Changes in the blood-nerve barrier after sciatic nerve cold injury: indications supporting early treatment
2015-01-18HaoLiJianpingJiaMinXuLeiZhang
Hao Li, Jian-ping Jia, Min Xu, Lei Zhang
1 Department of Neurology, the First People’s Hospital of Yibin, Yibin, Sichuan Province, China
2 Department of Neurology, Xuanwu Hospital Capital Medical University, Beijing, China
3 Department of Pharmacy, the First People’s Hospital of Yibin, Yibin, Sichuan Province, China
Changes in the blood-nerve barrier after sciatic nerve cold injury: indications supporting early treatment
Hao Li1,*, Jian-ping Jia2, Min Xu2, Lei Zhang3
1 Department of Neurology, the First People’s Hospital of Yibin, Yibin, Sichuan Province, China
2 Department of Neurology, Xuanwu Hospital Capital Medical University, Beijing, China
3 Department of Pharmacy, the First People’s Hospital of Yibin, Yibin, Sichuan Province, China
Severe edema in the endoneurium can occur after non-freezing cold injury to the peripheral nerve, which suggests damage to the blood-nerve barrier. To determine the effects of cold injury on the blood-nerve barrier, the sciatic nerve on one side of Wistar rats was treated with low temperatures (3—5°C) for 2 hours. The contralateral sciatic nerve was used as a control. We assessed changes in the nerves using Evans blue as a fuid tracer and morphological methods. Excess fuid was found in the endoneurium 1 day after cold injury, though the tight junctions between cells remained closed. From 3 to 5 days after the cold injury, the fuid was still present, but the tight junctions were open. Less tracer leakage was found from 3 to 5 days after the cold injury compared with 1 day after injury. The cold injury resulted in a breakdown of the blood-nerve barrier function, which caused endoneurial edema. However, during the early period, the breakdown of the blood-nerve barrier did not include the opening of tight junctions, but was due to other factors. Excessive fuid volume produced a large increase in the endoneurial fuid pressure, prevented liquid penetration into the endoneurium from the microvasculature. These results suggest that drug treatment to patients with cold injuries should be administered during the early period after injury because it may be more diffcult for the drug to reach the injury site through the microcirculation after the tissue fuid pressure becomes elevated.
nerve regeneration; peripheral nerve injury; sciatic nerve; hypothermia; blood-nerve barrier; Evans blue tracer; neural degeneration
Funding:The work was supported by a grant from Sichuan Province Medical Association, “SHIHUIDA” Subject, in China, No. SHD12-21; and the Scientific Research Project of Health Bureau of Yibin City in China.
Li H, Jia JP, Xu M, Zhang L (2015) Changes in the blood-nerve barrier after sciatic nerve cold injury∶ indications supporting early treatment. Neural Regen Res 10(3)∶419-424.
Introduction
Cold-induced damage to human tissue is known as cold injury. Two types of cold injury mainly occur: (1) freezing cold injury induced by temperatures below freezing, and (2) non-freezing cold injury induced by temperature from 0 to 10°C in wet conditions, such as frostbite, trench foot, and immersion foot. The effect of cold on peripheral nerves has been studied since the time of Hippocrates, and studies of the effects of cold temperature on peripheral nerves were reported mostly during military campaigns. For example, such studies were performed during Napoleon’s Russian Campaign, the Crimean War, the First and Second World Wars, the Korean conflict, and the Falklands War (Smith et al., 1915; Greene, 1941; Ungley and Durh, 1942; Blackwood, 1944; Lewis and Moen, 1952; Payne, 1984). Even during peacetime, similar studies were reported based on mountaineers (Marcus, 1979; Imray and Oakley, 2005; Imray et al., 2009), fshermen (Mills and Mills, 1993; Cattermole, 1999), and drunkards in winter (King et al., 1958; Cattermole, 1999), among others. During the China snowstorms in 2008, the incidence of non-freezing cold injury reached 12.78% in Ningbo city, accounting for 67.28% of all kinds of injuries. Although this topic has been studied for a long time, the mechanism of peripheral nerve cold injury is still only poorly understood, resulting in ineffective treatments (Liu et al., 2007; Ying et al., 2009). Early studies suggested that cold-induced nerve injury was primarily caused by ischemia (Denny-brown and Adams, 1945). However, subsequent investigators found that ischemia alone could not account for the peripheral nerve damage observed in non-freezing cold injury (Jia et al., 1998). In fact, non-freezing cold injury was reported to be an “ischemia plus” syndrome, which includes multiple factors such as reperfusion injury, abnormal sodium channels, and inactive transport of temperature-sensitive enzymes (Jia et al., 1998).
