Synthesis and characterization of colored layered double hydroxides for thermal stabilizer
2015-03-01LiuXunjunZhangYuchaoWangJuanLeiLixu
Liu Xunjun Zhang Yuchao Wang Juan Lei Lixu
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)
Synthesis and characterization of colored layered double hydroxides for thermal stabilizer
Liu Xunjun Zhang Yuchao Wang Juan Lei Lixu
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)
Abstract:Colored layered double hydroxides (LDHs) can be synthesized by introducing colored cations such as Fe3+and Cr3+, which can be used as thermal stabilizers for polyvinyl chloride (PVC). The yellowish Mg/Fe and bluish Mg/Cr LDHs are prepared by the co-precipitation method. The results show that the Mg3Cr-CO3and Mg3Fe-CO3colored layered double hydroxides can stabilize PVC for more than 30 min under the thermal aging temperature of 180 ℃. The preparation can use cheap Mg(OH)2instead of MgCl2, which produces a much smaller amount of the by-product NH4Cl. It is known that NH4Cl is a cheap fertilizer that is difficult to sell; therefore, the preparation is much greener and more economic than the one using magnesium salt.
Key words:colored layered double hydroxides; magnesium hydroxide; ferric chloride; chromic chloride; thermal stabilizer; polyvinyl chloride
Received 2015-01-04.
Biographies:Liu Xunjun (1988—), male, graduate; Lei Lixu (corresponding author), male, doctor, professor, lixu.lei@seu.edu.cn.
Foundation items:The Fundamental Research Funds for the Central Universities, the Scientific Innovation Research of College Graduates in Jiangsu Province (No.CXLX12-0105), the Analysis and Test Fund of Southeast University (No.201226).
Citation:Liu Xunjun, Zhang Yuchao, Wang Juan,et al.Synthesis and characterization of colored layered double hydroxides for thermal stabilizer[J].Journal of Southeast University (English Edition),2015,31(4):566-571.[doi:10.3969/j.issn.1003-7985.2015.04.023]

In the previous studies, the most common precipitating agent was sodium hydroxide; however, a great amount of sodium salt will be produced as a by-product. As we know, sodium salts are cheap and difficult to sell, so we have to decrease the formation of the sodium salt. An attempt has been noted by us, which uses ammonia as the precipitant, and also, Mg(OH)2is used instead of MgCl2or Mg(NO3)2as the source of Mg2+[24]. By this method, the only by-product, agricultural fertilizer NH4Cl is formed with only 1/3 amount of previous route, which greatly alleviates the pressure from the by-product. This can be seen from the preparation of [Mg3Fe(OH)8]2CO3from Mg(OH)2:
6Mg(OH)2+2FeCl3+4NH3·H2O+(NH4)2CO3=
[Mg3Fe(OH)8]2CO3+6NH4Cl
However, if it is prepared from MgCl2, the reaction is
6MgCl2+2FeCl3+16NH3·H2O+(NH4)2CO3=
[Mg3Fe(OH)8]2CO3+18 NH4Cl
It can be seen that three times as much NH4Cl is formed and four times NH3·H2O is consumed if MgCl2is used as a starting material when the same amount of [Mg3Fe(OH)8]2CO3is formed.
As we know, plastics can be degraded if they are exposed to sunlight for a long time. It is known that the UV light in the sunlight causes the degradation. Transition metals and their compounds such as Fe3+, Cr3+, Zn2+can absorb ultraviolet light well, thus they may protect plastics from ultraviolet radiation. Therefore, it is possible to make multifunctional additives if we introduce transition metals into the LDHs. This paper reports the syntheses of two colored layered double hydroxides, [Mg1-xFex(OH)2]2CO3(short as MgxFe-CO3) and [Mg1-xCrx(OH)2]2CO3(short as MgyCr-CO3), and their properties.
1Materials and Experimental Methods
1.1 Chemicals
All the chemicals used were of analytical grade, which were produced by Sinopharm Chemical Reagent Co., Ltd.
