TAM receptors: two pathways to regulate adult neurogenesis
2015-01-18KassandraJohnson,RuiJi
TAM receptors: two pathways to regulate adult neurogenesis
Introduction of neurogenesis:Neurogenesis is a process characterized by the production and differentiation of new neurons from neural stem cells (Emsley et al., 2005). This was previously thought to occur in prenatal and early postnatal development only; however, several studies have shown that it occurs continuously in our adult brains as well, mainly in the lateral ventricles of the brain, the lining of the subventricular zone (SVZ), and the subgranular zone (SGZ) of the dentate gyrus (part of the hippocampal complex). Neurogenesis may also be induced in the adult brain by injury or degeneration of the central nervous system (CNS). In this instance, new neurons have been found in other parts of the brain, such as in the neocortex, amygdala, substantia nigra and tegmentum of the midrain, the brain stem and spinal cord (Wang and Jin, 2014). There are numerous steps involved in differentiating neural stem cells into fully-grown neurons, starting with stem cell proliferation, then migration and survival, followed by commitment to neuronal lineage, and lastly the assimilation of the new neurons into existing brain circuits, ranging from up in the neocortex to down in the spinal cord. Neurogenesis is a dynamic process that is modulated by several factors, both intrinsic and extrinsic, such as growth and transcriptional factors, cell surface receptors, signal transduction molecules, and cytokine or chemokines. In adult brains, physiological as well as pathological conditions can affect neurogenesis. Neural stem cell proliferation and neuronal differentiation can be inhibited by infection or invoked infammation. The interruption of neurogenesis in adult brains leads to hippocampus-dependent learning and behavior impairment (Yan et al., 2007).
Introduction of TAM receptors:Recently, a close relationship between adult neurogenesis and a subfamily of protein tyrosine kinases (PTK), which includes Tyro3, Axl, and Mertk (TAM), was found in our lab (Ji et al., 2014b). These are all expressed on cellular plasma membranes with an extracellular N-terminus, serving as ligand-binding domains located outside of the cellular surface (Lemke and Rothlin, 2008). The intracellular C-terminus of the protein contains a large kinase domain, as well as a short sequence with some tyrosine residue. The activation of the tyrosine residue on the C-terminal causes it to become phosphorylated, which allows it to act like a docking site for downstream signaling molecules. Structurally similar proteins, growth-arrest-specifc 6 (GAS6) and protein S, have both been identifed as TAM receptor ligands. When these two proteins bind to TAM receptors, dimerization and activation of the receptor are triggered, resulting in the recruitment, phosphorylation, and activation of several downstream signaling proteins. This causes changes in gene expression as well as biological responses. TAM receptors along with their ligands are expressed broadly in several systems, including the immune, nervous, vascular, and reproductive systems. These TAM receptors and ligands have been identified as growth trophic receptors for cell survival in many cell types and cell lineages, which include vascular smooth muscle cells, endothelial cells, mesangial cells, fibroblasts, peripheral macrophages, testicular cells, and lens epithelial cells. Along with these growth trophic effects of the TAM signaling pathway, anti-apoptotic effects have been found in a number of cell types in the nervous system as well, including neurons, Schwann cells, and oligodendrocytes.
TAM receptors maintain adult neurogenesis by inhibiting microglia and astrocyte:It has been hypothesized that TAM receptors inhibit prolonged and unrestricted inherent immune responses of macrophages and dendritic cells. They do this by regulating the expression of suppressors of cytokine signaling proteins (SOCSs) and/or twist proteins, which either terminates cytokine receptor-mediated signaling or inhibits nuclear factor kappa B (NF-κB) transcriptional activity. Microglia, which are resident macrophages of the brain and spinal cord, express a wide range of cytokine receptors, Toll like receptors (TLRs), as well as all parts of the IKKNF-κB signaling pathway. Microglia also serve as immunocompetent cells to the brain and spinal cord and are responsible for protecting the CNS from numerous pathogenic factors, such as age-related protein aggregates, damaged neurons, and infectious agents. Chronic activation of the microglia cells, though, causes damage to neurons by releasing neurotoxic molecules. Such molecules include proinflammatory cytokines, complement proteins, and reactive oxygen species, and are detrimental to normal neural function and to the process of neurogenesis. Microglia can also be activated by proinflammatory cytokines, tumor necrosis factors (TNF-α) and lipopolysaccharide (LPS), among others, after which they can produce many proinfammatory mediators. Microglial infammation due to LPS has been found to cause proinfammatory cytokines to be released. This affects neural stem cell proliferation and neuronal differentiationin vitro, and inhibits adult neurogenesis in the hippocampus (Monje et al., 2003).
