THE FUNCTIONAL SIGNIFICANCE OF THE PLASMA MEMBRANE IN COLD ACCLIMATION OF TWO FISH SPECIES: THE TILAPIA (OREOCHROMIS NILOTICUS) AND THE PARADISE FISH (MACROPODUS OPERCULARIS)
2015-03-01DUANZhiGangWUJinYingGEYanYanZHANXuLiangandLIWenSheng
DUAN Zhi-Gang, WU Jin-Ying, GE Yan-Yan, ZHAN Xu-Liang and LI Wen-Sheng
(State Key Laboratory of Biocontrol, Institute of Aquatic Economic Animals and Guangdong Provincial Key Laboratory for Aquatic Economic Animals, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, China)
THE FUNCTIONAL SIGNIFICANCE OF THE PLASMA MEMBRANE IN COLD ACCLIMATION OF TWO FISH SPECIES: THE TILAPIA (OREOCHROMIS NILOTICUS) AND THE PARADISE FISH (MACROPODUS OPERCULARIS)
DUAN Zhi-Gang, WU Jin-Ying, GE Yan-Yan, ZHAN Xu-Liang and LI Wen-Sheng
(State Key Laboratory of Biocontrol, Institute of Aquatic Economic Animals and Guangdong Provincial Key Laboratory for Aquatic Economic Animals, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, China)
In this study, we initially compared the cold tolerance of the Genetically Improved Farmed Tilapia (GIFT) strain of Nile tilapia (Oreochromis niloticus) and the paradise fish (Macropodus opercularis) with a series of scientific indices, such as the temperature at death (TAD), the cumulative degree hours (CDH) and the survival curves. Next, we systematically studied the effects of low temperatures on a series of plasma membrane-related physiological functions, such as membrane fluidity, endocytosis and the activity of the membrane protein Na+, K+-ATPase in both species. The results showed that the paradise fish had a better cold tolerance than did the GIFT. The plasma membrane-related physiological functions were all sensitive in low temperatures of both fishes. However, these sensitivities were different between the hardy species (paradise fish) and the non-hardy species (GIFT). The patterns and tendencies concerning sensitivity changes during varied temperatures were consistent in each species. All these interspecific differences and intraspecific consistence highlighted the importance of the membrane fluidity during the cold acclimation. These results suggest that the plasma membrane, particularly membrane fluidity, may play an important role in cold acclimation of the two fish species.
Tilapia; Paradise fish; Low temperatures; Plasma membrane; Membrane fluidity; Endocytosis; Na+, K+-ATPase
Tilapia is an important aquaculture species but has poor cold tolerance[1]. Severe mortalities occur during the winter in temperate climate countries[2], which greatly restrains the yield of this species in aquaculture. Specifically, acute temperature decreases cause more damage to tilapia than do long periods of predictable cold during the winter. An acute temperature decrease does not leave sufficient time for the fishery to take protective measures, and the consequent losses have been frequently reported in the news. Recently, extreme weather events have increased significantly, which makes the relevant research even more urgent.
Efforts have been performed to solve cold tolerance problems in tilapia. However, little information is available on the mechanism that is the greatest contributor to cold injuries in tilapia. Based on previous research, cold temperature has comprehensive and profound effects on fish from macroscopic levels, such as behavior performance[3], to cellular and molecular levels, such as membrane fluidity, metabolism and material transport[4, 5], the activities of enzymes[6, 7], and the transcription of particular genes[8—12]. Among these effects, many interesting reports appeared to have commonalities. For example, it was reported that a low temperature might lead to the failure of osmoregulation in tilapia[13, 14]. In addition, when the temperature decreased, the oxygen consumption[15], the activities of liver glucose dehydrogenase (GDH) and glucose-6-phosphate dehydrogenase (G6PD)[16, 17], and the transcription levels of Δ-6 and Δ-9 desaturase increased[18, 19]. These findings collectively suggest the role of the plasma membrane in response to temperature changes because: 1) the failure of osmoregulation may derive from plasma membrane dysfunction and the consequent disequilibrium of ions inside and outside the cells; 2) the high transcription levels ofgenes related to energy metabolism may be a complementary reaction to the dysfunction of transmembrane transport of metabolic materials; 3) the high transcriptions levels of Δ-6 and Δ-9 desaturase obviously aim to adjust the lipid composition of the cellular membrane.
Moreover, many studies concerning the effects of temperature on fish indicated that the gene expressions related to lipid transport and membrane metabolism[20], membrane fluidity[21—23], endocytosis[24]and the activities of membrane proteins[22]changed significantly with temperature. These studies demonstrated that the cell membrane could be a common target. It is therefore necessary to examine the physiological changes related to the plasma membrane under different temperatures.
More importantly, two questions are raised: are such plasma membrane-related physiological changes species-specific? Second, are the functions represented by such changes worse in non-hardy species than in hardy species? In other words, these membrane-related functions in tilapia may be more sensitive to temperature changes than in a hardy species and may consequently contribute to tilapia’s poor cold tolerance.