1.2 Mg/Fe and Mg/Cr layered double hydroxide samples preparation
1.2.1Preparation of MgxFe-CO3
Mg(OH)2and FeCl3·6H2O were mixed in 150 mL water, which is then added into 100 mL of ammonia water containing ammonium carbonate under stirring. To make Mg3Fe-CO3with the Mg/Fe molar ratio of 3, the molar ratio of Mg(OH)2, FeCl3·6H2O, NH3·H2O, (NH4)2CO3was 3∶1∶2∶0.5. The temperature of the reaction mixture was then raised to make the water reflux, and the pH of the suspension was maintained between 7 and 9, which was continuously stirred for 2 h. After that, the suspension was filtered. Solids were washed with deionized water, and then dried in an oven at 120 ℃ for 48 h.
[MgFe(OH)8]2CO3and [Mg2Fe(OH)8]2CO3were synthesized similarly with the molar ratio of Mg and Fe being changed according to the chemical figure.
1.2.2Preparation of MgyCr-CO3
MgyCr-CO3was synthesized by the same method as above, but it was not quite successful. Therefore, MgCl2was used instead of Mg(OH)2; all others were the same except that the amount of ammonia water was increased according to the chemical equation. Therefore, the molar ratio of MgCl2, CrCl3·6H2O, NH3·H2O, and (NH4)2CO3was 3∶1∶8∶0.5 for the preparation of [Mg3Cr(OH)8]2CO3.
[MgCr(OH)8]2CO3and [Mg2Cr(OH)8]2CO3were also synthesized by changing only the amount of MgCl2according to the chemical figure.
1.3 Characterization
2Results and Discussion
2.1 The XRD patterns of the LDHs
Fig.1 shows the X-ray diffraction patterns of samples prepared under the exploratory conditions (aged for 2 h at the refluxing temperature of water). For MgxFe-CO3, it is observed that the intensities of diffraction peaks increase with the increase of Fe3+content. When the molar ratio of Mg and Fe is 1, the diffraction peaks are almost unobservable (see Fig.1(a)). This was observed and believed that more Mg2+could have contributed to the formation of laminar structure before[25].
However, it was not very successful when we attempted to synthesize MgyCr-CO3in the same method. Fig.1(b) shows the result of the attempt. It can be seen that there are the characteristic peaks of the proposed LDHs, but there are still impurity peaks from Mg(OH)2which appeared at around 18°[26]. Thus, the Cr3+cannot react with Mg(OH)2as smoothly as Fe3+. Perhaps this is ascribed to the insolubility of Mg(OH)2and the inertness in ligand substitution reactions of Cr3+.

(a)

(b)

(c)Fig.1 Powder X-ray diffraction patterns of MgxFe-CO3 products and MgyCr-CO3(y=1, 2 and 3). (a) MgxFe-CO3 synthesized from Mg(OH)2; (b) Mg3Cr-CO3 synthesized from Mg(OH)2; (c) MgyCr-CO3 synthesized from MgCl2
Therefore, we use MgCl2instead of Mg(OH)2to synthesize MgyCr-CO3, which is very successful. Fig.1(c) shows the related XRD patterns. Similarly, the MgxFe-CO3, MgyCr-CO3crystallized better with the increase of the Mg2+∶Cr3+molar ratio, but they are less well-crystallized than MgxFe-CO3. As we have discussed before, this may be also related to the inertness in ligand substitution reactions of Cr3+.
2.2 The IR spectra of as-prepared LDHs
Fig.2 shows the infrared spectrum of two typical samples. It is clear that both samples display similar characteristics, which is in accordance with the general knowledge on LDHs. The strong and broad band at around 3 442 cm-1is attributed to the hydroxyl groups stretching mode from both the layer hydroxyl groups and the interlayer water molecules[27]. The band observed at around 1 633 or 1 639 cm-1is ascribed to theδOHbending vibration[28]. The sharp absorption band observed at around 1 384 cm-1is attributed to the interlayer carbonate[29-30]. The lattice vibrations of metal-oxygen bonds vibrations likely result from the appearance of the strong bands at 588 cm-1.