The TAM receptors, which are expressed by microglia, negatively regulate dendritic cells and macrophages, and keep the microglia from becoming hyperresponsive to activation (Ji et al., 2013). Without TAM receptors, microglia produce increased amounts of proinflammatory cytokines when activated by poly I:C, LPS, and CpG (acting through TLR3, TLR4, and TLR9, respectively), which suggests that TAM receptors serve as negative mediators in regulating microglial innate immune responses. Consistent with these fndings, the conditioned medium from LPS-stimulated TAM TKO (Triple knockout) displayed more severe neurotoxicity for neural stem cells as can be seen by an increase in apoptosis, and a decrease in proliferation of the neural stem cells as well as neuronal differentiation. The key component in the medium conditioned by the TKO microglia has been identifed as interleukin 6 (IL-6). This is because either the IL-6 neutralizing body, or the IL-6-/-TKO microglial conditioned medium, can reverse the TKO microglial conditioned medium’s neurotoxic effects on the neural stem cell. Furthermore, by knocking out the IL-6 gene in the TAM TKO background, the decreased adult hippocampal neurogenesis seen in the TKO mice was reversed to the level compatible to that of the wild-type control mice. These results suggest that IL-6 plays an important role in the TKO microglia, and when upregulated, proves neurotoxic to neurogenesis in the adult hippocampus in the TKO mice. Another proinfammatory cytokine, TNF-α, was also upregulated in the activated TKO microglia. However, neither neutralizing antibody nor TNF receptor knockout in the TKO background restored neurogenesis, excluding the TNF-α as a mediator linking the hyperreactive TKO microglia and halted neurogenesis.
Astrocyte is another kind of immune modulating cell in the CNS and is able to produce inflammatory neurotoxic mediators. Like microglia, astrocytes express TLRs, the three TAM receptors, and IL-1β receptors. The cultured TKO astrocytes, in response to LPS activation, released a greater amount of IL-6 when compared to wild type cells. This suggests that TAM receptors negatively regulate in astrocytes as well. Because astrocytes express IL-1β receptors, when compared to wild type cells, IL-1β stimulation in the TKO astrocytes evoked a stronger IL-6 and IL-1β expression. Most likely, astrocytes are further stimulated to produce more proinfammatory cytokines by the heightened production of IL-1β by TKO microglia, which is detrimental to neurogenesis in the adult hippocampus.
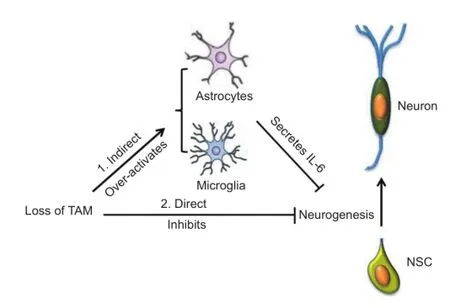
Figure 1 A schematic description of how TAM receptors regulate adult neurogenesis.
The overwhelming activation of MAP kinases in response to pathogenic reagents stimulation is the likely cause of the negative impact of TAM receptors on activated microglia and astrocytes. P38 has been indicated as a key MAP kinase in microglia for regulating proinflammatory cytokine production. Upon TLR activation, the TKO microglia exhibited a stronger activation of p38 when compared to wild type cells. The enhanced activation of p38, with possible help from other MAP kinases like pERK1/2, is most likely responsible for the amplifed production of proinfammatory cytokines.
TAM receptors maintain adult neurogenesis by supporting neural stem cells:As mentioned before, overly reactive microglia in TKO mice produced an increased level of proinfammatory cytokines, which are harmful to neural stem cell proliferation as well as neuronal differentiation. When both cell types were pretreated with a LPS-treated microglial conditioned medium, though, compared to wild type neural stem cells, the number of β-tubulin III+neurons differentiated from the TKO neural stem cells displayed even more drastically decreased neuronal differentiation. This is also true fromin vivostudy in which the TKO brain showed further decline with regard to NSC proliferation and differentiation into neurons than did the wild-type brains that had undergone the LPS-induced infammation. These fndings imply that the TAM receptors may be essential in neural stem cell proliferation and differentiation.
