A comparative corrosion behavior of Mg,AZ31 and AZ91 alloys in 3.5% NaCl solution
2015-02-16*
*
CSIR-Advanced Materials and Processes Research Institute,Bhopal 462026,M.P.,India
A comparative corrosion behavior of Mg,AZ31 and AZ91 alloys in 3.5% NaCl solution
I.B.Singh*,M.Singh,S.Das
CSIR-Advanced Materials and Processes Research Institute,Bhopal 462026,M.P.,India
The corrosion behavior of Mg,AZ31 and AZ91 has been evaluated in 3.5%NaCl solution using weight loss,electrochemical polarization and impedance measurements.Corrosion rate derived from the weight losses demonstrated the occurrence of steeply fast corrosion reaction on AZ91 alloy after three hours of immersion,indicating the start of galvanic corrosion.An increase of corrosion rate with immersion time was also observed for AZ31 but with lesser extent than AZ91 alloy.Whereas Mg metals showed a decrease of corrosion rate with immersion time, suggesting the formation of a protective layer on their surfaces.In contrast,the corrosion current density(Icorr)derived from the Tafel plots, exhibited their corrosion resistances in order of Mg>AZ91>AZ31.Electrochemical charge transfer resistance(Rct)and double layer capacitance measured by electrochemical impedance spectroscopy(EIS),are well in accordance with the measured Icorr.EIS measurements with time and microstructural examination of the corroded and uncorroded samples are helpful in elucidation of results measured by electrochemical polarization.
Mg and alloys;Weight loss;Electrochemical measurements;SEM
1.Introduction
Magnesium based alloys possess an attractive combination of low density and high strength/weight/ratio which are quite useful for their applications in automotive and aerospace industries.However,they are very reactive in the humid or wet atmosphere as their loosen oxide layer make them highly corrosive.Their corrosion reaction becomes much more pronounced when they are exposed in aqueous marine environment.Occurrence of the galvanic corrosion is another problem with Mg alloys as magnesium has the lowest electrode potential and acts as anode in the alloy[1-4].Though alloying of Al and Zn with Mg increases their strength but make them venerable to galvanic corrosion.Mg-Al alloys are the most common category of magnesium alloy.There are different opinions about the role of Al on the corrosion resistance of magnesium alloy.Lunder et al.[5]observed that when aluminum content reaches 8%(mass fraction),the corrosion resistance of magnesium alloy improve in a noticeable level. Warner et al.[6]reported that even 5%addition of Al in magnesium alloys is benefcial in improving their corrosion resistance.Whereas Hemann et al.[7]indicated that 9%and above Al is helpful in improving of corrosion resistance of magnesium alloy.However,no one among them studied the approximate exposure period at which this alloy becomes suxcitible for galvanic corrosion at neutral pH.
Alloying effect of Al and Zn on corrosion of Mg is latively less studies.Cao et al.[8]investigated the corrosion behavior of Mg and AZ91 alloy in 0.1 M NaCl solution using electrochemical studies.They observed a highly corrosive nature of AZ91 alloy as compared to Mg metal.Cheng et al.[9]studied the infuence of alloying elements like Zr,Al on the corrosion resistance of Mg in 1 M NaCl solution using electrochemical measurements.They observed AZ91 alloy shows the worst corrosion resistance among AZ31,AZ91,AM60 and ZK60 alloys. Though, electrochemical measurements including electrochemical impedance spectroscopy are quite useful in the determination corrosion kinetics and diffusion phenomena at the metal/electrolytes interfaces,weight loss measurement is still considered most authentic method for corrosion rate determination in free corrosion condition.As per our knowledge no one has made corrosion rate determination using weight loss measurement especially for Mg and their alloys in chloride bearing corrosive system.
With this view,the present work was aimed to make a detail corrosion studies of the Mg metal and their alloys,AZ31 and AZ91 in 3.5%NaCl solution using weight loss,electrochemical and microstructural studies.
2.Experimental
The materials used in the present study,were commercial grade Mg(~99%pure)metal and as cast AZ31(Al 3 wt%,Zn 1 wt%,balanced Mg)and AZ91(Al 9 wt%,Zn 1 wt%, balanced Mg)alloys.The casting of the alloys was made in the oxygen protective atmosphere.In weight loss measurement rectangular samples of dimension 2.5 cm×2.5 cm×0.25 cm were used.Samples were polished well with different grades of emery paper.Before immersing,samples were degreased using trichoroethylene solution.In weight loss measurement, pre-weighted samples were immersed vertically in 3.5%NaCl with the help of polypropylene make thread tightened at one end of the sample through drilled hole.Immersed samples were removed from the solution after different intervals of time.After removing,samples were washed carefully,dried in air and again re-weighted.The corrosion rate was derived using following Eq.(1)
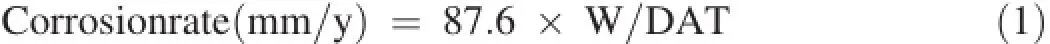
where corrosion rate is in millimeter per year,W,weight loss in mg,D,density and T,time in hour.