Jia and Pollock (1997) reported that there may be damage to the blood-nerve barrier after non-freezing cold injury to the peripheral nerve, found severe edema in endoneurium, possibly indicating the damage to the blood-nerve barrier.However, related research did not continue in-depth at the time. Similar to the effects of the blood-brain barrier on the central nervous system, the blood-nerve barrier plays an important role in maintaining the homeostasis of the neural microenvironment in the peripheral nervous system. Structural damage and changes in the permeability of the bloodnerve barrier have been reported in many autoimmune diseases of the peripheral nervous system including Guillain-Barre syndrome, chronic inflammatory demyelinating polyneuropathy, and multifocal motor neuropathy (Kanda et al., 2000, 2004, 2013). However, few reported studies on non-freezing cold injury to peripheral nerves have examined the changes in the blood-nerve barrier. Therefore, the aim of this study was to determine the effects of non-freezing cold injury on the blood-nerve barrier in a peripheral nerve of a male Wistar rat as a model of non-freezing cold injury.
Materials and Methods
Animals
A total of 48 specifc-pathogen-free adult male Wistar rats weighing 300—400 g and aged 3—4 months (Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China; certificate No. SCXK (Jing) 2002-003) were used in this study. All rats were housed at 25°C and in a relative humidity of 50%, and the experiments were carried out according to the Chinese guidelines for animal care and use. The experimental protocols were approved by the Animal Ethics Committee of Beijing Capital Medical University, China.
Establishing the cold injury model
A cooling model was created as previously described by Jia and Pollock (1997). At 1, 3, and 5 days after cold injury, the injured and non-injured sciatic nerves were observed and compared. The cooling cuff was custom made from copper tubing coated with epoxy resin and shaped into a hollow semicircle. A distance of 15 mm separated the water inlet and outlet tubes. Focal cooling of the sciatic nerve was achieved by circulating cold water through the cuff with an electric circulator (model: F12-ED; Julabo GmbH, Seelbach, Germany) (Figure 1).
First, the rats were anesthetized with an intraperitoneal injection of pentobarbital sodium in distilled water (50 mg/kg). In brief, the sciatic nerves were exposed in the mid-thigh region in order to unilaterally apply the cooling cuff closely beneath the nerve. When the cooling apparatus was running, the local temperature of the sciatic nerve was maintained between 3—5°C for 2 hours. The contralateral sciatic nerve was exposed, but not cooled. The rectal temperature and heart rate of the rats were monitored during the cold injury.
Measurement of Evans blue tracer
At 1, 3, and 5 days after cold injury, eight rats were anesthetized with an intraperitoneal injection of phenobarbital sodium (50 mg/kg) and were then intravenously injected with Evans blue (1 mL per 100 g). The bilateral sciatic nerves were harvested 1 hour after the injection, pressed against a flter paper to remove excess fuid, and weighed. Following a previous method (Saria and Lundberg, 1983), the nerves were then each placed in 1 mL of formamide and incubated for 24 hours in a water bath at 50°C. Colorimetric measurements were made in a microplate reader (model: UV-1800; Suzhou City Branch of Shimadzu Corporation of Japan, Jiangsu Province, China) at the absorption maximum for Evans blue (630 nm). The optical density was converted into a concentration using a standard curve of Evans blue in formamide.
Location of the Evans blue tracer
Following the same method as described above, the bilateral sciatic nerves were each harvested and divided into 5 mm segments. The segments were immediately embedded in Tissue-Tek Optical Cutting Temperature Compound (Sakura Finetek, Torrance, CA, USA), rapidly frozen in liquid nitrogen, and stored at —80°C until use. Transverse 10-µm-thick frozen sections were cut and mounted in glycerine-saline (1:1, v/v) buffer. The fresh sections were viewed under a florescence microscope (model: CX41-32RFL; Olympus, Tokyo, Japan) to measure the intensity of the red fuorescence.
