Adsorption and photocatalytic degradation of dye by N-doped TiO2/chitosan composited films
2015-04-01CHENJianxinZHANGNaLIYinhuiLIRuijuan
CHEN Jianxin, ZHANG Na, LI Yinhui, LI Ruijuan
(1School of Marine Science and Engineering,Hebei University of Technology,Tianjin300130,China;2School of Chemical Engineering,Hebei University of Technology,Tianjin300130,China)
Introduction
Chitosan (CS) is synthesized from the deacetylation of chitin. As an abundant natural biopolymer, it is widely applied in the field of waste water treatments[1-6],separation membranes[7], drug delivery systems[8-9]for its excellent film forming abilities, biodegradable,nontoxicity, water permeability, and high mechanicalstrength[10-12]. In addition, CS is soluble in acetic acid aqueous solution, and the viscous solution can be used to make functional films. It has been combined with various inorganic photo catalysts such as CdS[13]and ZnO[14], due to the unique adsorption and chelating properties.
Recent studies have shown that the TiO2and CS composite materials presented a versatile performance in heterogeneous photo catalysis technologies[15-17].All kinds of organic pollutants can be degraded and mineralizedviaTiO2photocatalytic[18]. However, the band gap of TiO2is 3.2 eV, which means that only ultraviolet light (hv<390 nm) can be utilized in sunlight[19]. In order to improve the utilization of solar energy, it is necessary for researchers to modify the semiconductor band gap. Asahi successfully demonstrated that N-doped TiO2can facilitate visible light absorption[20]. The reason that causes visible light absorption of N-doped TiO2has been carried out by numerous researchers[21-22]. In summary, the additional nitrogen atoms form an occupied mid-gap (N 2p) level above the TiO2(O 2p) valence band. This mid-gap acts as a step between the valence and the conduction band of TiO2, thence, the band gap of the semiconductor can be narrowed[23-24].
However, some shortcomings limit the application of nanosized TiO2powders, such as the tendency to aggregate in aqueous solution, the difficulty of separation from wastewater and the low ability of adsorption.Nevertheless, the adsorption-photocatalytic process was enhanced when TiO2combined with CS[25-27].Meantime, Nawi,et alconsider that CS in the bilayer TiO2/CS system can be oxidized to a chemically more stable and optically active form under irradiation of indoor fluorescent lamp without altering much of its polymeric structure[17,28]. At present, N-TiO2together with CS as a novel material in the wastewater treatment system draws a wide range of concern among people.
Moreover, some researchers have been explored the mechanism of dyes degradation under visible lights or solar lights. The electron hole (h+) and electron(e-) can be generated when TiO2was irradiated.Detailed mechanism of dye degradation under visible light irradiation is described in Fig.1[4,29].

Fig.1 Mechanism of dyes degradation under solar light
This paper introduced a mild sol-gel technique for the synthesis of N-doped TiO2nanoparticle. In order to overcome the defects of the powders, an organicinorganic nanocomposite film was deposited on transparent glass slide. The N-doped TiO2/CS composited films were characterized using conventional techniques.Methyl Orange (MO) was chosen as a model pollutant in the present study. Additionally, it is an environmental friendly system without the use of super oxidizing agents and high-energy light sources.
1 Experimental
1.1 Materials
CS with >85% of deacetylation degree and high molecular weight was supplied by Jinke Marine Biochemistry Co., Ltd., Zhejiang, China. Tetrabutyl titanate (TBOT, chemical grade) and methyl orange (AR)were all supplied by Guang Fu company in Tianjin,China. Urea (AR) was obtained from Tianjin Chemical Reagent No.1 Plant, China. Acetic acid and absolute ethanol were analytical reagent. All reagents were employed as received without further purification. Distilled water was used throughout the entire experiments.
