新型3-氨基-α-羟基-β-酯基-3-季碳氧化吲哚类化合物的合成及其抗肿瘤活性
2015-04-23黄俊飞余章彪刘雄利
杨 俊,黄俊飞,陆 毅,杨 超,余章彪,周 英,赵 致,刘雄利
(贵州大学药学院贵州省中药民族药创制工程中心,贵州贵阳 550025)
氧化吲哚是一类重要的杂环化合物,受到学者们的热切关注[1]。3-季碳氧化吲哚广泛存在于天然活性产物中,具有良好的生物活性[2]。根据活性结构拼合原理合成的含3-季碳氧化吲哚母核的新化合物,同样具有良好的生物活性[3-4]。目前,合成3-季碳氧化吲哚的方法主要为:以3-取代氧化吲哚作亲核试剂,与亲电试剂反应合成[5-15]。
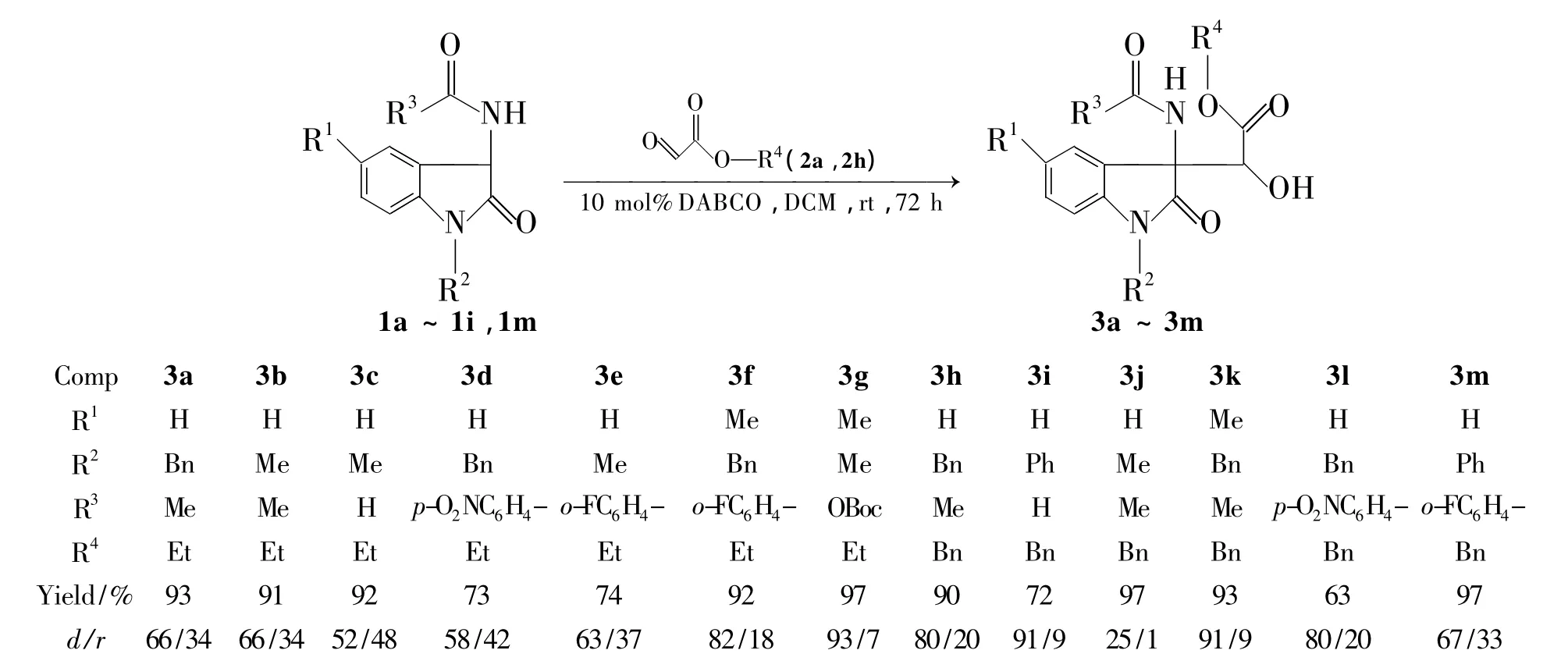
Scheme 1
为进一步丰富3-季碳氧化吲哚的合成方法,满足活性筛选和新药研发的需求,本文以1,4-二氮杂二环[2.2.2]辛烷(DABCO)为催化剂,二氯甲烷为溶剂,3-氮取代氧化吲哚(1a~1i和1m)和乙醛酸酯(2a,2h)经Aldol反应合成了13个新型的3-氨基-α-羟基-β-酯-3-季碳氧化吲哚类化合物(3a ~3m,Scheme 1),产率 63% ~97%,其结构经1H NMR,13C NMR和HR-ESI-MS表征。讨论了底物上的取代基对反应非对映选择性的影响。并用MTT法考察了3a~3m对人白血病细胞(K562)和人肺癌细胞(A549)的体外抑制活性。
1 实验部分
1.1 仪器与试剂
Bruker 400 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);Bruker Bio TOF III Q型质谱仪。
所用试剂均为分析纯;无水溶剂使用前均按标准程序干燥。
1.2 3a~3m的合成(以3a为例)
在反应瓶中加入N-(1-苄基-2-氧化吲哚-3-基)乙酰胺(1a)280 mg(1.0 mmol),乙醛酸乙酯(2a)153 mg(1.5 mmol)和二氯甲烷 15 mL,搅拌使其溶解;加入10 mol%DABCO 11.2 mg,于室温反应72 h(TLC检测)。反应液经硅胶柱层析[V(乙酸乙酯)∶V(石油醚)=1∶15]纯化得黄色固体3a。
用类似的方法合成黄色固体3b~3m。
3a:1H NMR δ(major+minor):0.88(t,J=3.6 Hz,2.9H,major),1.14(t,J=3.6 Hz,1.5H,minor),2.01 ~2.02(m,4.9H,major+minor),2.06(br s,0.7H,major),3.77(br s,0.5H,minor),3.87 ~ 3.90(m,2H,major),4.12 ~4.15(m,2H,major+minor),4.60 ~4.62(m,1.5H,major+minor),4.80(d,J=8.0 Hz,1H,major),4.92(d,J=8.0 Hz,0.5H,minor),5.06(d,J=8.0 Hz,0.5H,minor),5.16(d,J=8.0 Hz,1H,major),6.64(d,J=4.0 Hz,0.5H,minor),6.67(d,J=4.0 Hz,1H,major),6.97 ~7.00(m,1.5H,major+minor),7.08(d,J=3.6 Hz,0.5H,minor),7.15 ~ 7.18(m,1.5H,minor),7.22(s,1H,major),7.25 ~7.29(m,2.6H,major+minor),7.32 ~ 7.37(m,3.3H,major+minor),7.44(d,J=4.0 Hz,1H,minor),7.52(d,J=3.6 Hz,1.9H,major);13C NMR δ(major+minor):13.6,13.8,22.6,44.3,44.5,62.3,62.5,62.6,63.1,73.1,73.4,109.2,109.5,122.5,122.6,122.9,123.4,125.4,125.6,127.1,127.3,127.5,128.6,129.5,135.4,143.5,143.9,169.0,169.3,169.7,170.5,173.5,174.0;HR-ESI-MS m/z:Calcd for C21H22N2O5Na{[M+Na]+}405.142 6,found 405.142 8。
