧基}-6-甲氧基喹唑啉-4-胺(3d):白色固体,产率 71.3%,m.p.147℃ ~149 ℃;1H NMR δ:2.29 ~2.33(m,2H,b-H),3.49 ~3.52(t,J=6.3 Hz,2H,a-H),3.79(s,3H,OCH3),3.82(s,3H,OCH3),3.96(s,3H,OCH3),4.28 ~4.31(t,J=7.45 Hz,2H,c-H),7.03 ~7.06(m,1H,ArH),7.20(m,1H,ArH),7.33 ~7.35(m,1H,ArH),7.40(s,1H,ArH),7.47 ~7.4(m,1H,ArH),7.78 ~7.80(m,2H,ArH),8.11 ~8.13(m,1H,ArH),8.49(s,1H,d-H),9.51 ~9.52(s,1H,NH);13C NMR δ:28.98,31.55,56.11,56.15,56.81,67.05,102.35,108.48,109.35,110.05,112.47,116.94,117.11,119.20,119.36,121.70,122.80,123.93,137.32,147.40,149.55,151.88,152.68,153.13,153.87,156.47,164.72,168.45;IR ν:3 442,2 943,1 625,1 579,1 498,1 458,1 421,1 325,1 217 cm-1;Anal.calcd for C28H25N5O4S2FCl(614.1):C 54.76,H 4.10,N 11.40;found C 54.38,H 4.00,N 10.72。
N-(3-氯-4-氟苯基)-6-甲氧基-7-{3-[5-(4-硝基苯基)-1,3,4-噻二唑-2-巯基]丙氧基}喹唑啉-4-胺(3e):黄色固体,产率63.6%,m.p.110 ℃ ~113 ℃;1H NMR δ:2.32 ~ 2.35(m,2H,b-H),3.56 ~3.59(t,J=6.85 Hz,2H,a-H),3.79(s,3H,OCH3),4.28 ~ 4.29(t,J=5.7 Hz,2H,c-H),7.16 ~7.17(d,J=4.55 Hz,1H,ArH),7.42 ~7.47(m,1H,ArH),7.75 ~7.79(m,1H,ArH),7.95(s,1H,ArH),8.06 ~8.11(m,3H,ArH),8.31 ~8.32(m,2H,ArH),8.46 ~8.47(d,J=6.3 Hz,1H,d-H),9.48(s,1H,NH);13C NMR δ:29.19,56.78,60.30,66.90,102.28,108.36,109.25,116.94,122.82,123.95,124.90,125.04,128.06,129.01,137.24,147.32,149.03,149.41,153.09,152.70,153.82,156.40,164.25,170.92;IR ν:3 432,2 933,1 614,1 560,1 498,1 458,1 421,1 217 cm-1;Anal.calcd for C27H22N6O4S2FCl(613.0):C 52.89,H 3.62,N 13.71;found C 52.86,H 3.57,N 13.68。