1.2 Preparation of N-doped TiO2
Here in, the N-doped TiO2were prepared by sol-gel method using Tetrabutyl titanate (TBOT) as the precursor. Typically, TBOT (10 ml) was dissolved in the mixed solution of absolute ethanol (23 ml) and acetic acid (6.9 ml) and stirred vigorously for 20 min at room temperature. As the same time, 0.0265 g urea was dissolved in 8.5 ml distilled water, and then 11.4 ml absolute ethanol was added into the later solution until blended. Then, dropwise add the later solution into the former and stirred for 3 h in a thermostat water bath (30℃), a uniform sol was obtained. After gelling, the gel was dried in vacuum oven for 12 h at 65℃. The samples were calcined for 3 h at 500℃with a heating rate of 2℃·min-1. At the same time,pure TiO2was prepared with the same method without urea. The production TiO2, N-TiO2powders were grinded for further photocatalysis and characterization.
1.3 Preparation of N-TiO2/CS composited film
The schematically of the preparation of N-doped TiO2/CS film was represented in Fig.2. CS solution was prepared by dissolving 2.04 g CS flakes in 100 g 1% acetic acid solution at room temperature. The viscous solution was stirred continuously for 12 h to fully dissolve the chitosan flakes. Then, 0.1 g of N-doped TiO2powder was added into 0.5 g of the bubbles-free viscous CS solution, then ultrasonic dispersion for 20 min. The slurry solution was casted onto glass slides,each of dimensions 2.5 cm×7.6 cm. It was then heated in an oven for 12 h at 80℃ to remove the acetic acid. In addition, pure TiO2/CS and CS films were prepared using the same method.

Fig.2 Schematic mechanism for preparation of N-doped TiO2/CS film
The average of the optimum thickness of N-doped TiO2/CS was determined by SEM analysis to be (80±2) μm. This was obtained by casting (0.11±0.01)g·cm-2of N-doped TiO2/CS onto glass plate.
1.4 Characterization of N-TiO2/CS composite films
The XRD patterns of the samples were obtained using a Bruker D8 FOCUS X-ray diffractometer with CuKαin the 2θrange from 10° to 80°. SEM analysis was performed with a Nova NanoSEM 430. FT-IR spectra of the films were taken with a Fourier-transform infrared spectrophotometer (BRUKER TENSOR 27).Pressed pellets were prepared by grinding the powder specimens with spectroscopic grade KBr for FT-IR spectra test. The spectra were recorded from 4000 to 400 cm-1.
1.5 Adsorption of MO
The N-doped TiO2/CS composited film and CS film were immersed into 50 ml MO solution (10 mg·L-1). The adsorption system was continuously stirred in dark. The concentration of MO was measured on a 722 visible spectrophotometer (Shanghai precision & scientific instrument Co., Ltd). The amount of MO adsorbed was calculated according to Eq. (1)[30]

Whereqtis adsorption capacity, mg·g-1;C0andCtare the initial and instantaneous concentrations of MO, mg·L-1, respectively,Vis the volume of the solution, L;mis the mass of photocatalyst used, g.
1.6 Photocatalytic experiment
The photocatalytic activities of the TiO2/CS,N-doped TiO2/CS composited films and CS films were estimated by photocatalytic decolorization of MO with a 150 W halogen lamp as a visible light resource. The distance between reactor and light source was set about 15 cm. In the photocatalytic experiments, 0.11 g of photocatalyst was added to 50 ml of MO aqueous solution (10 mg·L-1). The solution was continuously stirred as the photocatalytic decolorization progress.The residual concentration of MO were analyzed on a 722 visible spectrophotometer atλmax=464 nm.What’s more, the recovering experiments by N-doped TiO2/CS composited films have been investigated systematically. The degradation ratio (D) of MO was calculated according to Eq. (2)[30]

WhereC0andCiare the initial and subsequent solution concentrations, mg·L-1;A0andAiare the absorbance of solution that correspond withC0,Ci.
2 Results and discussion
2.1 XRD analysis of N-doped TiO2/CS film
The phase structures of the samples were investigated by XRD method and the results are shown in Fig.3. The peak at 2θ=20° in the XRD pattern of pure CS film was the typical characteristic peak for CS[31].It can be seen that both the N-doped TiO2and theN-doped TiO2/CS film exhibit only the characteristic peaks of anatase phase (the strongest peak at 2θ=25.31°,37.89°, 48.09°, 55.12°, 62.89° and 68.78°, JCPDS 21-1272).