3b:1H NMR δ(major+minor):0.98(t,J=1.8 Hz,3H,major),1.12(t,J=2.4 Hz,1.6H,minor),1.95 ~1.96(m,4.5H,major+minor),2.04(br s,1.3H,major+minor),3.24 ~ 3.27(m,4.5H,major+minor),3.77(br s,0.5H,minor),3.92 ~3.94(m,1.9H,major+minor),4.05 ~4.14(m,2H,major),4.55(s,1.4H,major+minor),6.81 ~6.85(m,1.5H,major+minor),7.00 ~7.03(m,1.5H,major+minor),7.11 ~7.32(m,4.5H,major+minor);13C NMR δ(major+minor):13.6,13.7,22.6,26.5,62.1,62.3,62.6,62.7,62.9,73.1,73.2,108.0,108.3,122.5,122.6,123.0,123.4,125.5,129.7,144.3,169.0,169.1,169.4,169.7,170.3,173.4,173.9;HR-ESI-MS m/z:Calcd for C15H18N2O5Na{[M+Na]+}329.111 3,found 329.111 3。
3c:1H NMR δ(major+minor):0.98(t,J=3.0 Hz,3H,major),1.14(t,J=2.0 Hz,2.7H,minor),2.05(br s,1.7H,major+minor),3.26 ~3.28(m,5.8H,major+minor),3.80(br s,0.8 H,minor),3.92 ~3.94(br s,1.95H,major+minor),4.13 ~4.17(m,2.5H,major+minor),4.60 ~4.61(m,1.75H,major+minor),6.83 ~6.87(m,1.7H,major+minor),7.01 ~7.06(m,1.8H,major+minor),7.14(d,J=3.6 Hz,1H,major),7.28(d,J=3.6 Hz,1H,major),7.37 ~7.50(m,3.8H,major+minor),8.03 ~8.07(m,1.8H,major+minor);13C NMR δ(major+minor):13.7,13.8,26.6,61.9,62.2,62.3,62.4,73.1,73.2,108.2,108.4,122.7,122.8,123.3,123.6,130.0,144.1,144.5,159.8,159.9,160.2,169.6,170.3,172.8,172.9,173.4;HR-ESI-MS m/z:Calcd for C14H16N2O5Na{[M+Na]+}315.095 7,found 315.095 4。
3d:1H NMR δ(major+minor):0.92(t,J=3.6 Hz,2.2H,minor),1.22(t,J=3.6 Hz,3H,major),1.76(br s,1.2H,major),3.47(br s,1H,major),3.94 ~ 3.96(m,1.3H,minor),4.06(br s,0.6H,minor),4.25 ~4.28(m,2H,major),4.72(d,J=2.0 Hz,0.6H,minor),4.83(d,J=2.0 Hz,1H,major),4.92 ~ 4.94(m,1.6H,major+minor),5.15 ~ 5.20(m,1.6H,major+minor),6.73(d,J=4.0 Hz,1H,major),6.78(d,J=4.0 Hz,0.6H,minor),7.00 ~7.03(m,1.6H,major+minor),7.10(d,J=4.0 Hz,1H,major),7.22 ~7.25(m,1.6H,major+minor),7.27 ~7.33(m,2.3H,major+minor),7.36 ~7.41(m,3.2H,major+minor),7.48(d,J=3.6 