Morphological observation
The cold-injured and contralateral control nerves were harvested from four rats at 1, 3, and 5 days after cold injury and immediately placed in fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M PBS at pH 7.4). The nerves were fxed for 2 hours at 4°C and then washed with 0.1 M PBS, post-fxed in 0.1 M PBS containing 1% osmium tetroxide for 2 hours, dehydrated in ethanol, immersed in propylene oxide, and then embedded in Epon812. Next, the specimens were cut into transverse semi-thin sections and stained with toluidine blue for observation under a light microscope (model: Eclipse 50i POL; Nikon, Melville, NY, USA). Super-thin 50-nm sections were cut with a diamond knife, stained with uranyl acetate followed by lead citrate, and then examined with an electron microscope (model: EM208s; Philips, Eindhoven, the Netherlands).
Statistical analysis
Statistical analyses were performed with SPSS 13.0 software (SPSS, Chicago, IL, USA). The data are presented as the mean ± SD. An analysis of variance with a random variable design was used to assess the effect of time. Further pairwise comparisons were performed using pairedt-test. Values ofP< 0.05 were considered statistically signifcant.
Results
Quantifcation of Evans blue tracer
At 1 day after cold injury, the Evans blue tracer concentration in the cold-injured nerves was signifcantly larger than that in the control nerves (P< 0.01). The tracer concentration decreased over time (P< 0.01), reaching levels similarto the contralateral nerves at days 3 and 5 (P> 0.05; Figure 2).
Changes in the permeability of the endoneurial vasculature after cold injury
The non-injured sciatic nerve from rats showed a bright red fuorescence that was only located in the lumen of the endoneurial blood vessels; none appeared outside the vascular walls after intravenous injection of Evans blue tracer. Under normal conditions, the endoneurial blood vessels appear to be impermeable to the Evans blue tracer. The cooled sciatic nerve segments were examined 1 day after the cold injury. Those sciatic nerves showed an intense red fuorescence within the endoneurium as well as outside of the vasculature. At 3 and 5 days after the cold injury, the red fuorescence was also detected within the endoneurium, but the intensity was clearly decreased compared with 1 day after injury (Figure 3).
Morphological changes in the sciatic nerves of rats after non-freezing cold injury
At 1 day after cold injury, the microscopic examination of the tissue revealed extensive myelinated fiber degeneration in the form of giant empty axons and shrunken dark axons. In addition, the swelling of endothelial cells narrowed the endoneurial capillary lumen. At 3 and 5 days after the cold injury, the myelinated fber degeneration became more acute and the endoneurial capillary lumen continued to narrow. In the control group, the myelinated fbers were occasionally empty or showed dark axons, and the endoneurial capillary remained normal (Figure 4).
The cold-injured sciatic nerves showed extensive myelinated fber degeneration and swollen endothelial cells when observed by electron microscopy. The unmyelinated fbers and tight junctions were preserved at 1 day after cold injury. However, at 3 and 5 days after cold injury, the myelinated fibers showed prominent axonal degeneration and myelin splitting. The endothelial cells remained swollen and the tight junctions were destroyed. Scattered unmyelinated fber degeneration was also observed at 5 days after cold injury (Figure 5).
discussion
In the central nervous system, the blood-brain barrier separates the brain tissue from the blood and regulates the exchange of various substances between the blood and the brain. In the peripheral nervous system, the nerve environment is also maintained by a similar barrier, the bloodnerve barrier, which consists of the endothelial cells of the endoneurial microvessels and the perineurium (Choi and Kim, 2008). The blood-nerve barrier has a low permeability to many substances, preventing some solutes, macromolecules, white blood cells, and bacteria from entering the peripheral nerves by passive diffusion. It also separates peripheral nerves from the circulatory system and has a protective effect (Yosef and Ubogu, 2013). The blood-nerve barrier adjusts the fow of certain ions, nutrients, and other exogenous substances into and out of the nerve tissue through a number of transport proteins; regulates axonal depolarization-repolarization by maintaining the sodium and potassium ion concentration gradient difference; and ensures normal conduction of nerve signals (Ubogu, 2013; Yosef and Ubogu, 2013; Kusunoki, 2014). In a number of common peripheral nerve disorders, including thermal injury, diabetes mellitus, nerve trauma, and nerve compression, changes in the blood-nerve barrier integrity have been observed (Dyck and Giannini, 1996; Omura et al., 2004). Similarly, injuries to the blood-nerve barrier can also be found after non-freezing cold injury.