N-(3-氯-4-氟苯基)-7-{3-[5-(4-氯苯基)-1,3,4-噁二唑-2-巯基]丙氧基}-6-甲氧基喹唑啉-4-胺(3f):白色固体,产率55.2%,m.p.98 ℃ ~102℃;1H NMR δ:2.33 ~2.35(m,2H,b-H),3.49 ~3.52(t,J=6.9 Hz,2H,a-H),3.94(s,3H,OCH3),4.28 ~ 4.31(t,J=6.3 Hz,2H,c-H),7.19(s,1H,ArH),7.43 ~ 7.47(t,J=9.2 Hz,1H,ArH),7.59 ~7.60(d,J=8.6 Hz,2H,ArH),7.75(s,1H,ArH),7.79 ~7.82(m,1H,ArH),7.89 ~ 7.90(d,J=8.6 Hz,2H,ArH),8.12 ~8.14(m,1H,ArH),8.48(s,1H,d-H),9.52(s,1H,NH);13C NMR δ:29.19,56.78,60.30,66.90,102.28,108.36,109.25,116.94,122.82,123.95,128.06,128.90,129.01,147.32,149.03,149.41,152.70,153.09,153.82,154.60,156.40,164.25,165.66,170.92;IR ν:3 434,2 934,1 653,1 569,1 588,1 498,1 458,1 412,1 217 cm-1;Anal.calcd for C27H22N5O3SFCl2(586.4):C 55.30,H 3.78,N 11.94;found C 55.26,H 3.75,N 11.90。
1.3 体外抗肿瘤活性测定[25]
以DMSO为空白对照,阿霉素为阳性对照,采用MTT法测定3a~3f对PC-3和Bcap-37细胞的抑制活性(每个实验重复3次,取平均值)。
MTT测定法:将癌细胞接种于96孔细胞培养板中,每孔2 200个细胞,在37℃,5%CO2和饱和湿度的条件下培养24 h。加入含处理因子的1 640培养基(200 μL/孔),设空白对照和溶媒对照,每组 4个平行,继续培养 72 h。加入 5 mg·mL-1MTT 20 μL 培养 4 h。在酶标仪上于570 nm处测定其吸光率(OD),按下式计算细胞增殖抑制率。
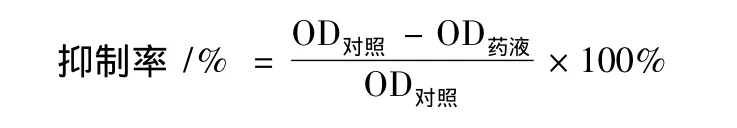
2 结果与讨论
2.1 表征
以3a为例,对其结构进行表征。1H NMR分析表明,CH2(b-H)受到两边CH2(a,c-H)影响裂分为多重峰;OCH3的吸收单峰位于δ 3.97,由于氧的电负性大于硫,所以OCH2(a-H)在δ 4.21~4.23 有三重峰,而SCH2(c-H)在 δ 4.26 ~4.29 出现三重峰;NH2在δ 7.30有一个单峰。受两个氮碳双键的影响,CH=(d-H)的吸收单峰出现在δ 8.50;δ 9.57的单峰为NH吸收峰。
IR 分析表明,3 275 cm-1~3 479 cm-1间有N -H的伸缩振动峰,1 630 cm-1~1 447 cm-1有Ar骨架振动峰;含氟化合物在1 310 cm-1~1 325 cm-1间有C-F的伸缩振动峰,含MeO化合物在1 270 cm-1附近有Ar-O-C的不对称伸缩振动,在1 110 cm-1附近有Ar-O-C的对称伸缩振动。
2.2 抗肿瘤活性
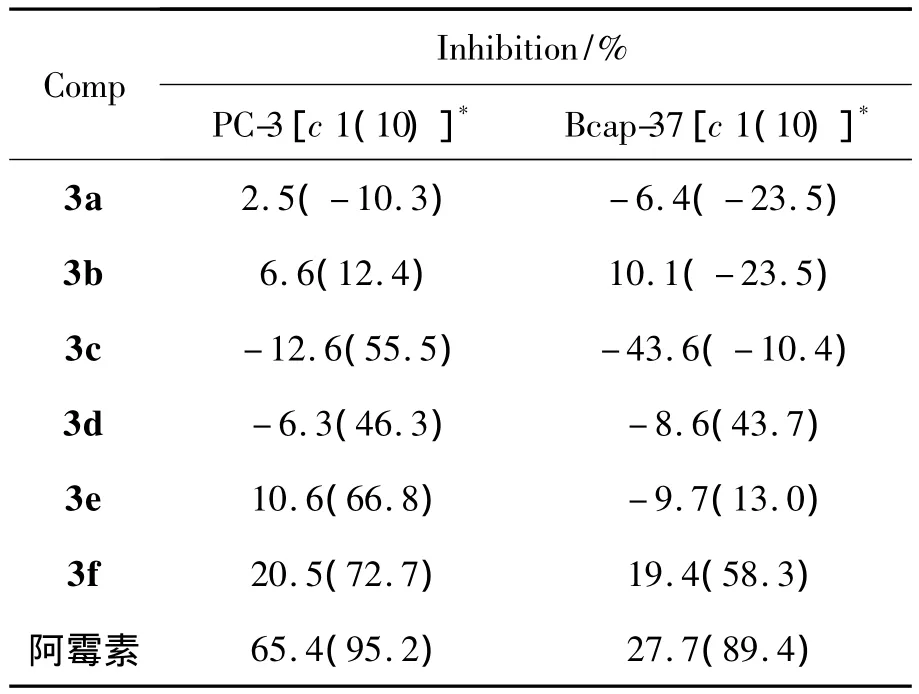
表1 3a~3g对PC-3和Bcap-37的抑制活性Table 1 Inhibition activities of 3a~3g against PC-3 and Bcap-37 cell in vitro
3a~3f对PC-3和Bcap-37的抑制活性结果见表1。从表1可见,在邻对位有吸电子基的化合物3e和3f对PC-3细胞具有相对较好的抑制活性,在用药量为10 μmol·L-1时其抑制率分别为66.8%和72.7%,具有一定的进一步优化和深入研究价值。
3e和3f对PC-3细胞的抑制活性与取代基的性质有关。由于O的电负性大于S,且对位受到NO2,Cl等吸电子基的影响,从而提高了他们与PC-3酶的亲和性。
致谢:感谢贵州大学精细化工开发中心重点实验室细胞生物学实验室对3a~3f的体外抗癌活性测试!
