自噬在雷帕霉素逆转脊髓吗啡耐受形成机制研究
2015-02-23朱少飞唐群新
朱少飞,唐群新
自噬在雷帕霉素逆转脊髓吗啡耐受形成机制研究
朱少飞,唐群新
目的 研究鞘内注射雷帕霉素对大鼠吗啡耐受形成的作用。方法 选择鞘内置管成功的成年雄性SD大鼠32只,随机分为M组、C组、MR组和R组(n=8),M组2次/d,连续7 d鞘内注射吗啡20 μg;C组2次/d,连续7 d鞘内注射生理盐水;MR组2次/d,连续7 d鞘内注射吗啡20 μg,并于第3天第2次注射吗啡同时鞘内注射雷帕霉素2.3 μg,连续3 d;R组2次/d,连续7 d鞘内注射生理盐水,并于第3天第2次注射生理盐水前鞘内注射雷帕霉素2.3 μg,连续3 d。于鞘内注射前及第1、3、5、7天,第2次鞘内注药后30 min,采用电子Von Frey测痛仪测定机械缩足反射阈值(MWT),最后一次MWT测定结束后,随机取4只大鼠L4~6脊髓背角,采用Western blot测定自噬标记蛋白LC3Ⅱ(LC3Ⅱ/LC3Ⅰ)及自噬调节信号相关蛋白Beclin-1的表达。结果 与M组比较,MR组鞘内注射第3、5、7天后,MWT均升高(P<0.05),虽然MR组MWT有降低趋势,但在第7天MWT仅比第1天下降43%,表明第3天后雷帕霉素与吗啡合用能部分逆转吗啡耐受的形成。Western blot结果:与C组比较,M组、R组和MR组第7天脊髓背角Beclin-1、LC3Ⅱ与LC3Ⅱ/LC3Ⅰ表达均上调(P<0.05)。与M组比较,R组和MR组第7天脊髓背角Beclin-1、LC3Ⅱ与LC3Ⅱ/LC3Ⅰ表达显著上调(P<0.01)。免疫组织化学结果:与M组比较,第7天MR组脊髓背角Beclin-1表达平均光密度值明显升高(P<0.01)。结论 吗啡耐受开始形成时合用雷帕霉素可以部分逆转脊髓吗啡耐受的形成。
吗啡耐受;自噬;雷帕霉素
0 引言
在疼痛药物治疗领域,阿片类药物占据着主导地位。吗啡是目前常用的强效镇痛药物,然而在慢性疼痛患者中,由于产生药物耐受、依赖,或药物不良反应而影响了其使用。目前吗啡耐受的确切机制仍不清楚,阿片受体脱敏、内吞和受体下游信号转导的调控是吗啡耐受形成的主要原因[1]。一氧化氮合酶(NOS)表达上调,N-甲基-D天冬氨酸(NMDA)受体结合活性增加也参与慢性吗啡耐受的形成[2]。
正常水平的自噬是维持细胞稳态、保护细胞的一种机制。在自噬的信号调节中,哺乳动物雷帕霉素靶蛋白(Mammalian target of rapamycin,mTOR)处于中心环节,其中PI3K-Akt-mTOR是最重要的信号通路[3]。雷帕霉素是一种大环内酯类免疫抑制剂,通过与亲免素FK506结合蛋白12(FKBP12)形成复合物特异性抑制mTORC1活性,激活自噬,是研究最多的自噬诱导剂[4]。既往有研究表明,特异性μ阿片受体激动剂DAMGO可通过PI3K信号通路激活Akt,并使其下游的S6K、4EBP1蛋白磷酸化,调节神经元蛋白翻译与突触可塑性[5]。最近研究表明,海马CA3区PI3K-Akt-mTOR-p70S6K信号通路的激活在慢性吗啡引起条件性位置偏爱(CPP)中起重要作用,应用PI3K抑制剂LY294002或者mTOR抑制剂雷帕霉素可以抑制PI3K-Akt信号通路的激活,从而抑制CPP的形成[6]。慢性应用吗啡导致神经元胞体减小、增加兴奋性和引起奖赏效应耐受与下调腹侧被盖区(VTA)多巴胺神经元Akt-mTORC2活性有关,虽然mTORC1活性增加,但与这些效应无关。自噬激活是应用吗啡后的早期反应,可能会有细胞保护作用[7-8]。自噬是否也参与吗啡耐受形成过程的调节,尚未见文献报道。