In this study, the Genetically Improved Farmed Tilapia (GIFT) and the paradise fish served as research objects. The paradise fish (Macropodus opercularis) is a tropical fish that belongs to Perciformes, similar to tilapia. However, different from tilapia, the paradise fish is widely considered to be a hardy fish[25—27]. In the first part of this study, the cold tolerance of the GIFT and the paradise fish were detected and compared with a same procedure to accurately quantify the difference of their cold tolerance. Then we systematically detected and compared a series of plasma membrane-related physiological indices under acute temperature decreases in the GIFT and the paradise fish to identify the role of plasma membrane during cold acclimation. The indices included membrane fluidity, endocytosis, and the activity of Na+, K+-ATPase.
1 Materials and Methods
1.1 Animals and rearing system
The GIFT (40±5.0) g were purchased from the Guangxi Institute of Fisheries. The paradise fish (6.0±0.5) g were purchased from the Yuanhu fish market in Nanning, Guangxi Province. The paradise fish was an improved species by the aquaculturists.
All fish were acclimated for 3 weeks to the laboratorial conditions in a controllable aquiculture system with recirculating water with continual aeration (24h) and a photoperiod of 12 L∶12 D before the experiment started. All experimental procedures were approved by the Institutional Animal Ethical Committee of Sun Yat-sen University and were conducted according to the corresponding protocols.
1.2 Extreme temperature tolerance
When the experiments began, the water temperatures of the chilling groups decreased by 1℃ per 24h. There were 3 replicates of 20 fish for each treatment (chilling and normal temperature). The survival rates of the GIFT and the paradise fish under different temperatures were recorded. Low-temperature tolerance of these two species was also quantified as CDH[28], TAD and LD50. CDH represents the sum of the hours the fish survived multiplied by the differences between the hourly temperatures. According to earlier studies[28, 29]and the performance observations of the fish, the threshold temperature for the calculation of CDH in each fish was 16℃ during the chilling procedure. where i = hours, T0= 16℃ as the threshold temperature, Ti= the temperature at the ith hour, and k = the hour of mortality.
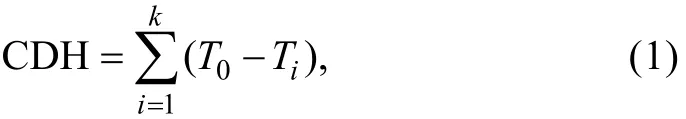
According to Charo-Karisa et al.[30], death was defined as the point at which the fish lost balance, fell on their sides and ceased fin, body and opercula movements and lost response to external stimuli.
The LD50for the lethal temperature of each species was calculated by the graphic method[1].
1.3 Membrane fluidity
For 1 month, the GIFT and the paradise fish were divided into two groups with 10 fish in each group, respectively. The water temperature of each group was either 15℃ (cold-reared groups) or 25℃ (normal temperature-reared groups). Here 15℃ was set as the temperature of cold-reared groups based on the observation of behavior and physical performance of the two fishes under cold temperatures. At 15℃, the GIFT and the paradise fish can still survive normally.
Isolation of hepatocytes
The hepatocytes were isolated from both species according to Part et al.[31]. Several modifications were performed for better cell activities. Briefly, all dissecting equipment was sterilized by high-temperature (180℃ for 4h). All procedures were performed in a sterile laminar flow clean bench. Solutions were sterilized by autoclaving and/or by passage through 0.2 µm Acrodisc syringe filters (PALL, USA), and all containers were either purchased sterile or were sterilized by autoclaving.
After the fish were eviscerated, the livers were macerated with phosphate buffer solution (PBS, Ca2+/Mg-free) for 5min and were minced. Hepatocytes were isolated from the liver by 3 cycles of trypsin digestion at 37℃ for 8min. Each cycle of trypsin digestion was stopped punctually by fetal bovine serum (FBS). The solution was filtered through cell strainers (BD-Falcon, USA), and the suspensions were washed 3 times at 700 rpm for 5min and were later resuspended with a stocking density of 3 × 105cells/mL in Ca2+/Mg2+-containing PBS. Trypan blue exclusion test indicated that the cell viabilities were more than 90% for all treatments.
The hepatocytes were then distributed in cell culture flasks (BD-Falcon, USA). The medium for the cell culture was Leibowitz L-15 supplemented with 2 mmol/L glutamine, 10% FBS, 100 IU/mL penicillin and 100 μg/mL streptomycin. All of the above-mentioned solutions were obtained from Gibco, USA.
Fluorescence polarization measurement
The plasma membrane fluidity of the hepatocytes in each fish was determined by the fluorescence anisotropy probe 1-[4-(trimethylamino)phenyl]-6-phenylhexa-1, 3, 5-triene (TMA-DPH, Sigma, USA), which was shown to be an effective and accurate probe[32]. The fluorescence anisotropy values are inversely proportional to membrane fluidity.
The measurements were conducted according to Ladha et al.[33]. Briefly, the samples were measured under steady-state conditions using a spectrofluorometer (LS-55, PerkinElmer, USA) coupled with polarisation accessories. TMA-DPH was prepared as a 2 mmol/L stock solution in dimethylformamide and was stored in a refrigerator and protected from light. The incubation temperatures of the cell suspensions without TMA-DPH varied from 25℃ to 5℃ with a thermostatic apparatus. Here 25℃ and 5℃ were set as the representative normal/cold temperatures based on our unpublished data. These data showed that the whole trend of the membrane fluidity change during the chilling process can be simply represented by comparing the values under the two terminal temperatures (25℃ and 5℃) in both fishes. 2 μL TMADPH stock solution was subsequently added to each 2 mL hepatocyte suspension and incubated at assay temperature (25℃ in all measurements) for 2min[34]before measurement. Excitation and emission wavelength were 360 and 435 nm, respectively. The results were corrected for visible light illumination. The equation used to determine anisotropy (r) is provided below:

IVVand IVHwere recorded by maintaining the excitation and emission polarizer at 0° (H) and 90° (V), alternatively. G is the instrumental correction factor (1.28).
