类Salen和β-二酮钆(III)配合物的合成和晶体结构
2015-03-19邹晓艳闫鹏飞董艳萍李光明
邹晓艳,闫鹏飞,董艳萍,李光明,*
(黑龙江大学a.《黑龙江大学工程学报》编辑部;b.功能无机材料化学省部共建教育部重点实验室;c.化学化工与材料学院,哈尔滨150080)
1 Introduction
Lanthanide complexes constructed from multidentate ligands are of considerable interest because of their unusual luminescent and magnetic[1-10].The well-known multidentate ligands,salen type are able to stabilize different metals in various coordination environments although their structures are often influenced by a variety of factors such as the radii of lanthanide ions,the structure of the ligands and the lanthanide counterions[11-15].In recent years,β-diketone ligand have also been employed to study the magnetic anisotropy due to their stable bidentate chelating modes coordinating to lanthanide ions and providing suitable ligand fields[16].Tang et al have reported a acetylacetonate(acac)complex[Dy(dppz)(acac)3]·CH3OH which the metal ions exhibit Ising-type ground states with a local symmetry of D4dand behave as SIM with anisotropy barriers enhanced reach up to 187K[17].
In view of the recent important progress on the structure,luminescence and magnetic of salen type andβ-diketones lanthanide complexes as well as our long-standing research on this domain[18-19],the rigid hexadentate salen-type ligand of N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine and acetylacetonate were employed to develop salen-type andβ-diketonate lanthanide complexes.As a result,a salen-type andβ-diketonate dinuclear lanthanide complex,namely,[Gd2L(acac)4(CH3OH)]4·3CH2Cl2(H2L=N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine;acac=acetylacetonate)has been synthesized.Crystal structure of the complex has been determined.
2 Experimental Section
2.1 Materials and Instrumentation
All chemicals and solvents except Gd(acac)3· H2O and H2L were obtained from commercial sources and used without further purification.The salen-type ligand H2L(Scheme 1)was prepared according to the literature and lanthanide precursors[20].Gd(acac)3·H2O was prepared according to a literature procedure previously described[21].Elemental(C,H and N)analyses were performed on a Perkin-Elmer 2400analyzer.FT-IR data were collected on a Perkin-Elmer 100spectrophotometer by using KBr disks in the range of 4 000~500 cm-1.UV spectra(in methanol)were recorded on a Perkin-Elmer 35spectrophotometer.Thermal analyses were carried out on a STA-6000with a heating rate of 10℃min-1in a temperature range from 30℃to 800℃in atmosphere.
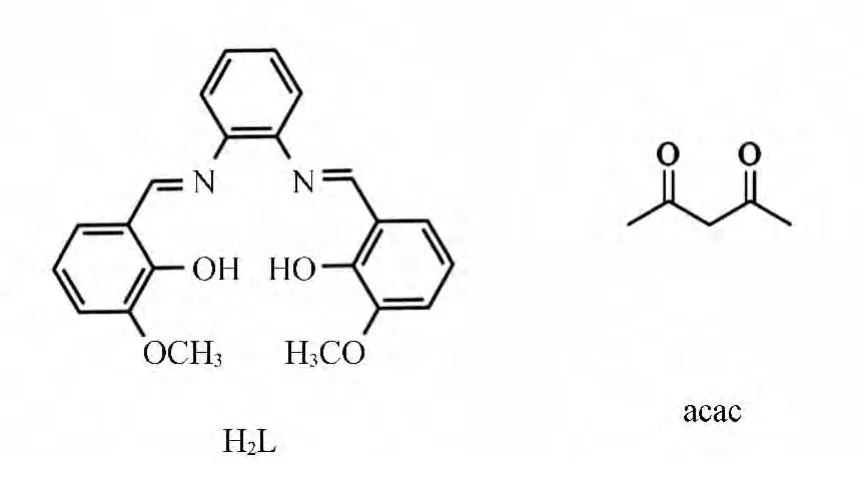
Scheme 1 Representation of the hexadentate salen-type and acac ligands
2.2 Crystallography
Single-crystal X-ray data of complex 1 was collected on a Rigaku R-AXIS RAPID imaging plate diffractometer with graphite-monochromated Mo Kα(λ=0.710 73Å)at 293K.Empirical absorption corrections based on equivalent reflections were applied.The structure of complex 1 was solved by direct methods and refined by full-matrix least-squares methods on F2using SHELXS-97 crystallographic software package[22].All non-hydrogen atoms are anisotropically refined.All crystal data and structure refinement details for complex 1 was summarized in Table 1.Contain the supplementary crystallographic data for complex 1 respectively.These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.