本研究观察慢性吗啡耐受过程中自噬标记蛋白LC3和自噬上游信号通路蛋白Beclin-1水平的变化,以及应用雷帕霉素激活自噬对慢性吗啡耐受变化的作用。
1 材料与方法
1.1 实验动物 雄性SD大鼠,体重250~300 g,由广东省医学实验动物中心提供,动物合格证号:SCXK(粤)2008-0002。动物每笼4只饲养,保持室温在24 ℃左右和50%~60%的相对湿度,自由摄食与饮水,隔天更换垫料。在安静、白天与黑夜12 h循环光照的环境中适应1周后开始实验。所有的实验步骤都按照有关实验动物的使用原则规范操作,尽量减轻动物的痛苦。
1.2 鞘内置管 根据经典的Yaksh法[9],参考大鼠腰部蛛网膜下腔置管法[10-11],进行大鼠腰段置管模型的制作。大鼠腹腔注射10%水合氯醛溶液(3 mL/kg)麻醉后,俯卧位,下腹部垫1个20 mL注射器,腰背部剪毛、消毒后,于背部正中线L3~4间隙纵向切开背部皮肤约2 cm,切开筋膜,钝性分离肌肉,暴露L3~4棘突间隙,切除部分L4棘突,用1 mL针头(针尖钝性处理,消毒)探查,穿破黄韧带和硬脊膜,大鼠尾巴突然侧摆或后肢出现抽动为成功标志,将充满生理盐水的PE-10导管经硬脊膜破口向头端插入2 cm,可见导管开口有脑脊液流出。将导管固定于肌肉上,然后在大鼠颈部皮肤切小口,将PE-10导管经皮下拉至颈部,穿出后固定,PE-10导管外露3 cm,不锈钢针芯封闭管腔。术毕局部肌肉注射4万单位青霉素钠。置管后第2天,蛛网膜下腔注射2%利多卡因20 μL,然后以10 μL生理盐水冲洗,如果插管位置在脊髓腰膨大处,大鼠立即出现双后肢瘫痪,30 min后逐渐恢复下肢活动,则证明置管位置正确,并且无明显脊髓损伤,可行下一步实验,在大鼠脊髓取材时可以确定。术后出现后肢瘫痪、运动功能障碍或导管位置不到位的大鼠从实验中排除。
1.3 实验分组 选择鞘内置管成功的成年雄性SD大鼠32只,随机分成4组,每组8只。M组(慢性吗啡耐受模型组):鞘内注射盐酸吗啡20 μg/10 μL,2次/d,给药时间:9∶00和18∶00,每次注药后以10 μL生理盐水冲管,连续7 d。C组(生理盐水对照组):鞘内注射生理盐水20 μL,2次/d,给药时间:9∶00和18∶00,连续7 d。MR组(慢性吗啡耐受+雷帕霉素组):鞘内注射盐酸吗啡同M组,于第3天第2次注射吗啡同时鞘内注射雷帕霉素2.3 μg/10 μL,连续3 d。R组(雷帕霉素对照组):鞘内注射生理盐水同C组,于第3天第2次注射生理盐水同时鞘内注射雷帕霉素2.3 μg/10 μL,连续3 d。