1.4 Endocytosis in hepatocytes
The characteristics of TMA-DPH also make it available as a quantitative membrane tracer for endocytosis and intracellular membrane traffic[32, 35]. The main principle is that after incubation with an external medium containing TMA-DPH for a specific time length, the cell layers are washed thoroughly and TMA-DPH is extracted from the plasma membrane. According to the partition equilibrium, the residual fluorescence is because of the TMA-DPH contained in the vesicles and the unavoidable and non-negligible contribution from the probe internalized metabolically. The endocytosis can thus be monitored by comparing the fluorescence intensity of the samples with that of the appropriate controls[36].
The hepatocytes were isolated with the identical method as shown in 2.1. The cells were later cultured until they reached confluence to detect the intact and relatively steady state of the membrane.
The experiment was modified based on Illinger et al.[36]. Before the measurements, the samples were briefly prepared as follows: after careful removal of the supernatant medium, the hepatocyte layers were covered with 0.8 mL L-15 medium containing 10% FBS and TMA-DPH at 2 μmol/L. The preparations were then incubated for various times at one of the two temperatures (12℃ and 25℃) under air. Here 12℃ was set based on the results of Extreme temperature tolerance (see section 2.1). 12℃ was chosen as an important temperature point because just below 12℃ the GIFT began to die. We proposed that some important physiological changes had happened at this temperature. After incubation, the supernatant medium was rapidly removed, and the hepatocyte layers were washed 5 times (for 30s each) with 0.5 mL PBS to ensure the complete extraction of TMA-DPH from the plasma membranes (the washing efficiency of the cell layers has been verified by Illinger et al.[36]). The cells were recovered with 0.5 mL PBS before quantification. The fluorescence intensity of TMA-DPH in the cell layers was measured with a Perkin-Elmer LS-55 Fluorescence Spectrophotometer (an excitation wavelength of 360 nm; an emission maximum of 435 nm) at room temperature under moderate stirring. The contribution of light scattering effects or of aspecific fluorescence was negligible.
1.5 Na+, K+-ATPase activity
Na+, K+-ATPase was only located in the plasma membrane. Therefore, needless to extract the purified plasma membranes, the hepatocyte homogenates can be directly used to measure the Na+, K+-ATPase activity.
The specific, Na- and K-dependent, ouabain-sensitive ATPase activity was measured in the crude hepatocyte homogenates as described by Flik et al.[37]. At least 3 fish of each species were involved. Homogenates of each fish (a final protein content of 1 mg/mL) were divided into triplicates, and 10 µL samples were incubated for 10min at one of the two temperatures (12 and 25℃)[22]. Here 12℃ was set based on the same reason described in section 1.4. The specific activity was calculated by subtracting the ouabain-sensitive ATPase activity from the total ATPase activity. ATP hydrolysis was assessed by the amount of inorganic phosphate formed in 1/min·mg of protein under each incubation condition.
The sample protein content was assayed using a commercial protein kit (Nanjing Jiancheng Bioengineering Institute).
1.6 Statistical analyses
The results were presented as the mean ± SEM. Statistical analyses were performed using a paired Student’s t-test to compare two groups from identical species, and an unpaired Student’s t-test was used after a demonstration of variance homogeneity with an F-test to compare two groups from the different species. A one-way ANOVA was performed to compare more than two groups, and the significance was accepted at P<0.05 (two-tailed test).
After the measurement of Na+, K+-ATPase activity, we calculated the ratio of the activity at 12℃ to the activity at 25℃ (expressed as a percentage) in each fish and compared the difference between the GIFT and the paradise fish with an unpaired Student’s t-test.
2 Results
2.1 Extreme temperature tolerance
Survival rates During the chilling procedure, the GIFT began to die at 11℃, whereas the paradise fish began to die at 5.5℃. The survival rate of the GIFT was zero at 7.6℃, and this parameter was 4.5℃for the paradise fish. These data showed that the lower lethal temperature of GIFT was significantly higher (P<0.01) than the paradise fish (Fig. 1). There were no fish died in the normal temperature groups.
TAD, CDH, LD50During the chilling procedure, the TAD and LD50of the paradise fish were significantly lower (P<0.01) than that of the GIFT, which were 3℃ lower for TAD and 2.87℃ lower for LD50. Furthermore, the CDH of the paradise fish was two times bigger (P<0.01) than that of the GIFT (Tab. 1). These results were consistent with the survival rate results.
2.2 Membrane fluidity
The membrane fluidity of the hepatocytes in both
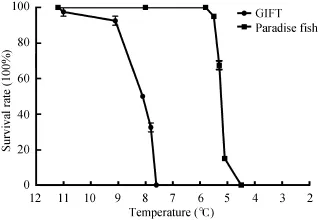
Fig. 1 Survival rates (%) of the GIFT and the paradise fish under chronic chilling (n=60)

Tab. 1 The TAD, CDH and LD50of the GIFT and the paradise fish during chilling procedure: (Significant difference shows as different letters)
When the temperature decreased from 25℃ to 5℃in the groups of cold-reared fish, the membrane fluidity of the paradise fish changed significantly (P<0.05), whereas the change was not significant for the GIFT (P>0.05). Additionally, the mean value of the membrane fluidity also changed far less in the GIFT thanthat in the paradise fish (Fig. 3). In both conditions, the GIFT showed a smaller amount of membrane fluidity compensation than did the paradise fish.