Table 1 Crystal data and structures refinement for complex 1
2.3 Synthesis of complex 1
A solution of Gd(acac)3·H2O(1.0mmol)in CH3OH(10mL)was added to a solution of H2L(0.5mmol)in CH2Cl2(25mL).The mixed solution was stirred for 4hat room temperature,and the filtrate was stored in the refrigerator to crystallize at low temperature(278K).Yellow crystal,suitable for single-crystal X-ray diffraction analysis was obtained after 2days.
[Gd2L(acac)4(CH3OH)]4·3CH2Cl2(1)Yield:0.432g(73.7%);Elemental analysis(%)calcd for C179H188N8O48Cl6Gd8(4 690.07):C,43.84;H,4.04;N,2.39;found C,43.40;H,4.28;N,2.33;IR(KBr,cm-1):3 421(s),2 957(w),1 654(s),1 648(s),1 616(s),1 521(s),1 471(m),1 439(m),1 198(w),756(w).UV-vis[MeOH,λ]:236,291nm.
3 Results and discussion
3.1 Spectral analysis
Infrared spectra of the ligand and complex 1 are showed in Fig.1,In a typical spectrum of com-plex 1,the broad weak O-H stretching vibration at 3 414cm-1disappeared,while a weak and broad band at about 3 421cm-1is newly generated from the N-H vibration.The strong v(C=N)bands occurring in the range of 1 648~1 654cm-1for complex 1 shifts to higher wavenumber in comparison with that for free H2L(1 636cm-1),due to the coordination of ionized OH groups,which reduces the strengthening of C=N groups.The UVVis spectra of the ligand and complex 1 are recorded in MeOH solution(Fig.1,right).For ligand,the typical absorptions at 215,240and 309nm are attributed to theπ-π*transition of the aromatic ring and azomethine chromophore.For complex1,the similar ligand-centered solution absorption bands(230,263,336nm)are observed and redshifted as compared to those(215,240and 309 nm)for ligand resulting from the changes in the energy levels of the ligand orbitals upon the coordination of the Gd(III)ions.
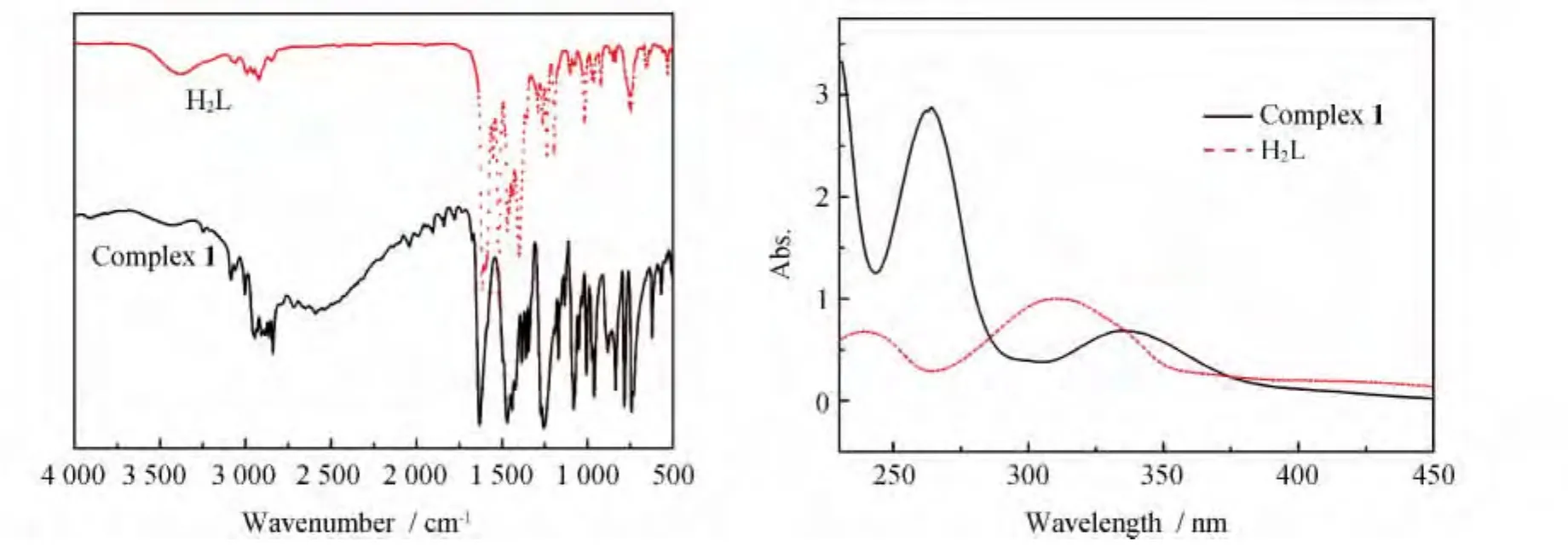
Fig.1 IR spectra of the H2L and complex 1(left);UV absorption spectra of the H2L and complex 1(right)
3.2 TG-DSC analysis
TG-DSC analysis of complex 1 is showed in Fig.2.In a typical curve of complex 1 exhibits a gradual weight loss of 5.96%in the range of 33~217℃,which corresponds to the loss of three dichloromethane molecules(calcd 5.44%).TG-DSC data further confirm that the crystalline dichloromethane molecules exist in complex 1.