1.4 Western blot检测和免疫组织化学检测 取脊髓组织置于1 mL细胞裂解液中,冰上匀浆。12 000 r/min 4 ℃离心15 min,取上清。取1 μL蛋白液,用BCA法测蛋白含量,细胞裂解液将上样蛋白浓度调成一致。样品按4∶1加入5×上样缓冲液,100 ℃变性5 min。15% SDS-聚丙烯酞胺凝胶电泳,湿法转至PVDF膜,室温封闭2 h,一抗(兔抗鼠LC3B 1∶700,兔抗鼠Beclin-1 1∶500,Abcam公司)与内参GAPDH(1∶400,武汉博士德公司)4C孵育过夜,HRP标记的羊抗兔二抗(1∶4 000,武汉博士德公司)室温孵育1 h,ECL显色,随后暗室曝光,显影,定影。采用Quantity One图像分析软件分析所得条带的光密度值,以靶蛋白光密度值/GAPDH光密度比值作为目的蛋白的相对表达量。
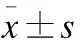
2 结果
2.1 机械痛阈的变化 各组大鼠机械痛阈基础值比较差异无统计学意义(P>0.05)。与C组、R组比较,M组第1天鞘内注射吗啡产生最大镇痛效应,随着注射时间增加,MWT逐渐下降,到第7天接近基础值水平,表明吗啡耐受已经形成。与M组比较,MR组鞘内注射第3、5、7天后MWT均升高(P<0.05),虽然MR组MWT有降低趋势,但在第7天MWT仅比第1天下降43%,表明第3天后雷帕霉素与吗啡合用能部分逆转吗啡耐受的形成。C组和R组各时点机械痛阈与基础值比较差异无统计学意义(见表1)。

表1 四组大鼠各时点机械痛阈的比较(g)
注:与C组、R组比较,*P<0.01;与M组比较,#P<0.05
2.2 Western blot结果
2.2.1 大鼠脊髓背角LC3蛋白水平的变化 与C组比较,M组、R组和MR组脊髓背角LC3Ⅱ与LC3Ⅱ/LC3Ⅰ表达均上调(P<0.05)。与M组比较,R组和MR组LC3Ⅱ与LC3Ⅱ/LC3Ⅰ表达显著上调(P<0.01)。R组和MR组比较无统计学差异。
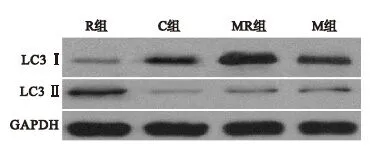
图1 四组大鼠第7天脊髓背角LC3蛋白表达
2.2.2 大鼠脊髓背角Beclin-1蛋白水平的变化 与C组比较,M组、R组和MR组脊髓背角Beclin-1表达显著增加(P<0.01)。与M组比较,R组和MR组Beclin-1表达量增加(P<0.01)。与R组比较,MR组Beclin-1表达轻度增高(P<0.05)。
3 讨论
哺乳动物雷帕霉素靶蛋白mTOR是自噬的负调控分子,在自噬信号调节中处于中心环节[12]。调节mTOR活性的主要信号通路是磷脂酰肌醇三磷酸激酶(PI3K)途径。生长因子或胰岛素与细胞膜表面相应受体结合激活Ⅰ型PI3K,使PIP2磷酸化生成PIP3,PIP3募集Akt/PKB及其激活剂PDK1,激活Akt,导致结节性硬化复合物TSC1/2的磷酸化。TSC1/TSC2复合物整合上游各种激酶的信号,包括Akt和细胞外信号调节蛋白激酶(ERK1/2)[13]。下游的Rheb是小GTP结合蛋白,直接与mTOR结合,并激活mTOR。TSC1/2磷酸化后,TSC1分裂出来,TSC1/2活性下降,抑制Rheb作用下降,负调节作用减弱,mTOR活性增强[14]。