Fig. 2 Effects of temperature on fluorescence anisotropy in heaptocytes of normal temperature-reared fish (n=3)
2.3 Endocytosis in hepatocytes
The rates (indicated by the slopes of the curves) and extents (indicated by the areas under the curves, AUC) of fluid uptake were calculated as indices of endocytosis.
After the data were analyzed with a one-way ANOVA, the ANOVA showed that the rates of endocytosis in the GIFT hepatocytes were sensitive to acute temperature changes (P<0.05), whereas the paradise fish showed a steady and high efficiency of endocytosis and partial temperature compensations that were optimal for the maintenance of the cell (P<0.05). After the AUC were calculated, the extents of endocytosis under varied temperature (12 and 25℃) showed a ratio of 1.90∶1.86 in the GIFT (Fig. 4). The same ratio was 2.22∶1.53 in the paradise fish (Fig. 5).

Fig. 3 Effects of temperature on fluorescence anisotropy in hepatocytes of cold-reared fish (n=3)

Fig. 4 Effects of temperature on endocytosis in hepatocytes of the GIFT (n=4)
Specifically at 12℃, the rate and extent of endocytosis showed an initial compensation and later decreased rapidly in the GIFT (Fig. 4), and the extent of endocytosis was 1.02 times at 12℃ than the extent of endocytosis at 25℃ during the measurement. According to the tendency of the curve, it can be predicted that the extent of endocytosis at 12℃ would be lower than the extent at 25℃ soon after 60min. In contrast to the GIFT, endocytosis continuously remained at a high rate and showed a durative compensation in the paradise fish (Fig. 5). The extent of endocytosis was 1.45 times greater at 12℃ than the extent at 25℃ during the measurement time (60min).

Fig. 5 Effects of temperature on endocytosis in hepatocytes of the paradise fish (n=4)
2.4 Na+, K+-ATPase activity
The activities of Na+, K+-ATPase were detected under different temperatures. In contrast to the activities at a normal temperature (25℃), the activities obviously decreased at 12℃ in both species (Fig. 6). However, the respective extents of change were different: the activity decreased 27.8% for the tilapia at 12℃, while it is only 21% for the paradise fish.

Fig. 6 Effects of temperature on activities of Na+, K+-ATPase in the GIFT and the paradise fish (n=3)
3 Discussion
In earlier studies, much work had been done to study the relationships between any two of the topicsconcerned in this study, such as the temperature and the membrane fluidity[21, 23], the membrane fluidity and intracellular transportation[38], the temperature and the activities of membrane proteins[39], and so on. However, few researches tried to investigate the combination of three or more topics of them. The limited combinations will restrict the studying range (only on the cellular level, etc.) and the amount of information which can be revealed. In our study, we tried to integrate as many topics as possible in one particular project to explore their interactions and relationships with the whole organism’s performance.
3.1 Extreme temperature tolerance
The method of interspecific comparison was adopted by many researchers to obtain meaningful research that concerns cold tolerance[24, 40]. Accordingly, it is better to choose the species that are not only closely related in taxonomy but also have similar temperature preference. In this study, both the GIFT and the paradise fish belong to the Perciformes and were tropical fish. It was reported that the GIFT had poor cold tolerance[1]. However, the paradise fish can live in both a cold and tropical aquarium. Many researchers treat the paradise fish as a hardy species[25—27]. Grodzinski indicated that the range of water temperatures for the survival of the paradise fish larvae was 8—41℃[41]. However, in this study, the cold tolerance of the GIFT and the paradise fish should be detected and compared with the same procedure. In this study, we confirmed that the paradise fish can survive at a temperature as low as 4.5℃ using a series of scientific indices (survival curves, TAD, CDH, and LD50) (Fig. 1, Table 1). More importantly, the significant difference between the cold tolerances of these two fish showed that the paradise fish can serve as an appropriate comparison model to the GIFT to study the mechanisms for chilling injuries.
3.2 Membrane fluidity
The membrane fluidity is essential for many biological phenomena, such as the activities of the membrane proteins, the proper processes of the transmembrane transportation[42](membrane fusion and fission et al.), and it even displays direct roles at the tissue level[43]. For example, it was also found that the activity of the Na+, K+-ATPase can be increased by simply fluidizing the plasma membrane with n-hexanol[44]. In addition, it was also suggested that the membrane fluidity had a decisive impact on the rates of operation of membrane proteins which were involved in the transmembrane transportation[38].
On the other hand, it has been well-documented that temperature has profound influence on the membrane fluidity[21—23], so it is suggested that extreme temperature can impede the above-mentioned life processes by disrupting the membrane fluidity. Therefore, the ability to regulate the membrane fluidity becomes especially important when the organisms face the extreme temperatures. Specifically, it was suggested that the fish can cope with the cold temperatures better if they can increase their membrane fluidities. This hypothesis was supported by many researches. For example, it was found that the increase of membrane fluidity was significant during the adaptation to low temperatures in species which managed to acclimatize to reduced temperatures[21, 23].