Fig.2 The FT-IR spectra of Mg3Fe-CO3 and Mg3Cr-CO3 samples
2.3 UV-vis spectrum characterization
The optical absorption behavior of the as-prepared Mg3Fe-CO3and Mg3Cr-CO3samples under the exploratory conditions are illustrated by the UV-vis absorption spectrum, as shown in Fig.3. Both materials show strong UV absorption at around 200 nm, which is assigned to the charge transfer of isolated Fe3+and Cr3+ions octahedrally coordinated in the brucite layered structure[31]. In the visible light region, the Mg3Fe-CO3shows absorption at above 400 nm, which is ascribed to the dd transition of Fe3+[32]. The Mg3Cr-CO3exhibits two bands at 419 and 588 nm which can be easily ascribed to spin-allowed, Laporte-forbidden transitions from the fundamental state4A2g(F)→4T1g(P) and4A2g(F)→4T2g(P), respectively, in its brucite layers[33-34].
2.4 Thermogravimetric studies
Fig.4 shows the TG-DTA curves of the samples. The Mg3Fe-CO3roughly undergoes two separate weight loss processes on heating, which is in agreement with studies

(a)
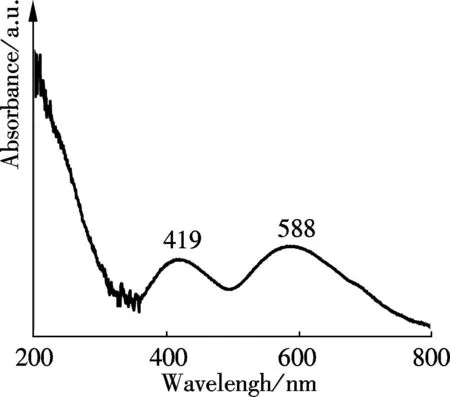
(b)Fig.3 The UV-vis spectrum. (a) Mg3Fe-CO3 sample;(b) Mg3Cr-CO3 sample