雷帕霉素是特异性mTOR抑制剂,通过与亲免素FK506结合蛋白12(FKBP12)形成复合物抑制mTORC1活性,从而激活自噬[15]。正常的自噬活性对于维持神经细胞稳态非常重要。最近研究表明,在神经退行性疾病,如亨廷顿病、帕金森病等,应用雷帕霉素可以增强自噬,通过清除大量有聚集倾向的蛋白,减轻毒性,减少神经元死亡[16-19]。Erlich等[20]在小鼠闭合性脑外伤后4 h应用雷帕霉素,明显抑制p70S6K磷酸化,减少小胶质细胞的活化,增加损伤处神经元的存活,改善神经功能恢复。Carloni等[21]研究显示,大鼠脑缺氧/缺血模型中,应用雷帕霉素是通过PI3K-Akt-mTOR信号通路促进自噬而产生的神经保护作用,该信号通路的受阻,如缺氧/缺血前20 min应用自噬拮抗剂渥曼青霉素(WM)、3-甲基腺嘌呤(3MA)抑制PI3K后,可增加细胞坏死。因此,应用雷帕霉素促进自噬活性,有神经保护作用。
然而,雷帕霉素抑制mTOR可以产生有利或有害的作用,关键在于疾病的模型与应用时机。研究表明,每天予雷帕霉素处理后,可加重肾脏缺血/再灌注损伤[22];缺血预处理前15 min应用雷帕霉素可以减弱心肌缺血预处理的心肌保护作用[23]。缺血再灌注前应用雷帕霉素,可以减少心肌梗死面积和细胞死亡,起到类似缺血预处理的心肌保护作用[16]。雷帕霉素的细胞保护作用可能是由于阻碍凋亡的信号通路,雷帕霉素可以通过激活自噬清除受损线粒体,减少胞浆内细胞色素C的释放,降低Caspase酶的激活[24]。如在缺氧/缺血性脑损伤模型上,雷帕霉素激活自噬可以减少活化的Caspase-3表达[25]。
既往对雷帕霉素在体内的药代动力学研究表明,雷帕霉素起效时间约2 h,半衰期较长,大约33~63 h[26-27]。也有相关研究认为,单次使用雷帕霉素可以影响自噬活性>24 h[28-29]。Sekiguchi等[30]在小鼠脊髓损伤后4 h单次腹腔内注射雷帕霉素1 mg/kg可以有效抑制24 h后mTOR信号途径的p70S6K的磷酸化,明显减低神经细胞死亡,提高运动功能评分。在福尔马林致痛的模型上,大鼠后足掌面皮下注射5%福尔马林前20 min鞘内注射雷帕霉素20 μL(250 μM,4.6 μg),与对照组相比,可以明显减轻福尔马林致痛的双相反应[31]。而在坐骨神经分支选择性损伤模型(SNI)上,于术后第6天鞘内注射10 μL(250 μM,2.3 μg)雷帕霉素,可以减轻SNI后继发的机械痛觉过敏[32]。本研究中,在吗啡耐受开始形成时(连续鞘内注射吗啡第3天)鞘内合用雷帕霉素2.3 μg/10 μL,连续3 d,能明显减轻吗啡耐受,脊髓背角LC3Ⅱ和Beclin-1蛋白均明显上调,而雷帕霉素对照组对痛阈无影响,说明了雷帕霉素逆转吗啡耐受的机制可能与上调自噬活性有关,但还不能证明是促进μ阿片受体的内吞,修复信号通路的动态变化,来减轻慢性吗啡耐受形成的。今后考虑研究自噬在吗啡耐受过程中阿片受体蛋白表达和mRNA表达中的作用,来进一步验证。
本研究显示,吗啡耐受开始形成时合用雷帕霉素,可能是通过抑制mTOR活性,促进自噬活性,从而部分逆转吗啡耐受的形成。
[1]Jaggi AS,Singh N.Analgesic potential of intrathecal farnesyl thiosalicylic acid and GW 5074 in vincristine-induced neuropathic pain in rats[J].Food and Chemical Toxicology,2012,50(5):1295-1301.