TAM receptors were originally considered as growth trophic receptors, since they were initially cloned from fast growing and transformed cells, as well as the fact that they are upregulated in these kinds of cells. TAM receptors also sustain cell growth and survival, and they support neuronal differentiation of PC12 cells when neuronal growth factor is stimulated. When comparing the neuronal predecessor to the offspring differentiated neuronal cells in a genome-wide analysis of the differential expressed genes, all three TAM receptors can be found in the embryonic cortical neuronal predecessor cells. Early differentiation and migration of the subventricular zone neural stem cells in mice is caused by the lack of Axl and Mertk. Also, knocking out Gas6, their common ligand, decreased the number of neural stem cells in the subventricular zone. All of this shows that TAM receptors may perform important fundamental functions in the maintenance of cortical neuronal predecessor cell identity, as well as in regulating neural stem cell survival, proliferation, and neuronal differentiation.
Neural stem cells that lack TAM receptors exhibited slow growth, decreased proliferation, decreased level of neuronal differentiation, and increased cell death. In our lab, we discovered that the TKO neural stem cells exhibited reduced NGF, but increased expression of TrkA, TrkB, and TrkC, which implies that the TAM receptors may operate in conjunction with neurotrphins in neural stem cells (Ji et al., 2014a). Moreover, according to some other studies, ERK pathway might be the main factor TAM receptors to regulate neural stem cell proliferation and differentiation. With a lack of TAM receptors, phosphorylation of ERK is upregulated and suppresses Krüppel-like factor (Klf4), which induces neural stem cells to cease self-renewal and begin differentiation (Kim et al., 2012).
Conclusion:TAM receptors are expressed in several facets of the CNS, including neural stem cells, microglia, and astrocytes (Figure 1). They have a dual role in regulating neurogenesis both directly by supporting neural stem cells, and indirectly by inhibiting glia. Based on our lab’s research and that of other researchers, there may be clinical implications for the TAM receptor mediated signaling pathway as a potential target for treatment in certain neurodegenerative diseases that are accompanied with neurogenesis loss. Activation of the TAM receptors by their ligands, Gas6 and Protein S, is postulated to improve neurogenesis in treatment of neurodegenerative diseases. Gas6 has been shown to activate several downstream signaling pathways including MAPK/ERK, PI3K/AKT, and JAK/ STAT. These pathways can inhibit severe infammation observed in neurodegenerative patients’ brains, but they can also activate classic oncogenic networks (Linger et al., 2008). Therefore, how to selectively activate TAM receptors so as to reduce chronic infammation and promote adult neurogenesis without activation of the oncogenic signal transduction networks is still a long way to go.
Kassandra Johnson, Rui Ji*
Department of Biochemistry and Molecular Biology, University of Louisville School of Medicine, Louisville, KY 40202, USA
*Correspondence to: Rui Ji, Ph.D., r0ji0001@louisville.edu.
Accepted:2015-02-06
Emsley JG, Mitchell BD, Kempermann G, Macklis JD (2005) Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol 75:321-341.
Ji R, Meng L, Jiang X, Cvm NK, Ding J, Li Q, Lu Q (2014a) TAM receptors support neural stem cell survival, proliferation and neuronal differentiation. PLoS One 9:e115140.
Ji R, Meng L, Li Q, Lu Q (2014b) TAM receptor defciency affects adult hippocampal neurogenesis. Metab Brain Dis doi: 10.1007/s11011-014-9636-y.
Ji R, Tian S, Lu HJ, Lu Q, Zheng Y, Wang X, Ding J, Li Q, Lu Q (2013) TAM receptors affect adult brain neurogenesis by negative regulation of microglial cell activation. J Immunol 191:6165-6177.
Kim MO, Kim SH, Cho YY, Nadas J, Jeong CH, Yao K, Kim DJ, Yu DH, Keum YS, Lee KY, Huang Z, Bode AM, Dong Z (2012). ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat Struct Mol Biol 19:283-290.
Lemke G, Rothlin CV (2008). Immunobiology of the TAM receptors. Nat Rev Immunol 8:327-336.
Linger R, Keating AK, Earp HS, Graham DK (2008) TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 100:35-83.
Monje ML, Toda H, Palmer TD (2003) Infammatory blockade restores adult hippocampal neurogenesis. Science 302:1760-1765.
Wang B, Jin K (2014) Current perspectives on the link between neuroinfammation and neurogenesis. Metab Brain Dis doi: 10.1007/s11011-014-9523-6.
Yan XB, Wang SS, Hou HL, Ji R, Zhou JN (2007) Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia. Behav Brain Res 177:282-289.
10.4103/1673-5374.153671 http∶//www.nrronline.org/
Johnson K, Ji R (2015) TAM receptors∶ two pathways to regulate adult neurogenesis. Neural Regen Res 10(3)∶344-345.
杂志排行
中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters
- Empowering sonic hedgehog to rescue brain cells after ischemic stroke