3.3 PXRD analysis
Powder X-ray diffraction(PXRD)patterns of complex 1is in agreement with the simulated ones(Fig.3).PXRD analysis further demonstrates that the crystal structure of complex 1 is truely representative of the bulk materials.The differences in intensity are due to the preferred orientation of the powder samples.
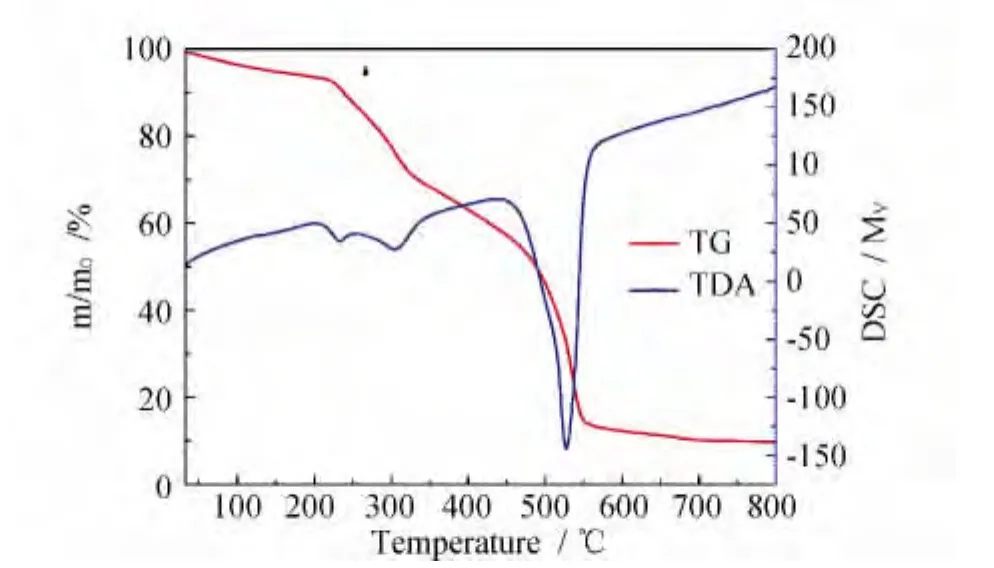
Fig.2 TG-DSC curves of complex 1
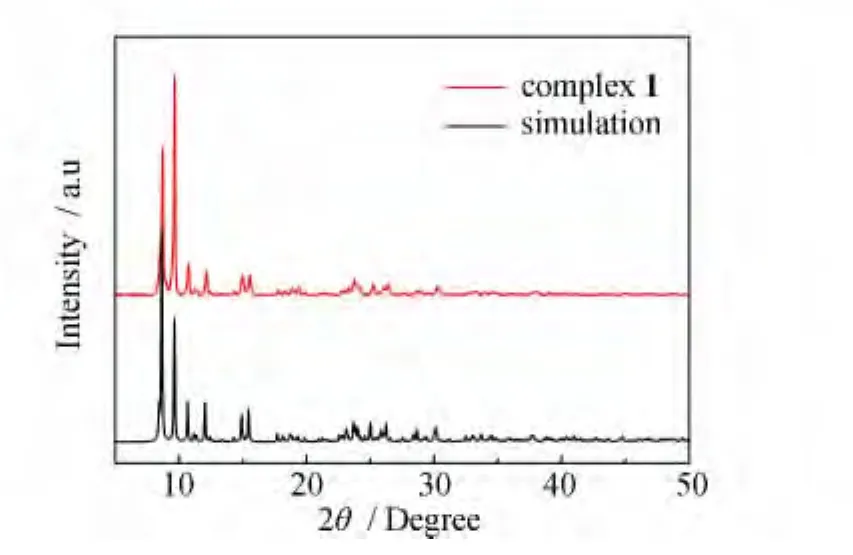
Fig.3 The powder X-ray diffraction patterns and the simulated patterns of complex 1
3.4 Structural descriptions of complex 1
X-ray crystallographic analysis reveals that complex 1 show a similar dinuclear core structure in which the positive charges of two Gd(III)cations are balanced by one L2-and four acac-.Complex 1 crystallizes in the monoclinic space group P21/c and as shown in Fig.4,complex 1 consist of four types of dinuclear lanthanide clusters.The Gd1(III)ion displays a eight-coordinate and is bonded to six oxygen atoms(four from the two top acac ligands and two from the phenolic oxygen of the salen-type ligand)and two nitrogen atoms(from the salen-type ligand)to form a square antiprism geometry.The Gd3(III)ion also displays a eight-coordinate and is bonded to eight oxygen atoms(two oxygen atoms from the phenolic oxygen of the salen-type ligand,four oxygen atoms from the two bottom acac ligands,and two oxygen atom from a methanol molecule)to form a distorted square antiprism geometry geometry(Fig.5).The Gd1(III)and Gd3(III)ions are bridged by the phenolic O19and O22atoms forming a rhombus{Gd1O19Gd3O22}core and the average Gd-O-Gd angle of 110.59,careful inspection of the packing arrangement reveals the closest intermolecular Gd1……Gd3distance of 7.961Å(Fig.4),which is similar to the tetra-dentate salen type dinuclear complex[Dy2(L4)2(acac)2(H2O)]·2CH2Cl2(H2L4=N,N′-bis(salicylidene)-1,2-cyclohexaendiamine,acac=acetylacetone)7cin which the two Dy(III)ions are bridged by two phenol oxygen atoms with a distance of 3.84Å[20].