[2]Hayashi Y,Koga Y,Zhang X,et al.Autophagy in superficial spinal dorsal horn accelerates the cathepsin B-dependent morphine antinociceptive tolerance[J].Neuroscience,2014,275:384-394.
[3]Toda N,Kishioka S,Hatano Y,et al.Modulation of opioid actions by nitric oxide signaling[J].Anesthesiology,2009,110(1):166-181.
[4]Bekhit MH.Opioid-induced hyperalgesia and tolerance[J].Am J Ther,2010,17(5):498-510.
[5]Esclatine A,Chaumorcel M,Codogno P.Macroautophagy signaling and regulation[J].Curr Top Microbiol Immunol,2009,335:33-70.
[6]P Headrick J,Pepe S,N Peart J.Non-analgesic effects of opioids:cardiovascular effects of opioids and their receptor systems[J].Current Pharmaceutical Design,2012,18(37):6090-6100.
[7]Viguier F,Michot B,Kayser V,et al.GABA,but not opioids,mediates the anti-hyperalgesic effects of 5-HT 7 receptor activation in rats suffering from neuropathic pain[J].Neuropharmacology,2012,63(6):1093-1106.
[8]Lamming DW,Ye L,Katajisto P,et al.Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity[J].Science,2012,335(6076):1638-1643.
The study suggested a possible role of IL-1β -511 C/T genotypes in the pathogenesis of pNETs since the presence of the IL-1β -511 CT and TT genotypes and the T allele was associated with an increased risk of pNET only.
[9]Cui Y,Zhang XQ,Cui Y,et al.Activation of phosphatidylinositol 3-kinase/akt-mammalian target of rapamycin signaling pathway in the hippocampus is essential for the acquisition of morphine-induced place preference in rats[J].Neuroscience,2010,171(1):134-143.
[10]Mazei-Robison MS,Koo JW,Friedman AK,et al.Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons[J].Neuron,2011,72(6):977-990.
[11]Zhao L,Zhu Y,Wang D,et al.Morphine induces Beclin-1 and ATG5-dependent autophagy in human neuroblastoma SH-SY5Y cells and in the rat hippocampus[J].Autophagy,2010,6(3):386-394.
[12]周晓菊,李宇宁.哺乳动物雷帕霉素靶蛋白在阿霉素诱导足细胞损伤中的作用[J].国际儿科学杂志,2014,41(6):644-647.
[13]Chawla SP,Staddon AP,Baker LH,et al.Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas[J].Journal of Clinical Oncology,2012,30(1):78-84.
[14]Benjamin D,Colombi M,Moroni C,et al.Rapamycin passes the torch:a new generation of mTOR inhibitors[J].Nature reviews Drug Discovery,2011,10(11):868-880.
[15]吕金,陈华,崔健君.慢性神经痛大鼠鞘内联合应用吗啡和氯胺酮在脊髓上水平的抗伤害性作用及对吗啡耐受的影响[J].实用药物与临床,2013,15(11):714-716.
[16]Barlow AD,Xie J,Moore CE,et al.Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2)[J].Diabetologia,2012,55(5):1355-1365.
[18]Perkey E,Fingar D,Miller RA,et al.Increased Mammalian target of rapamycin complex 2 signaling promotes age-related decline in CD4 T cell signaling and function[J].The Journal of Immunology,2013,191(9):4648-4655.
[19]Malagelada C,Jin ZH,Jackson-Lewis V,et al.Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson′s disease[J].J Neurosci,2010,30(3):1166-1175.
[20]Erlich S,Alexandrovich A,Shohami E,et al.Rapamycin is a neuroprotective treatment for traumatic brain injury[J].Neurobiol Dis,2007,26(1):86-93.
[21]Carloni S,Girelli S,Scopa C,et al.Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia[J].Autophagy,2010,6(3):366-377.
[22]Lui SL,Chan KW,Tsang R,et al.Effect of rapamycin on renal ischemia-reperfusion injury in mice[J].Transpl Int,2006,19(10):834-839.