Fig.3 XRD patterns of pure CS film (a), N-doped TiO2nanoparticle (b) and N-doped TiO2/CS film (c)
No peak ascribed to CS can be found in respect the CS content of the N-doped TiO2/CS film is below the detection limits of the XRD instrument. The average crystallite sizes of the samples can be calculated by applying the Scherrer formula on the anatase (101)diffraction peaks[32]

Wheredis the crystalline size;λis the wavelength of X-ray radiation (0.1541 nm);βis the peak width at half-maximum height (PWHMH);Kis the constant usually taken as 0.89. The only parameter determines the crystal size in the equation is the peak width. The crystalline size of N-doped TiO2that calculated by Eq. (3) was approximately 20 nm.
2.2 Surface studies
2.2.1Surface morphology of N-doped TiO2/CS filmThe SEM images of samples are shown in Fig.4. Fig.4(a) shows the surface morphology of pure CS film. It can be seen that the surface of the CS film was rather smooth, and the result is the same when the sample was amplified to 100000 times. However, the surface of the N-TiO2/CS composite film was relatively coarse with the introduction of N-doped TiO2. Obviously,many small particles had covered up the whole surface area and embedded in the cross-section area of N-doped TiO2/CS, which are speculated to be N-doped TiO2nanocrystal [Fig.4 (b)]. These result suggested that N-doped TiO2particles and CS were distributed compactly and well mixed.

Fig.4 SEM images of pure CS film (a), and N-doped TiO2/CS composite film (b)
2.2.2Color change of N-doped TiO2/CS filmFig.5(a) and (b) shows the color changes of unirradiated N-doped TiO2/CS thin film and irradiated N-doped TiO2/CS thin film, respectively. As can be seen, the color of irradiated N-doped TiO2/CS thin film obviously changed to a nigger-brown. This phenomenon also occurs in the TiO2-CS-Glass layer self-assembly system, which is attributed to the formation of the carbonyl chromophore group in the chemical structure of the photocatalytically oxidized CS. Furthermore,this result is consistent with previous studies[33].

Fig.5 Photographs of unirradiated N-doped TiO2/CS composite film (a) and irradiated N-doped TiO2/CS film (b)
2.3 FT-IR spectral analysis

Fig.6 FT-IR spectra of pure N-doped TiO2particles (a),unirradiated pure CS film (b), unirradiated N-doped TiO2/CS composited film (c) and irradiated N-doped TiO2/CS composited film (d)
Fig.6 shows the FT-IR spectra of unirradiated pure CS film, pure N-doped TiO2particles, unirradiated N-doped TiO2/CS composited film and irradiated N-doped TiO2/CS composited film. As it can be seen from the spectrum of (b), the characteristic bands of CS polysaccharide appeared at 3440 cm-1is the overlapping of the stretching vibration of -OH and -NH2[34-35].Characteristic band at 1637 cm-1corresponds to C O stretching of carbonyl group[35]. Meanwhile, adsorption band at 3440, 2854 and 1410 cm-1are attributed to the banding of hydroxyl groups, and the existence of TiO2-OH groups can be proved by hydroxyl band at 2852 cm-1[36]. Moreover, the characteristic band of TiO2is shown at 655 cm-1[36]. Absorption band for both the N-doped TiO2/CS composited films shows an intrigue characteristic of both CS and TiO2.
With the addition of N-doped TiO2nanoparticles,compared to chitosan the spectrums of (c) and (d) exhibited additional band 450 cm-1and 760 cm-1. This is because the envelope of phonon bands of Ti-O-Ti in CS matrix[36]. The result of spectrum (d) indicated that there is no obvious chemical structure modification of the irradiated film, except for the carbonyl band of the chitosan spectrum has shifted to 1642 cm-1which is ascribed to the photo-oxidation. The same FT-IR characterization results were detected by compliment experiments when pure CS films were subjected to the same irradiation condition of the photocatalytic experiment. The carbonyl band of the CS spectrum has shifted from 1637 to 1642 cm-1. In addition, the bands at 1077 cm-1and 1092 cm-1associated to the hydrogen bonding of Ti O with hydroxyl and amino groups of CS. Hence, hydrogen bonds and coordination bonds link Ti-O Ti inorganic network to CS macromolecules[36].