A number of tracers that can be injected intravenously and subsequently immobilized by chemical fixation of the tissue have been used to assess the permeability of the blood-nerve barrier. The use of fuorescent tracers such as trypan blue, sodium fluorescein, and Evans blue has been reported in these researches. In the present study, a fuorescent tracer (Evans blue) with a molecular weight of 66,200 Da was used to show albumin leakage as an indicator of the vascular permeability of different organs. Evans blue binds quantitatively to albuminin vivoandin vitroand emits an intense red fluorescence, allowing the Evans blue content to be determined by colorimetry at the absorbance maximum of 630 nm. Olsson (1990) showed that under normal circumstances, the endoneurial blood vessels appear to be impermeable to albumin labeled with Evans blue. However, in an injured nerve, such as by crushing or cutting, the endoneurial blood vessels become permeable to labeled albumin, which then leaks into the extracellular space through the injury site. This phenomenon suggests damage to the integrity of the blood-nerve barrier. Evans blue has been extensively used in the study of the loss of barrier function after thermal damage to peripheral nerves (Kiang and Wei, 1987; Berger et al., 2007; Patel et al., 2008), but has seldom been applied to the study of the loss of barrier function in peripheral nerves after non-freezing cold injury. Therefore, an intravenous injection of Evans blue was used here to observe changes in the sciatic nerve barrier function after non-freezing cold injury.
The results showed that on the 1stday of cold injury, an intense red fuorescence was observed in the endoneurium of the cooled segments of the sciatic nerves. At 3 and 5 days after the cold injury, the intense red fuorescence was still detected in the endoneurium. These results indicate that the endoneurial blood vessels became permeable to the labeled albumin, and that there was a large increase in Evans blue content outside of the blood vessels, likely caused by a breakdown of the blood-nerve barrier function. These results are further supported by the electron microscopy experiments, which clearly indicated impairment of the endothelial tight junctions at 3 days after cold injury. In addition, the unmyelinated fbers were preserved, while the large myelinated nerve fbers showed severe axonal degeneration.
In addition to damaging the blood-nerve barrier, the cold injury to the peripheral nerves increased the vascularpermeability, causing endoneurial edema. Endoneurial fuid can elastically stretch the perineurium, producing a large increase in the endoneurial fuid pressure (Myers et al., 1981). Elevation of the endoneurial fluid pressure decreases the diameter of the vascular lumen and may cause an increased resistance to blood fow. In addition, nerve edema may further reduce nerve blood fow, causing endoneurial hypoxia. This damage to the blood-nerve barrier is a complex process that occurs in two stages: an early stage (24 hours after cold injury) during which transportation occurs by pinocytotic vesicles, and a late stage (3 to 5 days after cold injury) characterized by passive inter-endothelial leakage through the damaged endoneurial capillary walls (Nukada et al., 1981). This may explain why we only found tight junction opening on the third day after cold injury, while the leakage of Evans blue-labeled albumin was observed on the first day. Although nerve blood flow gradually increases, returning to the baseline value (Jia and Pollock, 1997, 1999; Jia et al., 1998), it is unclear why there appeared to be quantitatively less leakage of Evans blue-labeled albumin on the third and ffth days than that on the frst day. We believe this may be explained by an elevated endoneurial fluid pressure that increased the absorption of fluid by the microvasculature (Myers et al., 1981).

Figure 1 Schematic of the system used to create the model of cold injury.

Figure 2 Concentration of Evans blue-labeled albumin in rat sciatic nerves at 1, 3, and 5 days after cold injury.