Electrochemical measurements like open circuit potential (OCP)variation with time,potentiodynamic polarizations and electrochemical impedance spectroscopy were performed in 3.5 wt%NaCl solution at room temperature(25°C)using a Solartron make 1280 Z corrosion measurement system.Three electrodes system,namely,a working electrode(area:1 cm2), a platinum counter electrode(area:2 cm2)and a saturated calomel electrode(SCE),as a reference electrode were employed in the present investigation.Open circuit potential (OCP)variations with time were recorded up to 30 min of exposure.Afterward,Tafel plot was obtained by carrying out partial potentiodynamic polarization in the potential range±150 mV from OCP at the potential scan rate of 1 mV/ s.In a separate experiment potentiodynamic polarization was made by scanning potential from-150 mV(SCE)from OCP to 1.5 V(SCE)in the anodic potential.EIS analysis was done in the frequency range of 20 mHz to 20 kHz using a sinusoidal ac signal of 10 mV at the open circuit potential.The reproducibility of the electrochemical test was verifed by carrying out two to three measurements on a fresh polished specimen. All the measurements have been performed in aerated solution under static condition.Different parameters of polarization curves and impedance diagrams were derived from software Corrware 2 and Z plot,respectively using curve ftting method. After completion of EIS measurement,were cleaned properly and used for their microstructural examination.
3.Results and discussion
Different corrosion reactions involved in magnesium and their alloys are similar neutral and alkaline media.The overall reaction[10]can be expressed as
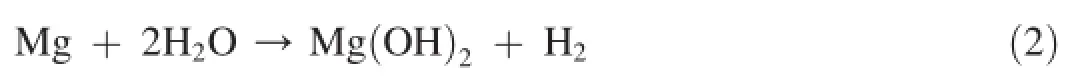
However,the above reaction presents a general description and does not explain the involvement of different stages of corrosion.For e.g.naturally formed oxide or passive flm present on Mg alloy can exhibit a high corrosion resistance up to certain exposure period.But the same alloy may show suddenly a very fast corrosion after passing of critical exposure period at which galvanic corrosion start.Therefore,the corrosion property of magnesium alloys is associated with the nature of naturally formed oxide flm present on their surfaces and also related to content of their cathodic alloying elements present in their matrix.Mg-Al-Zn alloy is the most common category of magnesium alloy where Al and Zn are the main cathodic elements which determine the corrosion of alloys.In both alloys,Zn content is same.Therefore,their entire corrosion property depends on the presence of Al content.On the other side it is well known that pure Mg metal posses better corrosion resistances than their alloys.Thus a comprehensive corrosion study comprises of weight loss and electrochemical measurements is carried out to understand the role of Al content on the critical exposure period at which galvanic corrosion starts and their comparative corrosion behavior in chloride bearing neutral solution.
3.1.Weight loss measurement
Weight loss measurement indicated that Mg metal demonstrates a very less weight loss as compared to AZ91 and AZ31 alloy up to 72 h of exposure.In comparison to AZ31, AZ91 alloy showed less weight loss up to initially 3 h of exposure.Thereafter,their steep increase of weight loss was measured.The corrosion rate(mm/y)derived as per Eq.(1),is plotted against time(Fig.1).From the trend one can see that the corrosion rate of AZ91 occurs comparatively much less than AZ31 alloy till initial 3 h of immersion.After this,there was a steep rise in their corrosion rate(6 mm/y)was measured. Whereas AZ31 alloy showed even less than one mm/y corrosion rate after 3 h of immersion.The increase in corrosion rate of AZ91 alloy was continued with fast rate till 32 h of immersion.After this period,a decline in their corrosion ratewas measured.After removing,specimens were observed visually porous structure.Due to decrease of dimension of the specimen,weight loss measurement was stopped after 48 h of immersion.