2.4 Adsorption of MO
According to previous researches, the degraded substrates are firstly adsorbed or transferred to the photocatalyst surface[37]. In order to detect the adsorption behaviors of the composited films to MO, dark adsorption experiments were performed.
The comparison between the adsorption of MO on CS film and N-doped TiO2/CS film is shown in Fig.7. The CS film and N-doped TiO2/CS film were immersed directly in 50 ml MO solution with 10 mg·L-1for 4.5 h, respectively. MO was adsorbed slightly better on CS than on N-doped TiO2/CS film during the first hour. This is because a portion of the adsorption active sites of CS were occupied by N-doped TiO2particles. However, the adsorption rate of N-doped TiO2/CS film was higher than CS about an hour later, which can also be ascribed to the presence of the N-doped TiO2particles. Since the addition of N-doped TiO2particles can enhance the roughness of the film, so the MO molecules can diffuse into the inner of the film.

Fig.7 Adsorption capacity of pure chitosan film and composited film for uptake of MO at ambient pH ~7.0
2.5 Photocatalytic activity and reusability of N-doped TiO2/CS film
Adsorption study of MO on N-doped TiO2/CS film was adopted as a probe to study the photocatalytic activity and reusability of N-doped TiO2/CS film.Consequently, photocatalytic experiments were carried out as previously mentioned in Experimental 1.6. The photocatalytic activities of TiO2/CS film,N-TiO2/CS film and CS film were compared for the degradation of MO dye and the photocatalytic activities of fresh and irradiated film for one cycle, two cycles and three cycles were also compared for the degradation of MOdye subsequently.
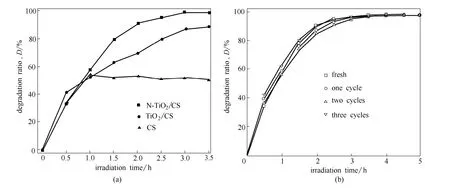
Fig.8 Degradation of MO by TiO2/CS, N-TiO2/CS and CS (a) and reusability of N-doped TiO2/CS film (b)(Under 150 W halogen lamp, [MO]0=10 mg·L-1, photocatalyst dosage:2 g·L-1)
Compared with the TiO2/CS film and CS film,N-doped TiO2/CS film has the best photocatalytic property, that was shown in Fig.8 (a). As can be seen from Fig.8 (b), fresh N-doped TiO2/CS film reached equilibrium at 3.0 h for the degradation of MO, the irradiated film for one cycle and two cycles reached equilibrium at 3.5 h. Besides, the irradiated film has a higher MO degradation ratio than the fresh one at the beginning of the experiments.
This is because the partial degradation of CS on the surface of N-doped TiO2, which means that more N-doped TiO2nanoparticle were exposed on the surface of the film. Furthermore, the degradation ratios were 99% and reached equilibrium after irradiated for 3.5 h, no matter to the fresh or irradiated film.
3 Conclusion
By a simple assemblage of N-doped TiO2/CS system, the photocatalytic decolorization of MO solution reached 99% after irradiated for 3.5 h. The N-doped TiO2/CS film exhibited enhanced photocatalytic activity under the simulated solar light irradiation compared with reported photocatalysts. The N-doped TiO2/CS film could be used repeatedly and the photocatalytic activity of the photocatalyst was found to maintain at 99% of initial decolorization rate after four batch reactions. The prepared N-doped TiO2/CS film may be promising photocatalytic material for decolorization treatment of dye-containing waste water. Furthermore, the visual color changed to a more intense brown was observed. The characterizations results of FT-IR and optical properties indicated that the photocatalytic-oxidation of CS took place without altering much of the overall polymeric structure of CS.The experimental results show that the N-doped TiO2/CS film can be considered as a convenient and environmentally friendly method for green chemistry application.