[23]Voleti B,Navarria A,Liu RJ,et al.Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling,synaptogenesis,and antidepressant behavioral responses[J].Biological Psychiatry,2013,74(10):742-749.
[24]Khan S,Salloum F,Das A,et al.Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes[J].J Mol Cell Cardiol,2006,41(2):256-264.
[25]Neff F,Flores-Dominguez D,Ryan DP,et al.Rapamycin extends murine lifespan but has limited effects on aging[J].The Journal of Clinical Investigation,2013,123(8):3272-3291.
[26]Long SA,Rieck M,Sanda S,et al.Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function[J].Diabetes,2012,61(9):2340-2348.
[27]王海莲,闫素英,刘扬.中国9家肿瘤专科医院2009-2012年应用镇痛药处方趋势分析[J].实用药物与临床,2014,17(9):1219-1223.
[28]Lamming DW,Ye L,Astle CM,et al.Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive[J].Aging Cell,2013,12(4):712-718.
[29]Sheng R,Zhang LS,Han R,et al.Autophagy activation is associated with neuroprotec-tion in a rat model of focal cerebral ischemic preconditioning[J].Autophagy,2010,6(4):482-494.
[30]Sekiguchi A,Kanno H,Ozawa H,et al.Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice[J].J Neurotrauma,2012,29(5):946-956.
[31]Bové J,Martínez-Vicente M,Vila M.Fighting neurodegeneration with rapamycin:mechanistic insights[J].Nature Reviews Neuroscience,2011,12(8):437-452.
[32]Caramés B,Hasegawa A,Taniguchi N,et al.Autophagy activation by rapamycin reduces severity of experimental osteoarthritis[J].Ann Rheum Dis,2012,71(4):575-581.
Effects of intrathecal rapamycin on the development of morphine tolerance
ZHU Shao-fei,TANG Qun-xin
(Department of Anesthesiology,Haikou People′s Hospital,Haikou 570208,China)
Objective To investigate the effects of intrathecal (IT) rapamycin on the development of morphine tolerance.Methods 32 healthy male SD rats with intrathecal (IT) catheter successfully placed were randomly divided into 4 groups (n=8) : group M was given morphine 20 μg IT twice daily for 7 d; group C was given normal saline(NS) 20 μL IT twice daily for 7 d; group MR was given morphine 20 μg IT twice daily for 7 d,and at the second injection from 3 d to 5 d,rapamycin 2.3 μg was added; group R was given normal saline 20 μL IT twice daily for 7 d,and at the second injection from 3 d to 5 d,rapamycin 2.3 μg was injected before the normal saline.Mechanical withdrawal thresholds (MWT) to von Frey filament stimulation were measured before intrathecal morphine or NS was given and at 30 min after second time of IT morphine or NS administration on 1,3,5,7 d.The rats were sacrificed after MWT measurement.The autophagy marker protein LC3Ⅱ (or LC3Ⅱ/LC3Ⅰ) and autophagy signal pathway related protein Beclin-1 expression in spinal dorsal horn was determined by Western blot.Results The analgesic effect of morphine decreased slowly in group MR,but higher than that of group M from 3 d to 7 d (P<0.05).Western blot results showed that LC3Ⅱ,Beclin-1 expression and LC3Ⅱ/LC3Ⅰ ratio were up-regulated in group M,group MR and group R compared with those in group C (P<0.05).The LC3Ⅱ,Beclin-1 expression and LC3Ⅱ/LC3Ⅰ ratio were higher in group MR and group R than those in group M (P<0.01).Immunohistochemistry results showed that the optical density of Beclin-1 in group MR was higher than that in group M (P<0.01).Conclusion Rapamycin could partly reverse the development of spinal morphine tolerance.
Morphine tolerance; Autophagy; Rapamycin
2015-01-21
海南省海口市人民医院麻醉科,海口 570208
10.14053/j.cnki.ppcr.201507005