4 Conclusions
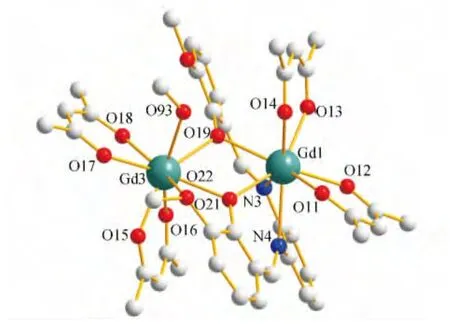
Fig.4 Molecular structure of complex 1.(Hydrogen atoms and solvent molecules are omitted)
Isolation of complex 1 demonstrates that syn-thesis of salen type dinuclear complex with rigid salen-type(H2L=N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine)andβ-diketonate(acac=acetylacetonate)ligands are possible,and the structure of the salen type ligand dominate the structures of the complexes and the coordination geometries of the Gd(III)ions.Complex 1is analogous possessing two unique lanthanide ions with same coordination geometries according to the microanalysis,TG-DSC and crystal analysis.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China(No.51402092);University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province(UNPYSCT-2015113).
Reference:
[1]Bogani L,Wernsdorfer W.Molecular spintronics using single-molecule magnets[J].Nature materials,2008,7(3):179-186.
[2]Sorace L,Benelli C,Gatteschi D.Lanthanides in molecular magnetism:old tools in a new field[J].Chemical Society Reviews,2011,40(6):3 092-3 104.
[3]Woodruff D N,Winpenny R E,Layfield R A.Lanthanide single-molecule magnets[J].Chemical reviews,2013,113(7):5 110-5 148.
[4]Yamanouchi M,Chiba D,Matsukura F.Current-induced domain-wall switching in a ferromagnetic semiconductor structure[J].Nature,2004,428(6 982):539-542.
[5]Liu Tianqi,Yan Pengfei,Luan Fang,et al.Near-IR luminescence and field-induced single molecule magnet of four salen-type ytterbium complexes[J].Inorganic Chemistry,2015,54,221-228.