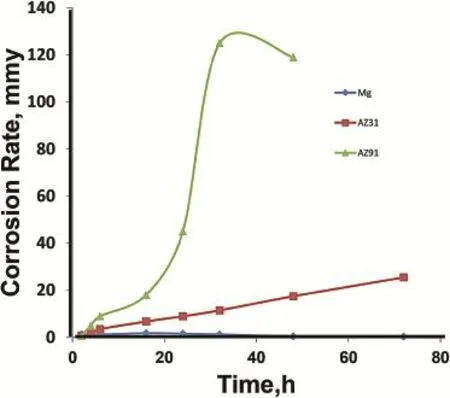
Fig.1.Corrosion rate variations with time for Mg,AZ1 and AZ91 alloys in 3.5%NaCl solution.
In case of AZ31 alloy,it showed a linear increase of weight loss till 72 h of immersion.Interestingly,Mg metal showed better corrosion resistances as compared to AZ91 alloy even after 72 h of immersion.But it showed very high corrosion rate as compared to Mg metal.Interestingly,a decrease in corrosion rate with exposure time was measured for Mg metal. The surface of the immersed Mg metal was intact even after 72 h of exposure.These this suggest that a protective nature of oxide flm forms at the Mg surfaces which reduces their corrosion signifcantly.A steep increase of corrosion rate after 3 h of immersion of AZ91 alloy evidences the occurrence of fast corrosion reaction that increases with exposure period possibly due to start of galvanic corrosion.Moreover,intensity of the galvanic corrosion appears to be quite high for AZ91 alloy.Presence of a higher Al content in the AZ91 alloy presumably increases the potential difference that results the start of galvanic corrosion with fast rate.
3.2.Electrochemical measurements
3.2.1.OCP behavior
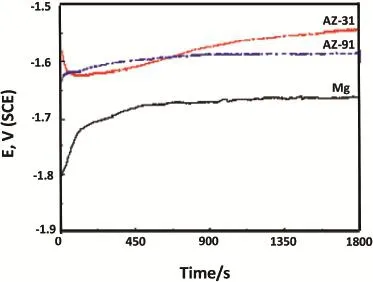
Fig.2.OCP measurement with time for Mg,AZ31 and AZ91 alloys in 3.5% NaCl solution.
The open-circuit potential(OCP)variations with time curves obtained for all the three studied substrates,are presented in Fig.2.From the trend,it can be noted that their initial OCP moves in negative direction till 5 min of measurement.Afterward,it increases toward positive direction and their increase rate slows down after 20 min of duration. However,their OCP could not attain steady state even after 30 min of measurement.Increase of OCP towards positive side is more for AZ31 alloy as compared to AZ91 and Mg metal. Observed OCP values indicate that OCP of Mg occurs more negative(-1.66 V)as compared to AZ31(-1.56 V)and AZ91(-1.6 V).Occurrence of a more negative OCP for Mg metal indicates their active behavior possibly their oxidation in to Mg2+ion that changes equilibrium potential more negative. Though the standard potential of the magnesium electrode is -2.37 V but measurement of their OCP as-1.66 V indicates the formation Mg(OH)2layer over it which is more cathodic in nature.In comparison,more positive OCP of AZ31 and AZ91 alloys may be due to the presence of beta phases which increases the free potential in the positive direction[11]. Observed OCP behavior is in accordance with the weight loss measurement as initially a higher corrosion rate was measured for Mg metal(Fig.1).Formation of mixed hydroxides of Al, Zn along with Mg can be explained similarly.Presence of pure hydroxide containing layer at the Mg surface seems to be more protective compared to mixed hydroxides layer forms on AZ31 and AZ91 alloy surfaces.