[1] Kong M, Chen X, Liu C, Liu C, Meng X, Yu L. Bactericidal mechanism of CS microspheres in a solid dispersing system againstE.coli[J].Colloid Surface B, 2008, 65 (2):197-202.
[2] Laus R, Fávere V T de. Competitive adsorption of Cu (Ⅱ) and Cd (Ⅱ)ions by chitosan crosslinked with epichlorohydrin-triphosphate [J].Bioresource Technology, 2011, 102 (19):8769-8776.
[3] Kushwaha S, Sudhakar P P. Adsorption of mercury (Ⅱ), methyl mercury (Ⅱ) and phenyl mercury (Ⅱ) on chitosan cross-linked with a barbital derivative [J].Carbohydrate Polymers, 2011, 86 (2):1055-1062.
[4] Zainal Z, Hui L K, Hussein M Z. Characterization of TiO2-chitosan/glass photocatalyst for the removal of a monoazo dyeviaphotodegradation-adsorption process [J].Journal of Hazardous Materials,2011, 164 (1):138-145.
[5] Ngah W S W, Fatinathan S, Yosop N A. Isotherm and kinetic studies on the adsorption of humic acid onto chitosan-H2SO4beads [J].Desalination, 2011, 272 (1-3):293-300.
[6] Huang X Y, Bu H T, Jiang G B, Zeng M H. Cross-linked succinyl chitosan as an adsorbent for the removal of Methylene Blue from aqueous solution [J].International Journal of Biological Macromolecules, 2011, 49 (4):643-651.
[7] Liu L F, Zhang P H, Yang F L. Adsorptive removal of 2,4-DCP fromwater by fresh or regenerated chitosan/ACF/TiO2membrane [J].Separation and Purification Technology, 2011, 70 (3):354-361.
[8] Kumari A, Yadav S K, Yadav S C. Biodegradable polymeric nanoparticles based drug delivery systems [J].Colloid Surface B, 2010, 75 (1):1-18.
[9] Wang H J, Zhao P Q, Liang X F, Gong X Q, Song T, Niu R F, Chang J. Folate-PEG coated cationic modified chitosan-Cholesterol liposomes for tumor-targeted drug delivery [J].Biomaterials, 2010, 31(14):4129-4138.
[10] Wan Ngaha W S, Teong L C, Hanafiah M A K M. Adsorption of dyes and heavy metal ions by chitosan composites [J].Carbohydrate Polymers, 2011, 83 (4):1446-1456.
[11] Mucha M, Wankowicz K, Balcerzak J. Analysis of water adsorption on chitosan and its blends with hydroxyl propyl cellulose [J].E-Polymers, 2007, 16:1-10.
[12] Lu B W, Chen W C. A disposable glucose biosensor based on drop-coating of screen-printed carbon electrodes with magnetic nanoparticles [J].Journal of Magnetism and Magnetic Materials,2006, 304 (1):e400-e402.
[13] Akpan U G, Hameed B H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts [J].Journal of Hazardous Materials, 2009, 170 (2-3):520-529.
[14] Wang X J, Wu Z, Wang Y. Adsorption–photodegradation of humic acid in water by using ZnO coupled TiO2/bamboo charcoal under visible light irradiation [J].Journal of Hazardous Materials, 2013,262:16-24.
[15] Nawi M A, Sabar S, Sheilatina. Photocatalytic decolourisation of Reactive Red 4 dye by an immobilized TiO2/chitosan layer by layer system [J].Journal of Colloid and Interface Science, 2012, 372 (1):80-87.
[16] Qian T T, Su H J, Tan T W. The bactericidal and mildew-proof activity of a TiO2-chitosan composite [J].Journal of Photochemistry Photobiology A, 2011, 218 (1):130-136.
[17] Nawi M A, Jawad A H, Sabar S, Ngah W S W. Photocatalytic-oxidation of solid state chitosan by immobilized bilayer assembly of TiO2-chitosan under a compact household fluorescent lamp irradiation [J].Carbohydrate Polymers, 2011, 83 (3):1146-1152.