Hz,1.9H,major),7.57 ~7.58(d,J=4.0 Hz,1.2H,minor),8.00 ~8.02(m,3.7H,major+minor),8.20 ~8.22(m,1H,major),8.27 ~ 8.28(m,3H,major+minor);13C NMR δ(major+minor):13.6,13.9,44.5,44.7,62.9,63.6,73.0,73.3,109.5,109.8,122.8,123.0,123.4,123.7,124.6,124.7,125.0,127.2,127.6,127.7,128.5,128.7,128.8,130.1,135.3,138.2,138.4,143.7,144.3,149.8,163.7,163.8,169.8,170.8,173.5;HR-ESI-MS m/z:Calcd for C26H24N3O7Na{[M+Na]+}490.161 4,found 490.162 0。
3e:1H NMR δ(major+minor):0.96(t,J=3.6 Hz,3H,major),1.25(t,J=3.6 Hz,1.8H,minor),1.74(br s,0.7H,major),3.13(br s,0.6H,minor),3.24 ~3.26(m,4.7H,major+minor),3.82(br s,1H,major),3.90 ~3.94(m,2H,major),4.24 ~ 4.31(m,1.1H,major),4.58(d,J=2.4 Hz,0.9H,major),4.63(d,J=4.0 Hz,0.6H,minor),6.79(d,J=4.0 Hz,1H,major),6.82(d,J=4.0 Hz,0.6H,minor),6.92 ~6.96(m,2.2H,major+minor),7.04 ~7.11(m,3.3H,major+minor),7.19 ~7.27(m,2.7H,major+minor),7.36 ~ 7.38(m,1.6H,major+minor),7.86 ~7.89(m,1.5H,major+minor),8.35 ~8.38(m,1.6H,major+minor);13C NMR δ(major+minor):13.7,13.9,26.6,62.5,62.9,63.0,72.8,73.3,108.1,108.3,115.8,115.9,116.0,119.5,119.6,119.7,122.5,122.7,122.8,123.5,124.6,124.7,124.8,125.4,129.9,130.0,132.1,132.3,133.8,133.9,144.4,145.3,160.2,161.4,161.7,169.8,170.7,173.2,173.6;HR-ESI-MS m/z:Calcd for C20H19N2O5FNa{[M+Na]+}409.117 6,found 409.117 9。
3f:1H NMR δ(major+minor):0.98(t,J=3.6 Hz,3H,major),1.28(t,J=3.6 Hz,0.7H,minor),1.74(br s,0.7H,major),2.26(s,3.7H,major+minor),3.13(br s,0.2H,minor),3.89(br s,0.7H,major),3.98 ~4.01(m,2H,major),4.29 ~4.45(m,1.1H,major),4.69(s,0.9H,major),4.81(s,0.2H,minor),4.89(d,J=7.6 Hz,0.2H,minor),4.92(d,J=8.0 Hz,1H,major),5.14(d,J=8.0 Hz,1H,major),5.23(d,J=8.0 Hz,0.3H,minor),6.58(d,J=4.0 Hz,0.2H,minor),6.63(d,J=4.0 Hz,1H,major),6.84(s,0.2H,minor),6.99 ~7.00(m,1.2H,major+minor),7.11(s,0.9H,major),7.16 ~7.19(m,1.2H,major+minor),7.22 ~7.24(m,1.2H,major+minor),7.28 ~7.31(m,1.2H,major+minor),7.35 ~ 