In this study, the membrane fluidity of the paradise fish was sensitive to temperature and showed a quick increase when the temperature decreased, which was consistent with earlier reports in other fish[21, 23]. However, the change extents (i.e., the ability of adjustment) were different between the hardy species (paradise fish) and the non-hardy species (GIFT). In the groups of normal temperature-reared fish, the paradise fish showed obvious better ability to adjust membrane fluidity than the GIFT under decreased temperatures. After being reared in 15℃ for 1 month, the GIFT did not show obvious improvement of such ability, and the paradise fish still exhibited better adjustment ability (see section 2.2). In the cold environment, the conformational freedom of the membrane proteins can be increased along with the increase of the membrane fluidity[44]. That will buffers the direct effects of temperature upon the motional properties of membrane-bound proteins and thereby enhances their functions such as catalysis and transport[44].
Based on all the importance mentioned, it can be hypothesized that the significant adjustment of the membrane fluidity was important during the paradise fish’s acclimation to acute temperature changes, while the poor adjustment of the GIFT may contribute to its difficulty of surviving in a changing thermal environment. The difference of such ability between the two species may attribute to the different compositions of the plasma membrane in each species.
Although the membranes of both species increased their fluidity after short-term chilling from 25℃ to 5℃, the membranes from cold-reared fish showed rigidification compared to their counterparts from normal temperature-reared fish under 25℃. Rigidification may have resulted from the change between the rearing temperature (15℃) and the assay temperature (25℃), which was consistent with the response in carp[21].
3.3 Endocytosis in hepatocytes
For cellular material transport, endocytosis is important for hepatocyte metabolism and subsequently the energy supply of the fish.
Because the major stages of endocytosis consist of the vesicles formation and the membrane fusion, changes of the membrane’s physical state also have marked effects on endocytic processes[45]. Therefore, it is likely that membrane fluidity has a direct influence on the efficiency of endocytosis. This hypothesis is supported by many earlier reports. For example, in diets that contain different contents of polyunsaturated fatty acid (PUFA), membrane fatty acid composition can influence the rates of endocytosis in isolated hepatocytes of rainbow trout (Oncorhynchus mykiss)[46]and rats[24], whereas the variance of the membrane fatty acid composition approximately represents an alteration of membrane fluidity[40, 47, 48]. Additionally, Temperature has a profound effect on endocytosis[49]. Therefore, it can be hypothesized that temperature may exert an influence on endocytosis via changes in membrane fluidity.
In this study, it was easy to detect and compare the endocytic performance of hardy and non-hardy fish with identical models. Endocytosis was sensitive to acute temperature decreases in hepatocytes of the GIFT and the paradise fish. More importantly, the sensitivity was species-specific. The low temperature had a bigger negative effect on the rates of endocytosis in the GIFT than in the paradise fish (Fig. 4, Fig. 5). Unlike the GIFT, the paradise fish showed a steady and high efficiency of endocytosis (Fig. 4). This high efficiency of endocytosis can be beneficial for the maintenance of the cell under acute temperature decreases.
In this study, the GIFT had relatively slower rates and lower compensations of endocytosis, which were in accordance with the results of the membrane fluidity section (see section 2.2). Considering the close relationship between the membrane fluidity and endocytosis, it implies that the relative worse performance of endocytosis in the GIFT may be observed because of the relative poor ability of tilapia to adjust membrane fluidity.
During acute chilling (12℃), the results of endocytosis and the macroscopic temperature tolerance (see section 2.1) showed identical patterns and tendencies in each fish species. Specifically, whether at the macroscopic or cellular level (endocytosis and membrane fluidity), the paradise fish obviously showed better performance than the GIFT during acute chilling. Additionally, based on the direct influence of membrane fluidity on material transport (endocytosis), it was well-supported that membrane fluidity played an important role during changes during low temperature acclimation.
3.4 Na+, K+- ATPase activity
Na+, K+-ATPase plays an important role in maintaining ion balance and resting potential. The structure of Na+, K+-ATPase is highly conserved in high organisms[50, 51]and is only located on the plasma membrane. The activity of Na+, K+-ATPase in the membranes required proper membrane fluidity for the rotational movement of the protein. This notion can be supported by many earlier reports. For example, it was reported that the specific activity of the Na+, K+-ATPase was remarkably affected by membrane fluidity[52]. In addition, it was found that the activity of Na+, K+-ATPase can be effected by changing the lipid composition[39]or the contents of PUFAs in the membrane[53, 54].
In this study, it was important to determine the different ratios of change between Na+, K+-ATPase activities in the GIFT and the paradise fish after identical temperature variations. The acute changes of Na+, K+-ATPase activity did not involve the alteration of gene expression. In light of the highly conserved structure of Na+, K+-ATPase among different species, we may attribute the different ratios of changes between Na+, K+-ATPase activities in the GIFT and the paradise fish partly to the membrane fluidity or the lipid composition, which are closely connected. In the paradise fish, although the activities of Na+, K+-ATPase were sensitive to low temperature, as observed in the GIFT, the relative ratio of change was significantly higher than in the GIFT. This result indicates that Na+, K+-ATPase of the paradise fish can maintain a bigger proportion of activity than Na+, K+-ATPase in the GIFT after temperature decrease.