[1]Zheng-Yang Gu,Tong-Hao Zhu,Jia-Jia Cao,et al.Palladium-catalyzed cascade reactions of isocyanides with enaminones:Synthesis of 4-aminoquinoline derivatives[J].ACS Catal,2014,4(1):49 -52.
[2]Raj R,Biot C,Carrère-Kremer S,et al.4-Aminoquinoline-β-lactam conjugates:Synthesis,antimalarial,and antitubercular evaluation[J].Chem Biol Drug Des,2014,83(2):191 -197.
[3]Jie Ren,Juan Zhao,Yong-Sheng Zhou,et al.Synthesis and antitumor activity of novel 4-aminoquinoline derivatives[J].Medicinal Chemistry Research,2013,22(6):2855-2861.
[4]Shashi Pandey,Pooja Agarwal,Kumkum Srivastava,et al.synthesis and bioevaluation of novel 4-aminoquinoline-tetrazole derivatives as potent antimalarial agents[J].European Journal of Medicinal Chemistry,2013,66:69 -81.
[5]T Vlaar,B U W Maes,E Ruijter,et al.Synthesis of 4-aminoquinolines by aerobic oxidative palladium-catalyzed double C-H activation and isocyanide insertion[J].Chemistry of Heterocyclic Compounds,2013,49(6):902-908.
[6]Solomon V R,Haq W,Srivastava K,et al.Design,synthesisof 4-aminoquinoline-derived thiazolidines and their antimalarial activity and heme polymerization inhibition studies[J].Enzyme Inhib Med Chem,2013,28(3):619 -626.
[7]Cha M Y,Lee K O,Kim J W,et al.Discovery of a novel her-1/her-2 dual tyrosine kinase inhibitor for the treatment of her-1 selective inhibitor-resistant nonsmall cell lung cancer[J].Journal of Medicinal Chemistry,2009,52:6880 -6888.
[8]Chandregowda V,Venkateshwara R G,Chandrasekhara R G.Convergent approach for commercial synthesis of gefitinib and erlotinib[J].Organic Process Research and Development,2007,11:813 -816.