7.40(m,2.4H,major+minor),7.48 ~7.50(m,1.7H,major+minor),7.57(d,J=4.0 Hz,1.9H,major),8.04~8.06(m,1H,major),8.53 ~8.55(m,1.2H,major+minor);13C NMR δ(major+minor):13.7,21.0,44.7,62.7,73.0,73.4,109.0,109.3,115.8,115.9,116.0,119.8,119.9,123.7,124.2,124.6,124.7,124.8,127.2,127.3,127.5,127.7,128.6,129.0,130.1,130.2,131.9,132.4,132.5,133.7,133.8,133.9,135.7,141.3,142.1,160.3,161.5,161.7,161.8,170.0,170.8,173.4;HR-ESI-MS m/z:Calcd for C27H25N2O5FNa{[M+Na]+}499.164 5,found 499.165 3。
3g:1H NMR δ(major+minor):1.02 ~ 1.04(m,13.1H,major+minor),1.75(br s,0.5H,minor),2.30(m,3H,major),3.20(m,3H,major),3.40(br s,0.5H,minor),3.97 ~4.00(m,0.9H,major),4.11(s,1.1H,major+minor),4.46 ~4.97(m,1H,major),5.91 ~ 6.01(m,1H,major),6.67 ~6.70(m,1H,major),6.95(s,0.5H,minor),7.08 ~ 7.11(s,1.4H,major+minor);13C NMR δ(major+minor):13.8,21.1,26.4,26.5,28.0,62.2,62.5,65.5,73.4,73.7,80.5,107.7,108.0,124.1,124.3,129.9,130.0,132.2,132.3,141.6,142.0,153.7,170.0,170.2,173.7,174.4;HR-ESI-MS m/z:Calcd for C20H26N2O8Na{[M+Na]+}445.158 7,found 445.158 7。
3h:产率90%,d/r=80/20;1H NMR δ(major+minor):1.64(br s,0.3H,minor),1.87(s,3.2H,major+minor),3.74(d,J=8.0 Hz,1H,major),3.96(br s,1.2H,major+minor),4.22 ~4.24(m,0.2H,minor),4.54(s,1.1H,major),4.77 ~4.81(m,2.2H,major+minor),4.87 ~4.89(m,1.2H,major+minor),5.02 ~5.05(m,0.2H,minor),6.29(d,J=3.6 Hz,1H,major),6.84(t,J=3.8 Hz,1H,major),7.00 ~7.04(m,4H,major+minor),7.11 ~7.17(m,2.4H,major+minor),7.19 ~ 7.22(m,2.2H,major+minor),7.25 ~ 7.31(m,5.2H,major+minor);13C NMR δ(major+minor):22.6,43.8,62.6,65.5,68.2,68.3,73.5,109.5,109.6,122.6,123.2,125.4,127.1,127.3,128.6,128.7,128.8,128.9,129.0,129.1,129.4,130.9,134.0,135.4,143.2,168.9,169.8,173.2;HR-ESI-MS m/z:Calcd for C2626H24N2O5Na{[M+Na]+}467.158 3,found 467.158 7。