3.2.2.Tafel plots
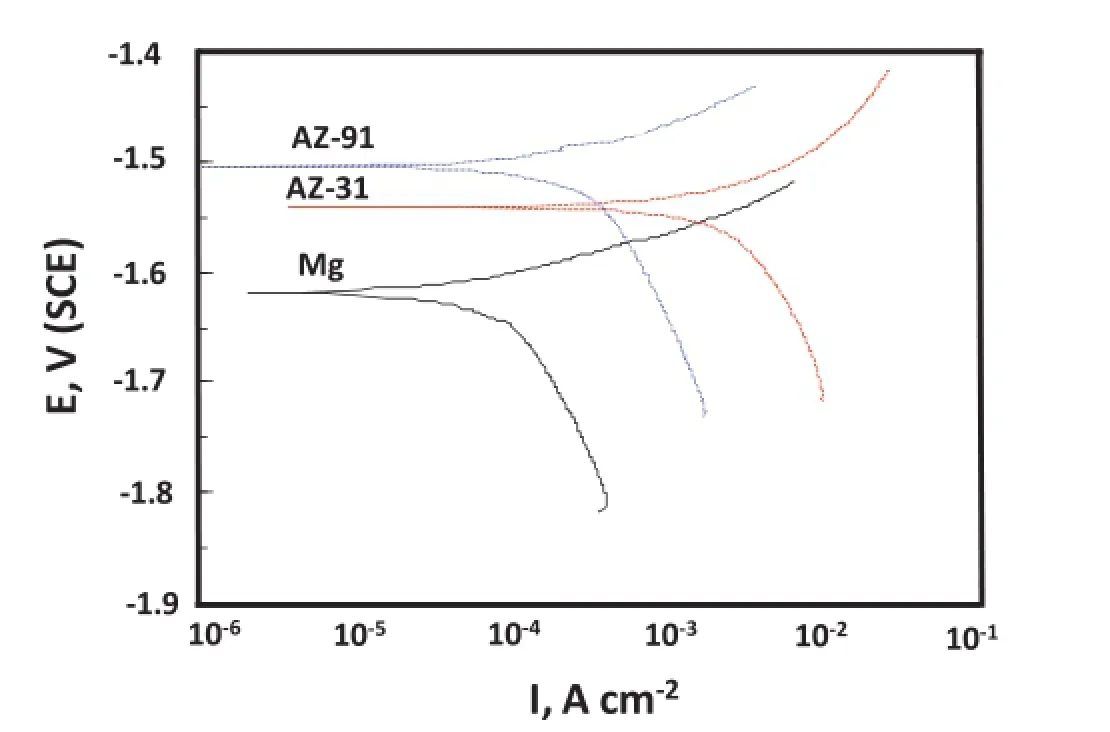
Fig.3.Tafel plots for Mg,AZ31 and AZ91 alloys in 3.5%NaCl solution.
Fig.3 shows the partial cathodic and anodic polarization curves obtained after carrying partial cathodic and anodicpolarization from OCP±150 mV.Different corrosion parameters like corrosion potential(Ecorr),corrosion current density(Icorr),anodic(βa)and cathodic(βc)Tafel constants derived by the Tafel extrapolation,are given in Table 1. Measured Icorrvalues attributes an order of magnitude lower Icorrfor Mg metal as compared to AZ31 and AZ91 alloys.Icorrof AZ91 alloy shows nearly fve times higher than Mg metal. On the other side Icorrof AZ31 alloy occurs nearly twenty times higher than Mg metal.Whereas AZ91 alloy posses three times higher Icorrthan AZ31 alloy.However,derived Icorrvalues make contradiction from the corrosion rates measured from weight losses as AZ91 alloy exhibited unexpectedly a higher corrosion rate as compared to Mg and AZ31 alloy after 3 h of immersion.Occurrence of a lower Icorrof AZ91 alloy than AZ31 alloy suggests their better corrosion resistance in the initial period of exposure.Measurement of a higher values of cathodic Tafel constant(βc)for Mg metal indicates the occurrence of a pronounced cathodic controlled reaction i.e. oxygen reduction reaction as per following reaction(3)
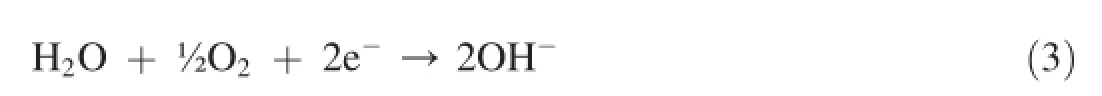
Since oxygen reduction is a main cathodic reaction,its control is one of the main factor in reducing of corresponding oxidation reaction(formation of Mg2+ion after discharge of two e-).Once hydrolysis reaction starts corrosion reaction proceeds bytheoxidationofMgasMg2+andtheir involvement with OH-ions that produced by the reduction of water molecule[12,13].ThisresultstheformationofMg(OH)2precipitates at the metal surfaces.The reaction involve in the formation of Mg(OH)2can be expressed as per following(reaction 4).
The overall reactions could be given as per following reaction.
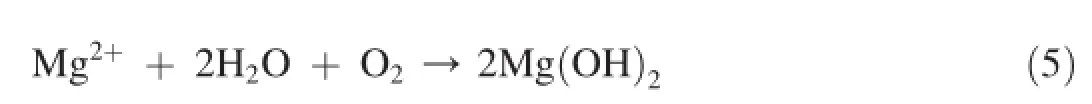
Similar reactions may also involve in the corrosion of Al and Zn of the alloys.Measurement of a lower βcvalues for AZ31 and AZ91 alloys than Mg metal indicates their lesser tendency in controlling of cathodic reaction.Due to increased cathodic reaction(reaction 3),OH-generation becomes more that increases the anodic oxidation reaction i.e metal dissolution.This is the main reason that anodic Tafel constant(βc) values increases for AZ31 and AZ91 alloys.