reported on other LDHs[35]. There are two obvious endothermic peaks at 177.9 and 362.4 ℃. The first-step weight loss of 10.8% at about 177.9 ℃ can result from the loss of interlayer water. The second-step weight loss of 25.9% at about 362.4 ℃ is due to the dehydroxylation of the layers to form oxides[36]. If the chemical figure of LDHs is [Mg3Fe(OH)8]2CO3·4H2O, the loss of the 4 crystal waters will be 10.9% of their weight,
森林中的树木是天然的除尘器。随着工业的不断发展,一些噪音和污染开始出现影响人们的生活,而进行营造林建设具有降低噪音和净化空气的作用。
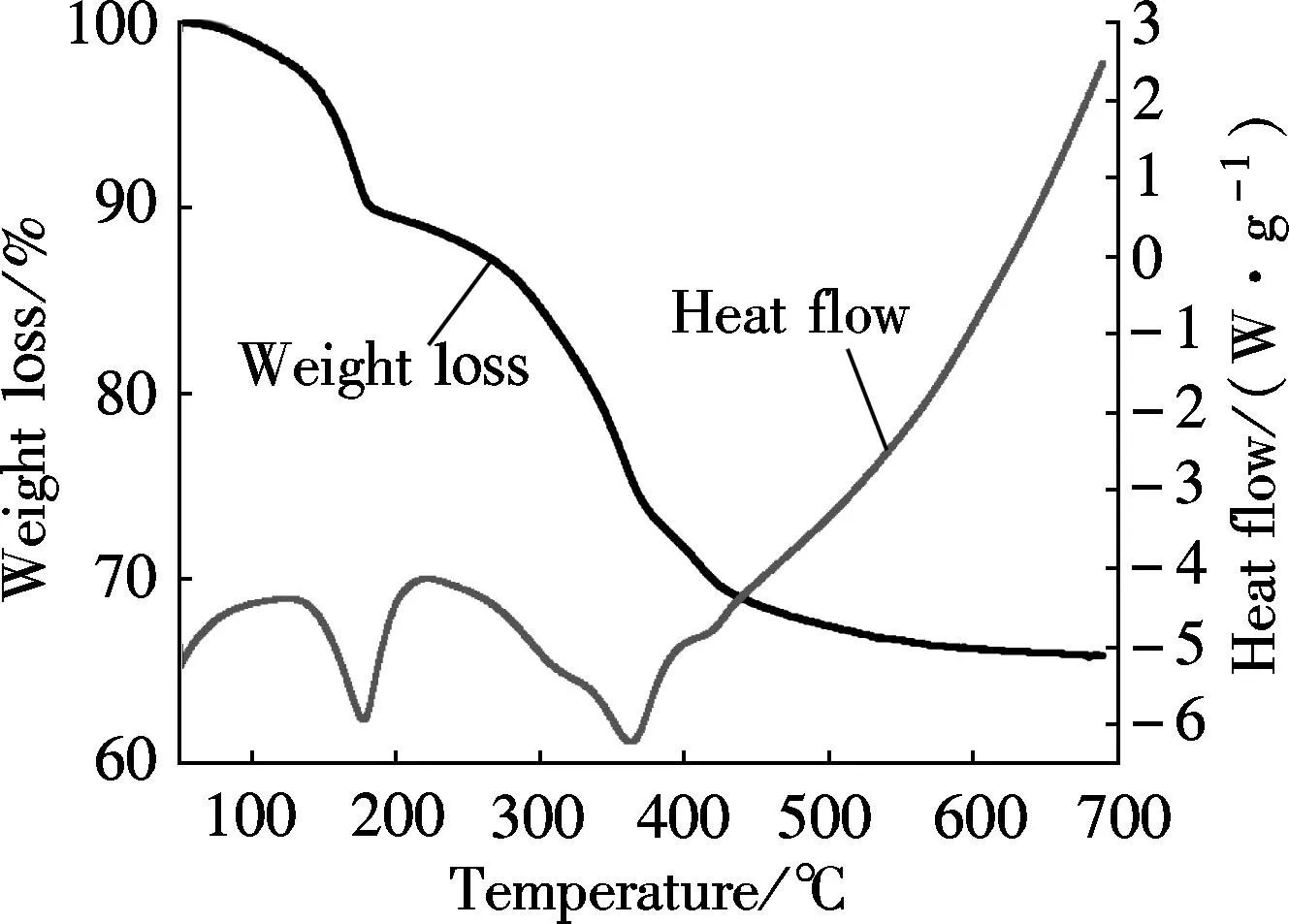
(a)
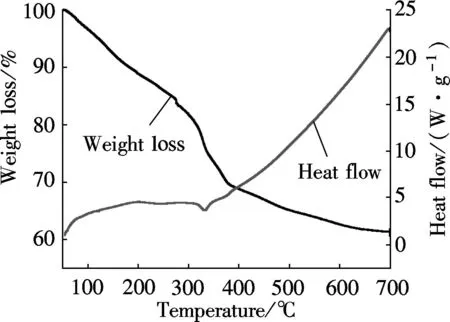
(b)Fig.4 TG-DTA curves. (a) Mg3Fe-CO3 sample; (b) Mg3Cr-CO3 sample
and the loss of 8 crystal waters from dehydroxylation will be 21.8% of weight. The total loss of weight will be 39.4%, which is very close to the experimental value of 36.7% if the carbonate also decomposes during the thermal decomposition[37]. The thermal decomposition analysis shows that there is a great loss of the crystal waters which will bring a large amount of heat away and a great deal of loss of water and CO2which also brings a large amount of heat away and H2O and CO2will dilute the concentration of O2on the surface of be polymer materials. Thus, LDH material can also be used as a flame-retardant.