3i:1H NMR δ(major+minor):1.60(br s,0.1H,minor),4.36(br s,1H,major),4.68(s,0.1H,minor),4.74(s,1H,major),4.89 ~4.97(m,2H,major),5.05 ~5.12(m,0.2H,minor),6.63(d,J=5.2 Hz,1H,major),6.66(d,J=5.2 Hz,0.1H,minor),6.98 ~ 7.08(m,3.3H,major+minor),7.13(d,J=4.8 Hz,2H,major),7.18 ~7.38(m,9.9H,major+minor),7.46(s,1.1H,major+minor),7.99(s,1.1H,major+minor);13C NMR δ(major+minor):62.0,68.5,73.5,109.7,123.3,124.0,125.0,126.5,128.1,128.7,128.8,128.9,129.5,129.9,131.0,134.0,134.2,144.4,160.2,170.0,172.4;HR-ESI-MS m/z:Calcd for C24H20N2O5Na{[M+Na]+}439.127 0,found 439.127 5。
3j:1H NMR δ:1.92(s,3H),2.79(s,3H),4.04(br s,1H),4.60(s,1H),4.84(d,J=3.6 Hz,1H),4.94(d,J=3.6 Hz,1H),6.56(d,J=5.2 Hz,1H),6.97(m,1H),7.06 ~ 7.10(m,3H),7.21(d,J=4.8 Hz,1H),7.23 ~7.35(m,4H);13C NMR δ:22.7,26.1,62.7,68.4,73.2,108.5,122.6,123.4,125.4,128.7,128.9,129.1,129.7,133.9,144.1,169.2,170.0,173.2;HR-ESI-MS m/z:Calcd for C20H20N2O5Na{[M+Na]+}391.127 0,found 391.127 6。
3k:1H NMR δ(major+minor):1.97(s,3.3H,major+minor),2.22(s,3.3H,major+minor),3.76(d,J=10.8 Hz,1H),3.99(br s,1H,major),4.62(s,1.1H,major+minor),4.86 ~4.95(m,3.1H,major+minor),5.06 ~5.15(m,0.2H,minor),6.26(d,J=5.2 Hz,1H,major),6.43(d,J=5.2 Hz,0.1H,minor),6.51~6.70(m,0.4H,minor+minor),6.87 ~6.90(m,1.1H,major+minor),7.02(s,1H,major+minor),7.08(s,1H,major+minor),7.13(d,J=4.8 Hz,2.2H,major+minor),7.18 ~7.21(m,1.1H,major+minor),7.25 ~7.41(m,8.3H,major+minor),7.52 ~7.54(m,0.1H,minor),7.71 ~7.73(m,0.1H,minor);13C NMR δ(major+minor):21.2,22.8,43.8,62.9,68.4,73.6,109.6,124.1,125.4,127.2,127.3,127.4,128.7,128.8,128.9,129.0,129.1,129.2,130.0,131.0,132.3,134.1,135.6,140.8,169.1,170.0,173.3;HR-ESI-MS m/z:Calcd for C27H26N2O5Na{[M+Na]+}481.173 9,found 481.173 9。
3l:1H NMR δ(major+minor):1.64(br s,1.3H,major+minor),3.92 ~3.97(m,2H,major),4.22 ~ 4.24(m,0.3H,minor),4.67(s,1H,major),4.85 ~4.93(m,3.3H,major+minor),5.15 ~5.21(m,0.33H,major+minor),6.42(d,J=4.0 Hz,1H,major),6.86 ~ 6.89(m,1H,major),7.08~7.10(m,2.6H,major+minor),7.13(d,J=3.6 