[9]Chandrika P M,Yakaiah T,Rao A R,et al.Synthesis of novel 4,6-disubstituted quinazoline derivatives,their anti-inflammatory and anti-cancer activity(cytotoxic)against U937 leukemia cell lines[J].European Journal of Medicinal Chemistry,2008,43(4):846 -852.
[10]Duncton M A J,Estiarte A M,Johnson R J,et al.Preparation of heteroaryloxetanes and heteroarylazetidines by use of a minisci reaction[J].Journal of Organic Chemistry,2009,74(16):6354 -6357.
[11]Fang H,Li M Y,Xia L.Pharmacophore-guided design,synthesis and evaluation of quinazoline-arylpiperazines as new aladrenoceptor antagonists[J].Chinese Chemical Letters,2007,18:41 -44.
[12]Gilday J P,David M.Process for the preparation of 4-(3-chloro-4-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline[P].WO 2 004 024 703,2006.
[13]Jung F H,Pasquet G,Brempt C L,et al.Discovery of novel and potent thiazoloquinazolines as selective aurora A and B kinase inhibitors[J].Journal of Medicinal Chemistry,2006,49:955 -970.
[14]Mortlock A A,Foote K M,Heron N M,et al.Discovery,synthesis,and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase[J].Journal of Medicinal Chemistry,2007,50:2213-2224.
[15]Okano M,Mito J,Maruyama Y,et al.Discovery and structure-activity relationships of 4-aminoquinazoline derivatives,a novel class of opioid receptor like-1(ORL1)antagonists[J].Bioorganic & Medicinal Chemistry,2009,17:119 -132.
[16]Portela Cubillo F,Scott J S,Walton J C.Microwavepromoted syntheses of quinazolines and dihydro quinazolines from 2-amino aryl alkanone O-phenyl oximes[J].Journal of Organic Chemistry,2009,74:4934 -494.
[17]Portela Cubillo F,Scott J S,Walton J C.Microwavepromoted syntheses of quinazolines and dihydro quinazolines from 2-amino aryl alkanone O-phenyl oximes[J].Journal of Organic Chemistry,2009,74:4934 -494.
[18] Sirisoma N,Pervin A,Zhang H,et al.Discovery of N-(4-methoxyphenyl)-N,2-dimethylquinazolin-4-amine,a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration[J].Journal of Medicinal Chemistry,2009,52:2341 -2351.
[19] Sirisoma N,Pervin A,Zhang H,et al.Discovery of N-(4-methoxyphenyl)-N,2-dimethylquinazolin-4-amine,a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration[J].Journal of Medicinal Chemistry,2009,52:2341 -2351.
[20]Stamos J,Sliwkowski M X,Eigenbrot C.Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-aminoquinzoline inhibitor[J].Journal of Biological Chemistry,2002,277(48):46256-46272.
[21]Strumberg D,Awada A,Hirte H,et al.Pooled safety analysis of BAY43-9006(sorafenib)monotherapy in patients with advanced solid tumors:Is rash associated with treatment outcome[J].European Journal of Cancer,2006,42(4):548 -556.
[22]Verhaeghe P,Azas N,Gasquet M,et al.Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquina-zolines[J].Bioorganic & Medicinal Chemistry Letters,2008,18:396 -401.
[23]Verhaeghe P,Azas N,Gasquet M,et al.Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquina-zolines[J].Bioorganic & Medicinal Chemistry Letters,2008,18:396 -401.
[24]李文举,徐海丽,欧阳贵平.新型4-氨基喹唑啉衍生物的合成及其抗肿瘤活性[J].合成化学,2015,23(1):17-22.
[25]陈舒忆,吕同杰,严和平,等.喹唑啉衍生物的合成及其抗肿瘤活性[J].合成化学,2013,21:092 -095.
[26]谢良辉,欧阳贵平.吉非替尼的合成工艺改进[J].合成化学,2010,18:523 -525.