The TG/DTA behavior of Mg3Cr-CO3is a slightly different from Mg3Fe-CO3. The weight loss is continuous but the rate is different. The endothermic peaks are not very clear (see Fig.4(b)), which reveals that there are different ways of decomposition. Perhaps it is related to its initial poor crystallization.
2.5 Thermal stability testing of prepared PVC composites
The PVC composite strips were prepared and made of 100 g PVC powder, 45 g DOP, 0.2 g Zn(st)2, 0.3 g Ca(st)2, Mg3Cr-CO3or Mg3Fe-CO3. The resulting PVC composite strips were placed in a thermal aging test box maintained at 180 ℃ to observe their color changes. The thermal stability of PVC is defined by the time when the strips become black. As shown in Fig.5, the strips

Fig.5 Thermal stability of composites of polyvinyl chloride. (a) Mg3Cr-CO3;(b) Mg3Fe-CO3
are colored by the LDHs. As the aging time is prolonged, the color of the strips changes. From Fig.5(a), it can be seen that the color of the blue PVC Mg3Cr-CO3composite becomes yellow when the time lasts 40 min, then deepens gradually, and finally turns red when the time lasts 90 min. The color of the pale red PVC-Mg3Cr-CO3composite becomes yellow when the time lasts 50 min, then deepens gradually, and finally turns brown when the time lasts 80 min. It was proposed by Lin et al.[38]that the stabilization of PVC by LDHs includes two steps. The first step is that HCl produced by the thermal dehydrochlorination of PVC reacts with the interlayer carbonate anions, and then more HCl reacts with the hydroxyl groups in the layers. Those processes remove the HCl which can catalyze the dehydrochlorination of PVC and thus stabilize the PVC material.
3Conclusion
[Mg3Fe(OH)8]2CO3has been successfully prepared from FeCl3·6H2O, Mg(OH)2, NH3·H2O, (NH4)2CO3in water under refluxing for 2 h. By using Mg(OH)2, the amount of by-product NH4Cl is reduced dramatically. Using Mg(OH)2to produce MgxCr-CO3is a little difficult, so it is better to use MgCl2as the starting material. The existence of Fe3+or Cr3+makes LDHs colored, and they also absorb UV lights. This may make plastics colored and UV-resistant. The decomposition of Mg3Fe-CO3leads to the weight loss due to the loss of water and CO2. Similarly, the decomposition of Mg3Cr-CO3leads to weight loss. This property can allow them to be used as a flame retardant as well as PVC thermal stabilizers.The as-prepared [Mg3Fe(OH)8]2CO3and [Mg3Cr(OH)8]2CO3were tested as the PVC thermal stabilizers, which show that the former can stabilize PVC for 50 min, and the latter for 30 min at 180 ℃.
References
[1]Wang L J, Xu X Y, Evans D G, et al. Synthesis of an N,N-Bis(phosphonomethyl)glycine anion-intercalated layered double hydroxide and its selective infrared absorption effect in low density polyethylene films for use in agriculture[J].IndEngChemRes, 2010, 49(11): 5339-5346.
[2]Feng Y J, Tang P G, Xi J M, et al. Layered double hydroxides as flame retardant and thermal stabilizer for polymers[J].RecentPatentsonNanotechnology, 2012, 6(3): 231-237.
[3]Ahamad A, Patil C B, Gite V V, et al. Evaluation of the synergistic effect of layered double hydroxides with micro-and nano-Ca2CO3on the thermal stability of polyvinyl chloride composites[J].JThermoplastComposMater, 2013, 26(9): 1249-1259.
[4]Yi S, Yang Z H, Wang S W, et al. Effects of MgAlCe-CO3layered double hydroxides on the thermal stability of PVC resin[J].JApplPolymSci, 2011, 119(5): 2620-2626.
[5]Wen R J, Yang Z H, Chen H Y, et al. Zn-Al-La hydrotalcite-like compounds as heating stabilizer in PVC resin[J].JRareEarths, 2012, 30(9): 895-902.