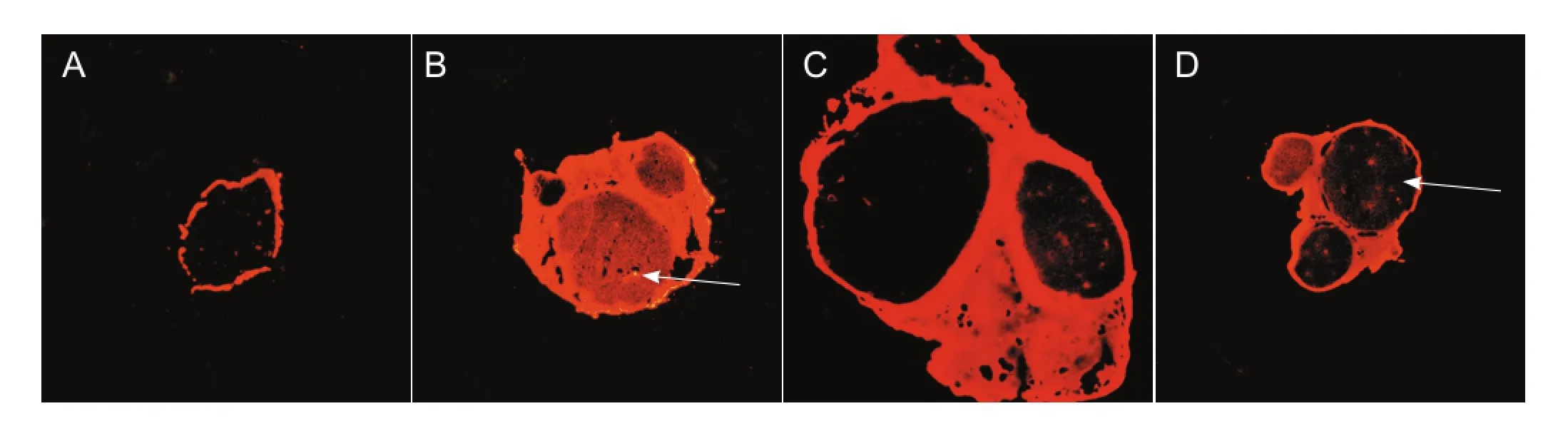
Figure 3 Changes in the permeability of Evans blue tracer in the rat sciatic nerve after cold injury (× 40).
Although the abnormal permeability of endoneurial vessels has been investigated in various experimental neuropathies, the mechanism for this increase remains unclear. Olsson (1966, 1968) suggested that this increase may occur caused by the release of vasogenic amines from degranulating mast cells in the endoneurium. Thus, the increased blood-nerve barrier permeability after cold injury may be attributed to vasogenic amines. We were not able to observe the release of vasoactive amines because of limitations in the experimental conditions, but this explanation should be investigated further.

Figure 4 Pathomorphological changes in transverse sections of rat sciatic nerves (toluidine blue staining, × 400).

Figure 5 Endoneurial vessels in sciatic nerve (electron microscope).
In conclusion, non-freezing cold injury to rat sciatic nerve results in the loss of the blood-nerve barrier function, causing endoneurial edema and possibly hypoxia. The severe nerve edema was more prominent at 1 day after the cold injury than at 3 or 5 days. This phenomenon may be caused by the elevated endoneurial fuid pressure, which increased the absorption of fuid by the microvasculature at 3 to 5 days after the cold injury. Based on these results, we conclude that drug treatments should be given to patients suffering from cold injuries during the early period of injury because it may become more difficult for the drug to reach the injury site through the microcirculation after the tissue fuid pressure becomes elevated.
Author contributions:We are very grateful to You Wu from Department of Neurology, Xinhua Hospital, Zhejiang Province, China for helpful work.
Author contributions:HL reviewed the literature and wrote this manuscript. JPJ, MX and LZ provided critical revision of the manuscript. All authors approved the final version of the paper.
Conficts of interest:None declared.
Berger J, Sprague SM, Wu Y, Davis WW, Jimenez DF, Barone CM, Ding Y (2007) Peripheral thermal injury causes early blood-brain barrier dysfunction and matrix metalloproteinase expression in rat. Neurol Res 29:610-614.
Blackwood W (1944) Studies in the pathology of human ‘immersion foot’. Br J Surg 31:329-350.
Cattermole TJ (1999) The epidemiology of cold injury in Antarctica. Aviat Space Environ Med 70:135-140.
Choi YK, Kim KW (2008) Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep 41:345-352.
Denny-brown D, Adams RD (1945) The pathology of injury to nerve induced by cold. J Neuropathol Exp Neurol 4:305-323.
Dyck PJ, Giannini C (1996) Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol 55:1181-1193.
Greene R (1941) Frostbite and kindred ills. Lancet 238:689-693.
Imray C, Grieve A, Dhillon S, Cauduell Xtreme Everest Reasearch Group (2009) Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J 85:481-488.
Imray CH, Oakley EH (2005) Cold still kills: cold-related illnesses in military practice freezing and non-freezing cold injury. J R Army Med Corps 151:218-222.
Jia J, Pollock M (1997) The pathogenesis of non-freezing cold nerve injury. Observations in the rat. Brain 120:631-646.