3.2.3.Current density vs time behavior
The corrosion current fows at OCP were measured under potentiostatically controlled condition.Fig.4 shows thevariations of current densities with time at OCP.From the trends,it can be seen that AZ31 alloy shows an increased anodic current with time,indicating the occurrence of anodic reaction(metal oxidation)under free corrosion condition.The current fows for Mg and AZ91 alloys,are appeared to less anodic,indicating the occurrence of a slow anodic reaction. Present current vs time implies the occurrence of a slow rate of corrosion reaction in Mg and AZ91 alloy as compared to AZ31 alloy.OCP measurements have also demonstrated similar trend of variation with time.
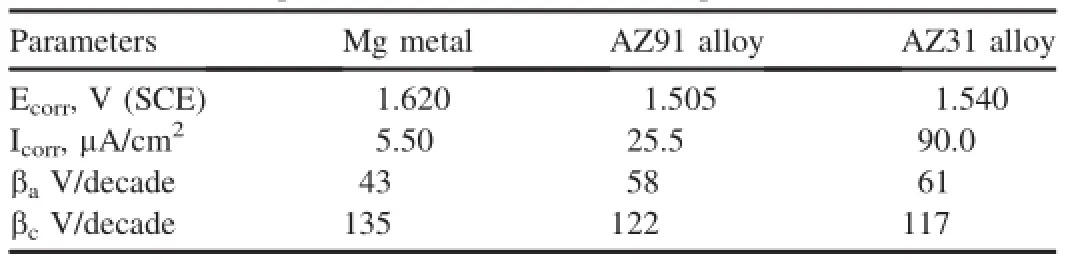
Table 1Different corrosion parameters derived from Tafel plots.
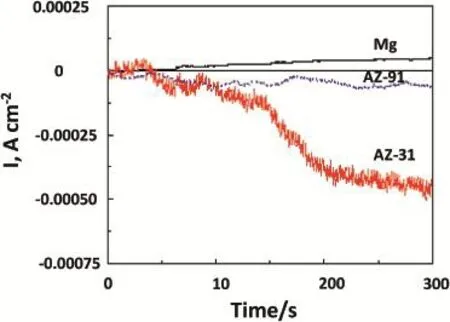
Fig.4.Potentiostatic current density behavior with time Mg,AZ31 and AZ91 alloys in 3.5%NaCl solution.
3.2.4.Potentiodynamic polarization
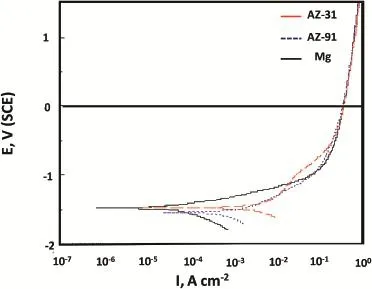
Fig.5.Potentiodynamic polarization Mg,AZ31 and AZ91 alloys in 3.5% NaCl solution.
Potentiodynamic polarization curves recorded in a wide anodic potential range(up to 1.5 V)are demonstrated in Fig.5.From the potentiodynamic polarization behavior,it can be seen that the nature of increase of current densities with potential,is similar for all the three types of electrodes. However,a limitation of current increase start at 1 V for Mgand AZ91 alloy while limitation in current increase occurs at 1.1 V for AZ31 alloy.Occurrence of limitations in current during anodic polarization is mainly due to the accumulation of corrosion product at the electrode surfaces that reduces the current increase further.It is therefore,polarization of metal in this potential region becomes potential independent.