[6]Xu S L, Zhang L X, Lin Y J, et al. Layered double hydroxides used as flame retardant for engineering plastic acrylonitrile-butadiene-styrene (ABS)[J].JPhysChemSolids, 2012, 73(12): 1514-1517.
[7]Shi L, Li D Q, Wang J R, et al. Synthesis, flame-retardant and smoke-suppressant properties of a borate-intercalated layered double hydroxide[J].ClaysClayMiner, 2005, 53(3): 294-300.
[8]Matusinovic Z, Wilkie C A, Fire retardancy and morphology of layered double hydroxide nanocomposites [J].JMaterChem, 2012, 22(36): 18701-18704.
[9]Guo Y W, Zhu Z L, Qiu Y L, et al. Adsorption of arsenate on Cu/Mg/Fe/La layered double hydroxide from aqueous solutions[J].JHazardMater, 2012, 239: 279-288.
[10]Mandal S, Mayadevi S. Adsorption of fluoride ions by Zn-Al layered double hydroxides[J].ApplClaySci, 2008, 40(1/2/3/4): 54-62.
[11]Zhao J K, Xie Y F, Yuan W J, et al. A hierarchical Co-Fe LDH rope-like nanostructure: facile preparation from hexagonal lyotropic liquid crystals and intrinsic oxidase-like catalytic activity[J].JMaterChemB, 2013, 1(9): 1263-1269.
[12]Dubey A. Synthesis and catalytic applications of CMK-LDH (layered double hydroxides) nanocomposite materials[J].GreenChem, 2007, 9(5): 424-426.
[13]Zeng L, Zhao T S, Li Y S. Synthesis and characterization of crosslinked poly (vinyl alcohol)/layered double hydroxide composite polymer membranes for alkaline direct ethanol cells[J].IntJHydrogEnergy, 2012, 37(23): 18425-18432.
[14]Yarger M S, Steinmiller E M P, Choi K S. Electrochemical synthesis of Zn-Al layered double hydroxide (LDH) films[J].InorgChem, 2008, 47(13): 5859-5865.
[15]Lei L X, Hu M, Gao X R, et al. The effect of the interlayer anions on the electrochemical performance of layered double hydroxide electrode materials[J].ElectrochimActa, 2008, 54(2): 671-676.
[16]Ghotbi M Y, Hussein M Z, Yahaya A H, et al. LDH-intercalated D-gluconate: generation of a new food additive-inorganic nanohybrid compound[J].JPhysChemSolids, 2009, 70(6): 948-954.
[17]Mandel K, Drenkova-Tuhtan A, Hutter F, et al. Layered double hydroxide ion exchangers on superparamagnetic microparticles for recovery of phosphate from waste water[J].JMaterChemA, 2013, 1(5): 1840-1848.
[18]Nyambo C, Songtipya P, Manias E, et al. Effect of MgAl-layered double hydroxide exchanged with linear alkyl carboxylates on fire-retardancy of PMMA and PS[J].JMaterChem,2008, 18(40): 4827-4838.
[19]Millange F, Walton R I, O’Hare D, Time-resolved in situ X-ray diffraction study of the liquid-phase reconstruction of Mg-Al-carbonate hydrotalcite-like compounds[J].JMaterChem, 2000, 10(7): 1713-1720.
[20]Costantino U, Coletti N, Nocchetti M, et al. Anion exchange of methyl orange into Zn-Al synthetic hydrotalcite and photophysical characterization of the intercalates obtained[J].Langmuir, 1999, 15(13): 4454-4460.
[21]Sun G B, Sun L N, Wen H, et al. From layered double hydroxide to spinel nanostructures: Facile synthesis and characterization of nanoplatelets and nanorods[J].JPhysChemB, 2006,110(27):13375-13380.
[22]Greenwell H C, Jones W, Rugen-Hankey S L, et al. Efficient synthesis of ordered organo-layered double hydroxides[J].GreenChem, 2010, 12(4): 688-695.