Hz,1H,major),7.16 ~7.18(m,1.3H,major+minor),7.24 ~7.33(m,8.5H,major+minor),7.81 ~7.90(m,3H,major),8.13(d,J=3.6 Hz,2H,major);13C NMR δ(major+minor):44.0,63.0,68.7,73.4,109.9,123.0,123.2,123.7,124.9,127.3,127.5,128.5,128.8,129.1,129.3,129.9,133.8,135.2,138.4,143.4,149.7,163.7,169.8,172.9;HR-ESI-MS m/z:Calcd for C31H25N3O7Na{[M+Na]+}574.159 0,found 574.159 4。
3m:1H NMR δ(major+minor):1.80(br s,1H,major),3.87(br s,1.5H,major+minor),4.73 ~4.80(m,1.5H,major+minor),4.90(d,J=6.0 Hz,1H,major),4.95(d,J=6.0 Hz,1H,major),5.03 ~5.28(m,3.7H,major+minor),6.62(d,J=4.0 Hz,0.9H,major),6.66(d,J=6.0 Hz,0.5H,minor),6.73(d,J=6.0 Hz,0.5H,minor),6.82(t,J=3.8 Hz,0.5H,minor),6.92 ~ 6.95(m,1H,major),7.00 ~7.04(m,3.2H,major+minor),7.07 ~7.17(m,7.3H,major+minor),7.20(t,J=3.6 Hz,1.5H,major+minor),7.24 ~7.38(m,13.4H,major+minor),7.42(d,J=2.4 Hz,2H,major),7.87 ~ 7.90(m,1.5H,major+minor),8.38 ~8.44(m,1.5H,major+minor);13C NMR δ(major+minor):62.8,67.7,68.6,73.2,73.6,109.5,109.6,115.9,116.0,123.1,123.2,123.7,124.6,124.7,125.2,126.5,126.9,127.9,128.6,128.7,128.8,128.9,130.0,132.2,132.3,133.7,133.8,133.9,134.3,134.4,144.6,160.2,161.4,161.9,170.1,170.6,172.6;HR-ESI-MS m/z:Calcd for C30H24N2O5FNa{[M+Na]+}511.166 9,found 511.166 7。
1.3 体外抗肿瘤活性测定
以阿霉素为阳性对照药,参考文献[1]方法,用MTT法测试3a~3h对人白血病细胞(K562)和人肺癌细胞(A549)的体外抗肿瘤活性。
2 结果与讨论
2.1 构效关系
底物取代基的位阻效应对反应的非对映选择性有一定影响,结果见Scheme 1。由Scheme 1可见,立体位阻较大的取代基有利于提高反应的非对映选择性。如氧原子为苄基取代时(3h~3m),非对映选择性明显高于氧原子为乙基取代(3a~3g)。
2.2 抗肿瘤活性
表1为3a~3m的抗肿瘤活性。由表1可见,3a~3m对A549和K562均有一定抑制活性,其中3f,3g和3h对K562具有较好的抑制活性;3b和3c对A549具有较好的抑制活性,它们均可作为先导化合物作进一步研究。

表1 3a~3m对K562和A549的抑制活性*Table 1 Inhibition rate of 3a~3m against K562 and A549
3 结论
合成了 13 个新型的 3-氨基-α-羟基-β-酯基-3-季碳氧化吲哚类化合物(3a~3m),收率63% ~97%。底物取代基对反应非对映选择性有一定影响。抗肿瘤活性实验表明:3d,3f和3m对K562具有较好的抑制活性;3f和3i对A549具有较好的抑制活性,均可作为先导化合物进一步研究其药理活性。
[1]杨俊,杨超,景德红,等.新型3-季碳胺甲基氧化吲哚类化合物的高效合成[J].合成化学,2015,23(6):495-498.