Because the results about the Na+, K+-ATPase were consistent with the performance of their respective membrane fluidity (see section 2.2), based on the detailed discussion in section 3.2, it was very possible that, in these two fish, there were tight relationships between membrane fluidity and the activity of Na+, K+-ATPase, which together affected the fish’s physiological functions during cold acclimation. Overall, the microenvironment of the membrane is important for optimal enzyme activity. It may be hypothesized that a superior adjustment of the membrane showed importance during the temperature adaptation. The abovementioned results were consistent with their macroscopic performances of temperature tolerance. The results provided further support concerning the importance of the plasma membrane (particularly membrane fluidity) during cold temperature acclimation.
4 Conclusions
We methodically detected the membrane-related functions in two fish species with distinct cold-resistant performance. The significant differences between the hardy (paradise fish) and non-hardy species (GIFT)relative to plasma membrane functions during chilling, the functional significance of the plasma membrane were notable during an acute temperature decrease. Furthermore, the processes of endocytosis and the Na+, K+-ATPase activities had a close relationship with membrane fluidity, in which the paradise fish exhibited better performance than the GIFT. These results may specifically note the importance of membrane fluidity during cold temperatures.
In reality, the results suggested that more studies should be performed to verify the importance of improving cell membranes in cold acclimation. In addition, the effects of temperature on fish are comprehensive. We only referred to one major perspective, i.e., the membrane-related functions. Additional research from other perspectives should be conducted in the future.
Acknowledgments:
We would like to thank Mr. Gan Xi, Yong-JuLuo and Jia-JieZhu (Guangxi Institute of Fisheries, China) for their help with the rearing of the fish.
[1] Li C H, Li S F. Study on low lethal temperature of different strains of Nile tilapia (Oreochromis niloticus) [J]. Fisheries Science & Technology Information, 1996, 23(5): 195—198[李晨虹, 李思发. 不同品系尼罗罗非鱼致死低温的研究.水产科技情报, 1996, 23(5): 195—198]
[2] Tave D, Jayaprakas V, Smitherman R O. Effects of intraspecific hybridization in Tilapia nilotica on survival under ambient winter temperature in Alabama [Z]. Blackwell Publishing Ltd, 1990, 21(3): 201—209
[3] Yuan X, Li L P, Tu Z Y, et al. The effect of temperature on fatigue induced changes in the physiology and swimming ability of juvenile Aristichthys nobilis (bighead carp) [J]. Acta Hydrobiologica Sinica, 2014, 38(3): 505—509 [袁喜,李丽萍, 涂志英, 等.温度对鳙幼鱼疲劳引起的生理变化和游泳能力的影响研究. 水生生物学报, 2014, 38(3): 505—509]
[4] Yan Y L, Xie X J. Mitochondrial metabolic compensation of the southern catfish, Silurus meridionalis Chen, in response to acclimation of temperature and photoperiod [J]. Acta Hydrobiologica Sinica, 2014, 38(3): 422—429 [闫玉莲, 谢小军. 温度及光照驯化对南方鲇线粒体代谢补偿调节的影响. 水生生物学报, 2014, 38(3): 422—429]
[5] Guo Q D, Wang Y J, Lü W Q. Combined effects of temperature and salinity on the physiological osmtic induction and antioxidant response in the juvenile Japanese flounder (Paralichthys olivaceus) [J]. Acta Hydrobiologica Sinica, 2014, 38(1): 58—67 [郭勤单, 王有基, 吕为群. 温度和盐度对褐牙鲆幼鱼渗透生理及抗氧化水平的影响.水生生物学报, 2014, 38(1): 58—67]
[6] Feng G P, Zhuang P, Zhang L Z, et al. Effects of water temperature on metabolic enzymeand antioxidase activities in juvenile chinese sturgeon (Acipenser sinensis) [J]. Acta Hydrobiologica Sinica, 2012, 36(1): 137—142 [冯广朋, 庄平, 章龙珍, 等. 温度对中华鲟幼鱼代谢酶和抗氧化酶活性的影响. 水生生物学报, 2012, 36(1): 137—142]
[7] He W, Chen B J, Cao Z D, et al. The effect of acclimation temperature on the activity of carbohydrate-metabolizing enzymes in five cyprinids [J]. Acta Hydrobiologica Sinica, 2015, 39(1): 203—208 [何伟, 陈波见, 曹振东, 等. 温度驯化对五种鲤科鱼类糖代谢酶活性的影响. 水生生物学报, 2015, 39(1): 203—208]
[8] Liu B, Wang M Y, Xie J, et al. Effects of acute cold stress onserum biochemical and immune parameters and liver HSP70 gene expression in GIFT strain of Nile tilapia (Oreochromis niloticus) [J]. Acta Ecologica Sinica, 2011, 31(17): 4866—4873 [刘波, 垚王美 , 谢骏, 等. 低温应激对吉富罗非鱼血清生化指标及肝脏 HSP70 基因表达的影响. 生态学报, 2011, 31(17): 4866—4873]
[9] Vergauwen L, Benoot D, Blust R, et al. Long-term warm or cold acclimation elicits a specific transcriptional response and affects energy metabolism in zebrafish [J]. Comparative Biochemistry and Physiology. Part A, Molecular and Integrative Physiology, 2010, 157(2): 149—157
[10] Logan C A, Somero G N. Transcriptional responses to thermal acclimation in the eurythermal fish Gillichthys mirabilis (Cooper 1864) [J]. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 2010, 299(3): R843—R852