3.2.5.Electrochemical impedance spectroscopy(EIS) studies
EIS measurement in the form of Nyquist plot,was carried out after 30 and 90 min of exposure.As shown in Figs.6 and 7,Nyquist plots demonstrate the occurrence of one capacitive semi circle from higher to the middle frequency region and an inductive loop in the lower frequency region for all the three electrodes.In contrast,Cao et al.[8]and Cheng et al.[9]reported the existence of two semicircles;one in the higher frequency and another one in the lower frequency regions. Though,their observed frst semicircle in higher frequency regions was almost indistinguishable.In the present case,the radius of the semicircle occurs considerably more for Mg than AZ91 alloy.In case of AZ31 alloy,it becomes comparatively very less(Fig.6).After the increase of immersion time to 90 min,the radius of semicircle increases further for Mg metal while it decreases for AZ31 and AZ91 alloys(Fig.7).It is reported that the high frequency part in the Nyquist plot represents the properties of the coatings,whereas the low frequency part associated with the Faradaic processes occurring on the bare metal[14,15].In case of Mg and their alloys, presence of thick oxide flm itself behaves as coating that protects metal up to certain level.Therefore,observation of capacitive behavior from higher to middle frequency region is associated with the resistance of the oxide flm.Since the oxide flm present at the Mg metal possess more corrosion resistance,shows a better capacitive behavior.Presence of a poor capacitive behavior for AZ31 and AZ91 alloys suggest their more corrosive behavior.Occurrence of capacitive loops at the low frequency region for AZ91 and AZ31 alloys indicate the continuation of Faradaic process i.e.diffusion process at the metal/solution interfaces.The appearance of a low frequency inductive loop is also related to relaxation of the adsorbed anions on the surface of the metal[15,16].Based on this,it may further be described that presence of Cl-in the corrosive media increases their adsorption at the AZ31 and AZ91 alloy.This results the increase of oxidation of metal by consuming electrons from the oxidized metal at the adsorb sites.This ultimately starts corrosion reaction at particular adsorbed site.Exhibition of comparatively less pronounced inductive loop for Mg metal suggests their lower susceptibility for adsorption Cl-ions.

Fig.6.EIS measurements(Nyquist diagram)in Mg,AZ31 and AZ91 alloys in 3.5%NaCl solution after 30 min of exposure.
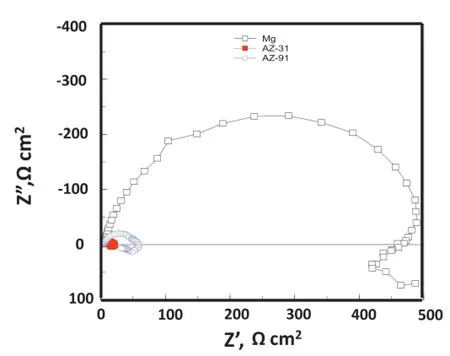
Fig.7.EIS measurements(Nyquist diagram)in Mg,AZ31 and AZ91 alloys in 3.5%NaCl solution after 90 min of exposure.
Different EIS parameters like Rct,solution resistance(Rs), and double layer capacitance(Cdl)as given in Table 1,are derived by curve ftting method.Due to presence of one semi circle,an equivalent circuit consisting of resistor connected in series to a parallely connected resistor and capacitor has been used for data analysis.From Table 2,it can be noted that Rctof Mg increases from 310 to 495 Ω cm2after 30 min-90 min exposure.An increase of charge resistance with time attributes the sequential growth of protective Mg(OH)2layer at the Mg surface.In case of AZ91 and AZ31 alloys Rctreduces from 76 to 42 Ω cm2and from 25 to 9.5 respectively after 30-90 min,suggesting decrease of their corrosion resistances with time.In opposite to Rct,an increase of Cdlvalues was measured for both alloy with time.As seen in Table 2,Cdlof AZ31 and AZ91 alloy increases nearly an order of magnitude higher after 30-90 min of exposure.Since Cdlis associated with the water/electrolytes uptake through the pores/defects of the surface flm[17,18],an increase Cdlwith time for AZ91 and AZ31 alloy implies the increase of porosities/defects in their oxide flm.Increase of porosities in the flm increases the diffusion reaction.A decrease of Cdlwith exposure time as observed for the Mg metal(Table 2),suggesting the decrease of porosities/defects in the oxide flm that increases their corrosion resistance.Measurement of Rctand Cdlvalues are well in accordance with the corrosion rate measured byweight loss and Tafel extrapolation as Mg metal showed much better corrosion resistance compared to than AZ31 and AZ91 alloys.
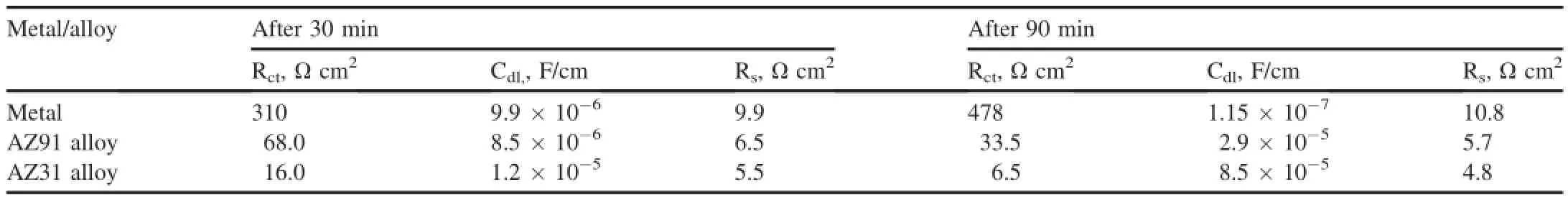
Table 2Different corrosion parameters derived from EIS diagrams.