[2] 黄璇,郭丰敏,刘雄伟,等.新型3-季碳羟甲基(或3-季碳胺甲基)氧化吲哚的合成[J].合成化学,2014,22(4):499 -503.
[3]Wen T W,Ming B Z,Jin H L,et al.Synthesis of oxindoles by iron-catalyzed oxidative 1,2-alkylarylation of activated alkenes with an aryl C(sp2)-H bond and a C(sp3)-H bond adjacent to a heteroatom[J].Angew Chem Int Ed,2013,52:3638 -3641.
[4]Artur P,Yan X J,Jie P Z,et al.Palladium-catalyzed enantioselective domino Heck-cyanation sequence:Development and application to the total synthesis of esermethole and physostigmine[J].Chem Eur J,2007,13:961-967.
[5]Hamashima Y,Suzuki T,Takano H,et al.Catalytic enantioselective fluorination of oxindoles[J].J Am Chem Soc,2005,127:10164 -10165.
[6]Ishimaru T,Shibata N,Nagai J,et al.Lewis acidcatalyzed enantioselective hydroxylation reactions of oxindoles and β-keto esters using DBFOX ligand[J].J Am Chem Soc,2006,128:16488 -16489.
[7]Tomita D,Yamatsugu K,Kanai M,et al.Enantioselective synthesis of SM-130686 based on the development of asymmetric Cu(I)F catalysis to access 2-oxindoles containing a tetrasubstituted carbon[J].J Am Chem Soc,2009,131:6946 -6948.
[8]Trost B M,Zhang Y.Mo-catalyzed regio-,diastereoand enantioselective allylic alkylation of 3-aryloxindoles[J].J Am Chem Soc,2007,129:14548 -14549.
[9]Hills I D,Fu G C.Catalytic enantioselective synthesis of oxindoles and benzofuranones that bear a quaternary stereocenter[J].Angew Chem Int Ed,2003,42:3921 -3924.
[10]Shaw S A,Aleman P,Christy J,et al.Enantioselective TADMAP-catalyzed carboxyl migration reactions for the synthesis of stereogenic quaternary carbon[J].J Am Chem Soc,2006,128:925 -934.
[11]Ogawa S,Shibata N,Inagaki J,et al.Cinchona-al-kaloid-catalyzed enantioselective direct aldol-type reaction of oxindoles with ethyl trifluoropyruvate[J].Angew Chem Int Ed,2007,46:8666 -8669.
[12]Ishimaru T,Shibata N,Horikawa T,et al.Cinchona alkaloid catalyzed enantioselective fluorination of allyl silanes,silyl enol ethers and oxindoles[J].Angew Chem Int Ed,2008,47:4157 -4161.
[13]He R,Ding C,Maruoka K.Phosphonium salts as chiral phase-transfer catalysts:Asymmetric michael and mannich reactions of 3-aryloxindoles[J].Angew Chem Int Ed,2009,48:4559 -4561.
[14]Bui T,Candeias N R,Barbas C F III.Dimeric quinidine-catalyzed enantioselective aminooxygenation of oxindoles:An organocatalytic approach to 3-hydroxyoxindole derivatives[J].J Am Chem Soc,2010,132:5574 -5575.
[15]Trost B M,Frederiksen M U.Palladium-catalyzed asymmetric allylation of prochiral nucleophiles:Synthesis of 3-allyl-3-aryl oxindoles[J].Angew Chem Int Ed,2005,44:308 -310.
[16]Mosman T.Rapid colorimetril assay for cellular growth and survival:Application to proliferation and cytotoxicity assays[J].J Immunol Methods,1983,65:55-63.