[11] Li L C, Li Q, Long Y, et al. Microarray analysis of temperature stress effects on transcriptional expression in zebrafish larvae [J]. Acta Hydrobiologica Sinica, 2012, 36(5): 882—891 [李林春, 李青, 龙勇, 等. 温度刺激对斑马鱼仔鱼基因转录表达的影响. 水生生物学报, 2012, 36(5): 882—891]
[12] Angilletta M J, Niewiarowski P H, Navas C A. The evolution of thermal physiology in ectotherms [J]. Journal of Thermal Biology, 2002, 27(4): 249—268
[13] Allanson B R, Bok A, Van Wyk N I. The influence of exposure to low temperature on Tilapia mossambica Peters (Cichlidae) [J]. Journal of Fish Biology, 1971, 3(2): 181—185
[14] Sardella B A, Brauner C J. Cold temperature-induced osmoregulatory failure: The physiological basis for tilapia winter mortality in the Salton Sea [J]? California Fish and Game, 2007, 93(4): 200—213
[15] Schnell A K, Seebacher F. Can phenotypic plasticity facilitate the geographic expansion of the tilapia Oreochromis mossambicus [J]? Physiological and Biochemical Zoology, 2008, 81(6): 733—742
[16] Tranulis M A, Christophersen B, Blom A K, et al. Glucosedehydrogenase, glucose-6-phosphate dehydrogenase and hexokinase in liver of rainbow trout (Salmo gairdneri). Effects of starvation and temperature variations [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 1991, 99(3): 687—691
[17] Shivkamat P, Roy R. Regulation of membrane lipid bilayer structure during salinity adaptation: A study with the gill epithelial cell membranes of Oreochromis niloticus [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2005, 142(1): 28—36
[18] Zerai D B, Fitzsimmons K M, Collier R J. Transcriptional response of delta-9-desaturase gene to acute and chronic cold stress in Nile tilapia, Oreochromis niloticus [J]. Journal of the World Aquaculture Society, 2010, 41(5): 800—806
[19] Vagner M, Santigosa E. Characterization and modulation of gene expression and enzymatic activity of delta-6 desaturase in teleosts: A review [J]. Aquaculture, 2011, 315(1—2): 131—143
[20] Chen Z Z, Chen C-H C, Zhang J F, et al. Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish [J]. Proceedings of the National Academy of Sciences, 2008, 105(35): 12944—12949
[21] Dey I, Farkas T. Temperature shifts induce adaptive changes in the physical state of carp (Cyprinus carpiot L.) erythrocyte plasma membranes in vitro [J]. Fish Physiology and Biochemistry, 1992, 10(4): 347—355
[22] Sardella B A, Cooper J, Gonzalez R J, et al. The effect of temperature on juvenile Mozambique tilapia hybrids (Oreochromis mossambicus x O. urolepis hornorum) exposed to full-strength and hypersaline seawater [J]. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2004, 137(4): 621—629
[23] Buda C, Dey I, Balogh N, et al. Structural order of membranes and composition of phospholipids in fish brain cells during thermal acclimatization [J]. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(17): 8234—8238
[24] Padron D, Bizeau M E, Hazel J R. Is fluid-phase endocytosis conserved in hepatocytes of species acclimated and adapted to different temperatures [J]? American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 2000, 278(2): R529—R536
[25] Darling R A. A directed research project investigating aggressive behavior in paradise fish [J]. Bioscene, 2003, 29(2): 3—7
[26] Ward R W. Ethology of the paradise fish, Macropodus opercularis I. differences between domestic and wild fish [J]. Copeia, 1967, 1967(4): 809—813
[27] Gervai J, Csányi V. Artifical gynogenesis and mapping of gene-centromere distances in the paradise fish, Macropodus opercularis [J]. Theoretical and Applied Genetics, 1984, 68(6): 481—485
[28] Behrends L L, Kingsley J B, Bulls M J. Cold tolerance in maternal mouthbrooding tilapias: heritability estimates and correlated growth responses at suboptimal temperatures. In: Pullin R S V, Lazard J, Legendre M, et al. (Eds.), The Third International Symposium on Tilapia in Aquaculture [C]. ICLARM Conference Proceedings. 1996
[29] Cnaani A, Gall G A E, Hulata G. Cold tolerance of tilapia species and hybrids [J]. Aquaculture International, 2000, 8(4): 289—298
[30] Charo-Karisa H, Rezk M A, Bovenhuis H, et al. Effects of rearing conditions on low-temperature tolerance of nile tilapia, Oreochromis niloticus, juveniles. In: Bolivar R, Mair G, Fitzsimmons K (Eds.), New Dimensions in Farmed Tilapia. Proceedings of the 6th International Symposium on Tilapia in Aquaculture, Manila [C]. 2004
[31] Part P, Norrgren L, Bergstrom E, et al. Primary cultures of epithelial cells from rainbow trout gills [J]. Journal of Experimental Biology, 1993, 175(1): 219—232
[32] Illinger D, Duportail G, Mely Y, et al. A comparison of the fluorescence properties of TMA-DPH as a probe for plasma membrane and for endocytic membrane [J]. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1995, 1239(1): 58—66