3.2.6.Microstructural examinations
Surface morphologies of the corroded and uncorroded specimens(after 90 min of exposure)were examined by SEM. Photomicrographs as shown in Fig.8,demonstrates uncorroded(a)and corroded Mg surface(b).As compared to uncorroded surface,corroded surface shows the accumulation of corrosion product over the grain boundaries.Since grain boundaries are thermodynamically more active,precipitation of corrosion product(Mg(OH)2)in this region reduces the corrosion attack.This is perhaps reason for owing better corrosion resistances by Mg metal.
The surface morphology of uncorroded surface of AZ31 alloy as depicted in Fig.9a,exhibit the presence of a larger area consisting of α phase(matrix)which is surrounded by β phase(Mg17Al12).In case AZ91 alloys,it is diffcult to distinguish between α and β phase as surface is covered by oxide layer(Fig.10a).On the other side,corroded surface of the both alloys shows a presence of thick corrosion product layer(Figs.9b and 10b)which is not protective in nature.
There is general opinion that presence of β phase in magnesium/alloys plays a major role in their corrosion resistances/ passivation.It acts as a cathode and shows a good passive behavior in broad pH ranges.After the dissolution of the anodic phase(α phase of the matrix),the surrounding phase(β phase)may play the role of a barrier layer in the inhibition of the corrosion reaction.According to the previous studies [19,20],the role of β phase in corrosion process related to its content,size and distribution.When the mass fraction of β phase is high,the grain size of magnesium alloy is small,and the β phase distributes continuously in the matrix containing α phase,so it may play the role of a barrier layer to deter corrosion.On the contrary,if the grain size is larger and the distance between β phases is enlarged,galvanic corrosion may occur that results the decline of the corrosion resistance. Presence of β phase enhances their potential differences which responsible for the generation of local galvanic cells.This ultimately starts corrosion reaction which increases with the time.Based on the above,it may therefore be concluded that presence of a substantial level of Al(~9 wt%)in AZ91 that posses more β phases,exhibits much pronounced galvanic corrosion as compared to AZ31 alloy.
4.Conclusions
·Weight loss measurement shows initially an increase of corrosion rate in order of Mg<AZ91<AZ31 in 3.5% NaCl solution.After 3 h of immersion,the corrosion rate of AZ91 becomes much more than AZ31 alloy due to start of galvanic corrosion.
·Measurement of a decrease of corrosion rate with immersion time for Mg metal indicates the formation of a continuous layer of Mg(OH)2at their surface that increases corrosion resistances.Mixed hydroxides of Mg,Al and Zn form at the alloys surface are not benefcial in improving of their corrosion resistance.
·Corrosion current density derived from the Tafel extrapolation indicates similar trend as corrosion rate measured by weight loss data during initial immersion period.
·Charge transfer resistancemeasurement indicates an increase of corrosion resistance in order as AZ31<AZ91<Mg which is well in accordance with the corrosion rate measurement by Tafel plots and weight loss measurement.Measurement of an increase of Rctwith time for Mg evidences the formation of protective layer over their surfaces while decrease of Rctwith time for both alloys indicates their corrosion with time.
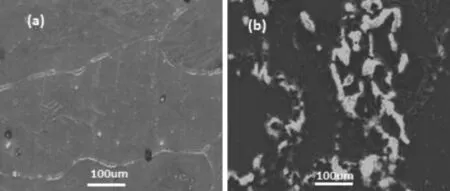
Fig.8.SEM photomicrographs of Mg uncorroded(a)and corroded after 90 min exposure(b).
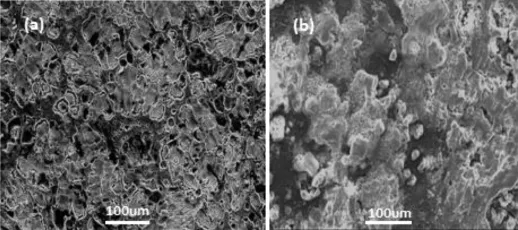
Fig.9.SEM photomicrographs of AZ31 alloy uncorroded(a)and corroded after 90 min exposure(b).