[18] Akpan U G, Hameed B H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts [J].Journal of Hazardous Materials, 2009, 170 (2-3):520-529.
[19] Nolana N T, Synnott D W, Seery M K. Effect of N-doping on the photocatalytic activity of sol-gel TiO2[J].Journal of Hazardous Materials, 2012, 211-212:88-94.
[20] Asahi R, Morikawa T, Oikawa K. Visible-light photocatalysis in nitrogen-doped titanium oxides [J].Science, 2001, 293 (5528):269-271.
[21] Emeline A V, Kuznetsov V N, Rybchuk V K, Serpone N. Visible-light-active titania photocatalysts:the case of N-doped TiO2-properties and some fundamental issues [J].International Journal of Photoenergy, 2008, 258:1-19.
[22] Spadavecchia F, Cappelletti G, Ardizzone S, Ceotto M, Falciola L.Electronic structure of pure and N-doped TiO2nanocrystals by electrochemical experiments and first principal calculations [J].The Journal of Physical Chemistry C, 2011, 115:6381-6391.
[23] Irie H, Watanabe Y, Hashimoto K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNxpowders [J].The Journal of Physical Chemistry B, 2003, 107 (23):5483-5486.
[24] Nakamura R, Tanaka T, Nakoto Y. Mechanism for visible light responses in anodic photocurrents at N-doped TiO2film electrodes [J].The Journal of Physical Chemistry B, 2004, 108 (30):10617-10620.
[25] Zainal Z, Hui L K, Hussein M Z, Abdullah A H, Hamadneh I R.Characterization of TiO2-chitosan/glass photocatalyst for the removal of a monoazo dyeviaphotodegradation-adsorption process [J].Journal of Hazardous Materials, 2009, 164 (1):138-145.
[26] Miller S M, Zimmerman J B. Novel, bio-based, photoactive arsenic sorbent:TiO2-impregnated chitosan bead [J].Water Research, 2009,44 (19):5722-5729.
[27] Miller S M, Spaulding M L, Zimmerman J B. Optimization of capacity and kinetics for a novel bio-based arsenic sorbent, TiO2-impregnated chitosan bead [J].Water Research, 2011, 45 (17):5745-5754.
[28] Jawad A H, Nawi M A. Oxidation of crosslinked chitosan-epichlorohydrine film and its application with TiO2for phenolremoval [J].Carbohydrate Polymers, 2012, 90 (1):87-94.
[29] Blake D M, Maness P C, Huang Z. Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cell [J].Separation and Purification Technology,1999, 28 (1):1-50.
[30] Zhu H Y, Jiang R, Fu Y Q. Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation [J].Desalination, 2012, 286 (1):41-48.
[31] Fakhreia A A, Enas I I, Khaled D K. Crystallinity, antimicrobial activity and dyeing properties [J].European Polymer Journal, 2014, 58:164-172.
[32] Khanna P K, Singh N, Charan S. Synthesis of nano-particles of anatase-TiO2and preparation of its optically transparent film in PVA [J].Material Letter, 2007, 61 (25):4725- 4730.
[33] Zainol I, Akil H M, Mastor A. Effect of γ-irradiation on the physical and chemical properties of chitosan powder [J].Materials Science and Engineering:C, 2009,29 (1):292-297.
[34] Chao A C, Shyu S S, Lin Y C, Mi F L. Enzymatic grafting of carboxyl groups on to chitosan-to confer on chitosan the property of a cationic dye adsorbent [J].Bioresource Technology, 2004, 91 (2):157-162.
[35] Monteiro Jr O A C, Airoldi C. Some thermodynamic data on copper-chitin and copper-chitosan biopolymer interactions [J].Journal of Colloid Interface Science, 1999, 212 (2):212-219.
[36] Khan R, Dhayal M. Electrochemical studies of novel chitosan/TiO2bioactive electrode for biosensing application [J].Electrochemistry Communications, 2008, 10 (2):263-267.
[37] Tang C, Chen V. The photocatalytic degradation of reactive black 5 using TiO2/UV in an annular photoreactor [J].Water Research, 2004,38 (11):2775-2781.