[33] Ladha S, Mackie A, Clark D. Cheek cell membrane fluidity measured by fluorescence recovery after photobleaching and steady-state fluorescence anisotropy [J]. Journal of Membrane Biology, 1994, 142(2): 223—228
[34] Kuhry J, Fonteneau P, Duportail G, et al. TMA-DPH: A suitable fluorescence polarization probe for specific plasma membrane fluidity studies in intact living cells [J]. Cell Biochemistry and Biophysics, 1983, 5(2): 129—140
[35] Kubina M, Lanza F, Cazenave J, et al. Parallel investigation of exocytosis kinetics and membrane fluidity changes in human platelets with the fluorescent probe, trimethylammonio-diphenylhexatriene [J]. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1987, 901(1): 138—146
[36] Illinger D, Kubina M, Duportail G, et al. TMA-DPH a fluorescent probe of membrane dynamics in living cells. How to use it in phagocytosis [J]. Cell Biophysics, 1989, 14(1): 17—26
[37] Flik G, Bonga S E W, Fenwick J C. Ca2+-dependent phosphatase and ATPase activities in eel gill plasma membranes--I. Identification of Ca2+-activated ATPase activities with non-specific phosphatase activities [J]. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1983, 76(4): 745—754
[38] Yuli I, Wilbrandt W, Shinitzky M. Glucose transport through cell membranes of modified lipid fluidity [J]. Biochemistry, 1981, 20(15): 4250—4256
[39] Metz J R, van den Burg E H, Bonga S E, et al. Regulation of branchial Na+/K+-ATPase in common carp Cyprinus carpio L. acclimated to different temperatures [J]. Journal ofExperimental Biology, 2003, 206(13): 2273—2280
[40] Palmerini C A, Mazzoni M, Giovinazzo G, et al. Blood lipids in Antarctic and in temperate-water fish species [J]. Journal of Membrane Biology, 2009, 230(3): 125—131
[41] Grodzinski Z. Thermal tolerance of the larvae of three selected teleost fishes [J]. Acta Biologica Cracoviensia Serie Zoologique, 1971, 14: 289—298
[42] Block E R, Edwards D. Effect of plasma membrane fluidity on serotonin transport by endothelial cells [J]. American Journal of Physiology-Cell Physiology, 1987, 253(5): C672—C678
[43] Connor W E. Importance of n-3 fatty acids in health and disease [J]. The American Journal of Clinical Nutrition, 2000, 71(1): 171S—175S
[44] Cossins A R, Bowler K, Prosser C L. Homeoviscous adaptation and its effect upon membrane-bound proteins [J]. Journal of Thermal Biology, 1981, 6(4): 183—187
[45] Mamdouh Z, Giocondi M C, Laprade R, et al. Temperature dependence of endocytosis in renal epithelial cells in culture [J]. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1996, 1282(2): 171—173
[46] Røsjø C, Berg T, Manum K, et al. Effects of temperature and dietary n-3 and n-6 fatty acids on endocytic processes in isolated rainbow trout (Oncorhynchus mykiss, Walbaum) hepatocytes [J]. Fish Physiology and Biochemistry, 1994, 13(2): 119—132
[47] Stubbs C D, Smith A D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function [J]. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes, 1984, 779(1): 89—137
[48] Chintalapati S, Kiran M D, Shivaji S. Role of membrane lipid fatty acids in cold adaptation [J]. Cellular and Molecular Biology, 2004, 50(5): 631—642
[49] Dunn W A, Hubbard A L, Aronson N J. Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver [J]. Journal of Biological Chemistry, 1980, 255(12): 5971—5978
[50] Razzaque M S. Klotho and Na+, K+-ATPase activity: solving the calcium metabolism dilemma [J]? Nephrology Dialysis Transplantation, 2008, 23(2): 459—461
[51] Brauer P R, Sanmann J N, Petzel D H. Effects of warm acclimation on Na+,K+-ATPase alpha-subunit expression in chloride cells of Antarctic fish [J]. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology, 2005, 285(1): 600—609
[52] Kimelberg H K. Alterations in phospholipid-dependent (Na++K+)-ATPase activity due to lipid fluidity: Effects of cholesterol and Mg2+[J]. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1975, 413(1): 143—156
[53] Djemli-Shipkolye A, Raccah D, Pieroni G, et al. Differential effect of ω3 PUFA supplementations on Na,K-ATPase and Mg-ATPase activities: possible role of the membrane ω6/ω3 ratio [J]. Journal of Membrane Biology, 2003, 191(1): 37—47
[54] Rodrigo R, Bächler J, Araya J, et al. Relationship between (Na + K)-ATPase activity, lipid peroxidation and fatty acid profile in erythrocytes of hypertensive and normotensive subjects [J]. Molecular and Cellular Biochemistry, 2007, 303(1): 73—81
Q178.1
A
1000-3207(2015)06-1150-10
10.7541/2015.151
Received date: 2014-03-18; Accepted date: 2015-01-05
Foundation item: The China Agriculture Research System (CARS-49); the Agriculture Research Special Funds (3-49-10)
Brief introduction of author: Duan Zhi-Gang (1985—), male, born in Zhengzhou, Henan Province, Master, Major in hydrobiology, E-mail: 4j7k23l@163.com
Wu Jin-Ying, Associate Professor, E-mail: lsswjy@mail.sysu.edu.cn