Jia J, Pollock M (1999) Cold nerve injury is enhanced by intermittent cooling. Muscle Nerve 22:1644-1652.
Jia J, Pollock M, Jia J (1998) Cold injury to nerves is not due to ischaemia alone. Brain 121:989-1001.
Kanda T (2013) Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry 84:208-212.
Kanda T, Numata Y, Mizusawa H (2004) Chronic infammatory demyelinating polyneuropathy: decreased claudin-5 and relocated ZO-1. J Neurol Neurosurg Psychiatry 75:765-769.
Kanda T, Yamawaki M, Iwasaki T, Mizusawa H (2000) Glycosphingolipid antibodies and blood-nerve barrier in autoimmune demyelinative neuropathy. Neurology 54:1459-1464.
Kiang JG, Wei ET (1987) Corticotropin-releasing factor inhibits thermal injury. J Pharmacol Exp Ther 243:517-520.
King RC, Parrish JA, Allibone A (1958) Trench-foot in peacetime England. Br Med J 1:1099-1102.
Kusunoki S (2014) How is the blood-nerve barrier involved in the pathogenetic mechanisms of multifocal motor neuropathy? J Neurol Neurosurg Psychiatry 85:473.
Lewis RB, Moen PW (1952) Experimental immersion leg. Am J Med Sci 224:529-539.
Liu YH, Yang WN, Yang GY, Chen XY (2007) Analysis on related factors of 200 cases’ frostbite resulting in disability in cold highland areas. Linchuang Junyi Zazhi 35:94-95.
Marcus P (1979) “Trench foot” caused by the cold. Br Med J 1:622.
Mills WJ, Mills WJ (1993) Peripheral non-freezing cold injury: immersion injury. Alaska Med 35:117-128.
Myers RR, Powell HC, Heckman HM, Costello ML, Katz J (1981) Biophysical and pathological effects of cryogenic nerve lesion. Ann Neurol 10:478-485.
Nukada H, Pollock M, Allpress S (1981) Experimental cold injury to peripheral nerve. Brain 104:779-811.
Olsson Y (1966) Studies on vascular permeability in peripheral nerves. 2. Distribution of circulating fuorescent serum albumin in rat sciatic nerve after local injection of 5-hydroxytryptamine, histamine and Compound 48/80. Acta Physiol Scand 42:284.
Olsson Y (1968) Mast cells in the nervous system. Int Rev Cytol 24:27-70.
Olsson Y (1990) Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol 5:265-311.
Omura K, Ohbayashi M, Sano M, Omura T, Hasegawa T, Nagano A (2004) The recovery of blood-nerve barrier in crush nerve injury-a quantitative analysis utilizing immunohistochemistry. Brain Res 1001:13-21.
Patel TH, Sprague S, Lai Q, Jimenez DF, Barone CM, Ding Y (2008) Blood brain barrier (BBB) dysfunction associated with increased expression of tissue and urokinase plasminogen activators following peripheral thermal injury. Neurosci Lett 444:222-226.
Payne R (1984) Lessons of the Falklands. World Med J 17:26-27.
Saria A, Lundberg JM (1983) Evans blue fuorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods 8:41-49.
Smith JL, Ritchie J, Dawson J (1915) Clinical and experimental observations on the pathology of trench frostbite. J Pathol Bacteriol 20:159-190.
Ubogu EE (2013) The molecular and biophysical characterization of the human blood-nerve barrier: current concepts. J Vasc Res 50:289-303.
Ungley CC, Durh MD (1942) Peripheral vasoneuropathy after chilling“immersion foot and immersion hand”. Lancet 240:447-451.
Ying YY, Zhu YC, Xu R, Dong HJ, Shi NF, Xu LR, Cai YB, Ma X, Ping JM, XU GZ (2009) Epidemiological investigation of damage caused by frozen rain and snow disaster in Ningbo area at the year 2008. Weisheng Yanjiu 38:463-464.
Yosef N, Ubogu E (2013) An immortalized human blood-nerve barrier endothelial cell line for in vitro permeability studies. Cell Mol Neurobiol 33:175-186.
Copyedited by McCarty W, Yajima W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*
10.4103/1673-5374.153690
http://www.nrronline.org/
Accepted: 2014-12-12
杂志排行
中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- TAM receptors: two pathways to regulate adult neurogenesis
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters