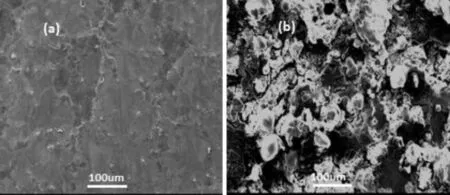
Fig.10.SEM photomicrographs of Mg uncorroded(a)and corroded after 90 min exposure(b).
Acknowledgment
Authors are thankful to director,CSIR-AMPRI for proving laboratory facilities.The present work has not been supported by any funding agency.
[1]J.I.Skar,Mat.Corros.50(1999)2-10.
[2]E.Boese,J.Gollener,A.Heyn,J.Strunz Mat.Corros.52(2001) 247-256.
[3]H.O.Teeple,ASTM,STP 175(1956)89-115.
[4]G.Song,B.Johnnsson,S.Hapugoda,D.Jhon,Corros.Sci.49(2007) 1245-1265.
[5]O.Lunder,J.S.Lein,T.Avenue,K.Nisanisanclolu,Corrosion 45(1989) 741-748.
[6]T.J.Warner,N.Thorne,G.Anussbaum,W.M.Stobbs,J.Surf.Int.Ana. 19(1992)386-392.
[7]F.Hehman,F.H.fross,W.Young,J.Met.39(1987)14-21.
[8]F.H.Cao,V.H.Len,Z.Zhang,V.H.Zhang,X.Zhang,Russ.J.Electrochem.43(2007)837-843.
[9]Y.L.Chang,T.W.Qin,H.M.Wang,Z.Zhang,Trans.Nonferr.Met.Soc. China 19(2005)517-524.
[10]H.W.Huo,Y.Li,H.N.Wang,F.H.Wang,Mater.Rev.15(2001)25-27.
[11]M.C.Zhao,P.J.Uggowitzer,M.Liu,P.Schmutz,G.Song,A.Altre, Trans.Tech.Public(2009)473-478.
[12]P.M.Brandford,B.Case,G.Dearnaley,J.F.Rurner,I.S.Woolseyz, Corros.Sci.169(1976)747-766.
[13]K.G.Cowan,J.A.Harrison,Electrochim.Acta 25(1980)899-912.
[14]N.Pebre,T.Picaud,M.Durprat,F.Dabosi,Corros.Sci.29(1989) 1073-1086.
[15]G.Ruhi,O.P.Modi,I.B.Singh,Surf.Coat.Technol.204(2009) 359-365.
[16]L.Tomcsanyi,K.Tomcsanyi,I.Varga,H.Batrik,E.Horanyi, E.Maleczki,Electrochim.Acta 34(1989)855-859.
[17]I.B.Singh,D.P.Mondal,M.Singh,S.Das,Corros.Sci.51(2009) 234-241.
[18]F.Bellucci,L.Nicodem,Corrosion 49(1993)235-240.
[19]G.L.Song,A.Andrej,D.Mathew,Corros.Sci.41(1999)249-273.
[20]G.L.Song,A.Andrej,X.L.Wu,B.Zhang,Corros.Sci.40(1998) 1769-1791.
Received 17 September 2014;revised 15 January 2015;accepted 2 February 2015 Available online 18 April 2015
*Corresponding author.Tel.:+91 755 2488260.
E-mail address:ibsingh58@yahoo.com(I.B.Singh).
Peer review under responsibility of National Engineering Research Center for Magnesium Alloys of China,Chongqing University.
http://dx.doi.org/10.1016/j.jma.2015.02.004.
2213-9567/Copyright 2015,National Engineering Research Center for Magnesium Alloys of China,Chongqing University.Production and hosting by Elsevier B.V.All rights reserved.
Copyright 2015,National Engineering Research Center for Magnesium Alloys of China,Chongqing University.Production and hosting by Elsevier B.V.All rights reserved.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Infuence of rolling parameters on dynamically recrystallized microstructures in AZ31 magnesium alloy sheets
- Effect of temperature on the mechanical abnormity of the quasicrystal reinforced Mg-4%Li-6%Zn-1.2%Y alloy
- Improving the corrosion resistance of AZ91D magnesium alloy through reinforcement with titanium carbides and borides
- Effects of Ti addition on the microstructure and mechanical properties of Mg-Zn-Zr-Ca alloys
- Phase stability,elastic properties and electronic structures of Mg-Y intermetallics from frst-principles calculations
- Dynamic compressive property and failure behavior of extruded Mg-Gd-Y alloy under high temperatures and high strain rates