[6]Ishikawa N,Sugita M,Ishikawa T,et al.Lanthanide double-decker complexes functioning as magnets at the single-molecular level[J].Journal of the American Chemical Society,2003,125(29):8 694-8 695.
[7]Zhao L,Wu J,Ke H,et al.Three dinuclear lanthanide(iii)compounds of a polydentate Schiff base ligand:Slow magnetic relaxation behaviour of the DyIII derivative[J].CrystEngComm,2013,15(26):5 301-5 309.
[8]AlDamen M A,Clemente-Juan J M,Coronado E.Mononuclear lanthanide single-molecule magnets based on polyoxometalates[J].Journal of the American Chemical Society,2008,130(28):8 874-8 875.
[9]AlDamen M A,Cardona-Serra S,Clemente-Juan J M,et al.Mononuclear lanthanide single molecule magnets based on the polyoxometalates[Ln(W5O18)2]9and[Ln(β2-SiW11O39)2]13-(LnIII=Tb,Dy,Ho,Er,Tm,and Yb)[J].Inorganic Chemistry,2009,48(8):3 467-3 479.
[10]Guo Y N,Chen X H,Xue S,et al.Modulating magnetic dynamics of three Dy2complexes through keto-enol tautomerism of the o-vanillin picolinoylhydrazone ligand[J].Inorganic Chemistry,2011,50(19):9 705-9 713.
[11]Jiang S D,Wang B W,Su G,et al.A mononuclear dysprosium complex featuring single-molecule-magnet behavior[J].Angew Chem Int Ed Engl,2010,49(41):7 448-7 451.
[12]Long J,Habib F,Lin P H,et al.Single-molecule magnet behavior for an antiferromagnetically superexchange-coupled dinuclear dysprosium(III)complex[J].Journal of the American Chemical Society,2011, 133(14):5 319-5 319.
[13]Shen S,Xue S,Lin S Y,et al.A triangular dysprosium with asymmetric central caps featuring ferromagnetic coupling and single-molecule magnet behaviour[J].Dalton Transactions,2013,42(29):10 413-10 416.
[14]Zou H H,Wang R,Chen Z L,et al.Series of edgesharing bi-triangle Ln4clusters with a mu4-NO3-bridge:syntheses,structures,luminescence,and the SMM behavior of the Dy4analogue[J].Dalton Transactions,2014,43(6):2 581-2 587.
[15]Zou xiaoyan,Yan Pengfei,Liu tianqi,et al.Synthesis and crystal structure of N,N′-bis(2-hydroxy-3-methoxybenzylidene)-1,3-propanediamine trinuclear chloride lanthanide complexes[J].Journal of Engineering of Heilongjiang University,2013,4(3):29-34.
[16]Bernot K,Luzon J,Bogani L,et al.Magnetic anisotropy of dysprosium(III)in a low-symmetry environment:a theoretical and experimental investigation[J].Journal of the American Chemical Society,2009,131(15):5 573-5 579.
[17]Chen G J,Guo Y N,Tian J L,et al.Enhancing anisotropy barriers of dysprosium(III)single-ion magnets[J].Chemistry,2012,18(9):2 484-2 487.
[18]Lin P H,Sun W B,Yu M F,et al.An unsymmetrical coordination environment leading to two slow relaxation modes in a Dy2single-molecule magnet[J].Chem Commun(Camb),2011,47(39):10 993-10 995.
[19]Sun W B,Han B L,Lin P H,et al.Series of dinuclear and tetranuclear lanthanide clusters encapsulated by salen-type andβ-diketionate ligands:single-molecule magnet and fluorescence properties[J].Dalton Transactions,2013,42(37):13 397-13 403.
[20]Koner R,Lee G H,Wang Y,et al.Two new diphenoxo-bridged discrete dinuclear CuIIGdIII compounds with cyclic diimino moieties:syntheses,structures,and magnetic properties[J].European Journal of Inorganic Chemistry,2005,2005(8):1 500-1 505.
[21]Stites J G,McCarty C,Quill L L.The rare earth metals and their compounds.VIII.An improved method for the synthesis of some rare earth acetylacetonates1a[J].Journal of the American Chemical Society,1948,70(9):3 142-3 143.
[22]Sheldrick G M.A short history of SHELX[J].Acta Crystallographica Section A:Foundations of Crystallography,2007,64(1):112-122.