[23]Guo Z, Feng J, Feng Y, et al. In situ synthesis of solid base catalysts for the regeneration of degradation products formed during the anthraquinone process for the manufacture of hydrogen peroxide[J].ApplCatalAGeneral, 2011, 401(1/2): 163-169.
[24]Zhang L H, Zhu J, Jiang X R, et al. Influence of nature of precursors on the formation and structure of Cu-Ni-Cr mixed oxides from layered double hydroxides[J].JPhysChemSolids, 2006, 67(8):1678-1686.
[25]Vulic T, Reitzmann A, Ranogajec J, et al. The influence of synthesis method and Mg-Al-Fe content on the thermal stability of layered double hydroxides[J].JThermAnalCalorim, 2012, 110(1):227-233.
[26]Sierra-Fernandez A, Gomez-Villalba L S, Milosevic O, et al. Synthesis and morpho-structural characterization of nanostructured magnesium hydroxide obtained by a hydrothermal method[J].CeramicsInternational, 2014, 40(8): 12285-12292.
[27]Kagunga W, Baddour-Hadjean R, Kooli F, et al. Vibrational modes in layered double hydroxides and their calcined derivatives[J].ChemPhys, 1998, 236(1/2/3): 225-234.
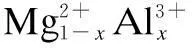
[29]Du L C, Qu B L. Structural characterization and thermal oxidation properties of LLDPE/MgAl-LDH nanocomposites[J].JMaterChem, 2006,16(16): 1549-1554.
[30]O’Leary S, O’Hare D, Seeley G. Delamination of layered double hydroxides in polar monomers: new LDH-acrylate nanocomposites[J].ChemCommun2002,(14): 1506-1507.
[31]Heredia A I C, Oliva M I, Zandalazini C I, et al. Synthesis, characterization, and catalytic behavior of Mg-Al-Zn-Fe mixed oxides from precursors layered double hydroxide[J].IndEngChemRes, 2011, 50(11): 6695-6703.
[32]Parida K, Satpathy M, Mohapatra L. Incorporation of Fe3+into Mg/Al layered double hydroxide framework: effects on textural properties and photocatalytic activity for H2generation[J].JMaterChem, 2012, 22(15): 7350-7357.
[33]Liu J W, Chen G, Li Z H, et al. Electronic structure and visible light photocatalysis water splitting property of chromium-doped SrTiO3[J].JSolidStateChem, 2006, 179(12): 3704-3708.
[34]Guo Y, Zhang H, Zhao L, et al. Synthesis and characterization of Cd-Cr and Zn-Cd-Cr layered double hydroxides intercalated with dodecyl sulfate[J].JSolidStateChem, 2005, 178(6): 1830-1836.
[35]Spratt H J, Palmer S J, Frost R L, Thermal decomposition of synthesised layered double hydroxides based upon Mg/(Fe,Cr) and carbonate[J].ThermochimicaActa, 2008, 479(1/2): 1-6.
[36]Wang Y L, Wu P X, Li B, et al. In-depth study on intercalating threonine into layered double hydroxides[J].ApplClaySci, 2011, 53(4):615-620.
[37]Moscowitz H, Lando D, Cohen H, et al. Bishophite chlorination[J].IndEngChemProdResDev, 1978, 17(2): 156-160.
[38]Lin Y J, Li D Q, Evans D G, et al. Modulating effect of Mg-Al-CO3layered double hydroxides on the thermal stability of PVC resin[J].PolymDegradStab, 2005, 88(2): 286-293.
doi:10.3969/j.issn.1003-7985.2015.04.023
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Mitigation of inter-cell interference in visible light communication
- Modified particle swarm optimization-based antenna tiltangle adjusting scheme for LTE coverage optimization
- Distribution algorithm of entangled particles for wireless quantum communication mesh networks
- Kernel principal component analysis networkfor image classification
- CFD simulation of ammonia-based CO2 absorption in a spray column
- Simulation and performance analysis of organic Rankine cycle combined heat and power system
