RAFting the rapids of axon regeneration signaling
2015-01-18JianZhong
RAFting the rapids of axon regeneration signaling
The functional regeneration of damaged axons and severed connections in the mature central nervous system (CNS) remains a challenging goal of neurological research. Mature CNS neurons are refractory to axon regeneration for two major reasons, one, because the activity of cell-intrinsic mechanisms that drive axon growth during development is low — and often further suppressed after an injury — and two, because certain molecules that are part of mature extracellular matrix and myelin act as strong inhibitors of axon growth. Genetic removal of growth inhibitory molecules can increase axon sprouting, but is not sufficient to enable long-range axon growth. Since axon growth is robust during early developmental stages, it has long been hypothesized that mature injured neurons may be “reprogrammed” to the earlier growth state by re-activation of the intracellular growth signaling cascades that drive axon elongation in the developing fetus.
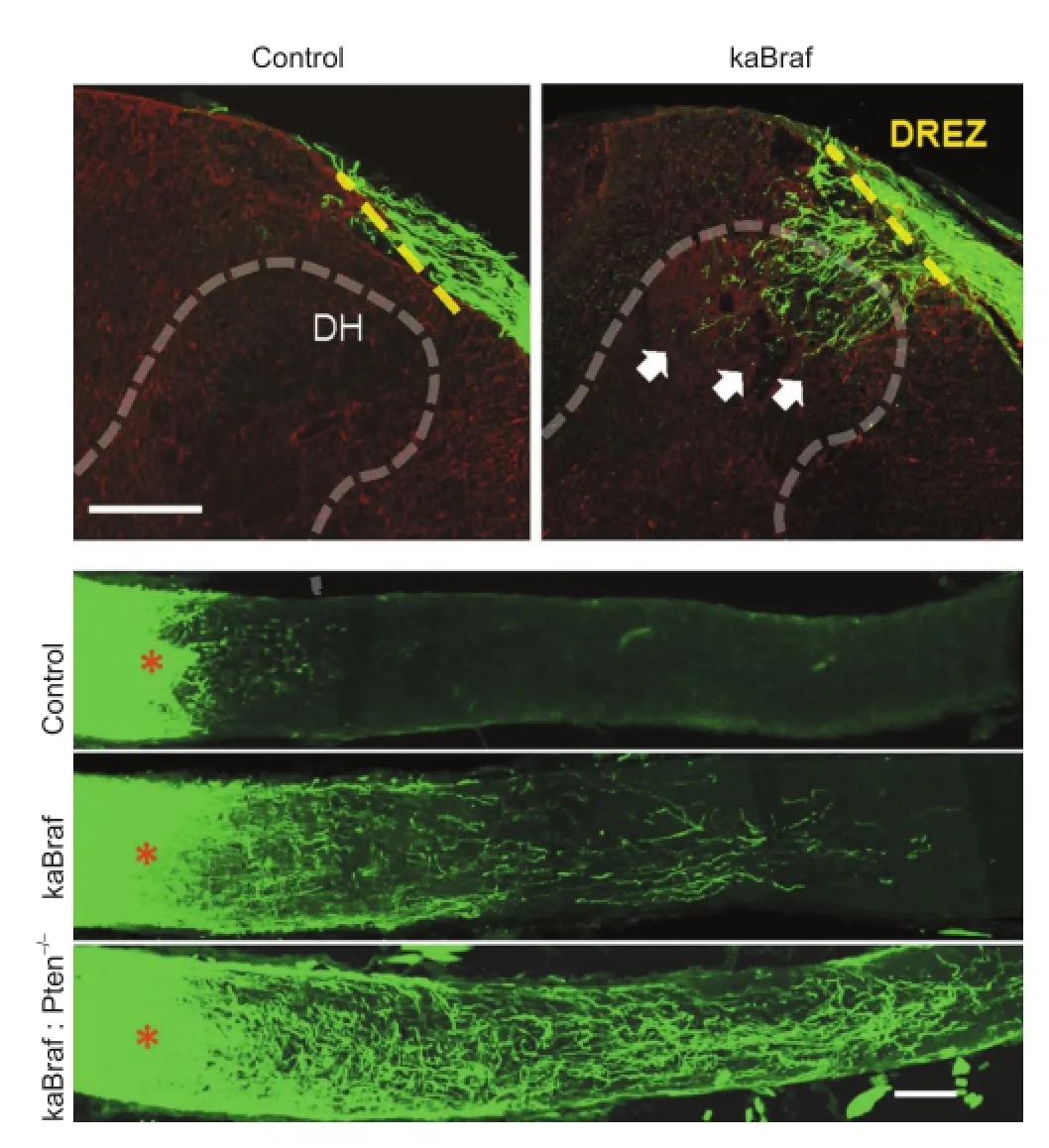
Figure 1 B-RAF signaling promotes robust axon regeneration in the spinal cord and in the optic nerve.
Many aspects of developmental axon growth mechanisms, especially in the periphery, are now well understood. The most prominent examples are the peptide growth factors of the neurotrophin family, acting on Trk family receptor tyrosine kinases to trigger multiple interlinked signaling cascades in developing sensory neurons. Among these cascades, the rapidly accelerated fbrosarcoma (RAF)-mitogen-activated protein kinases (MEK)-extracellular signal-regulated kinases (ERK) pathway has been strongly implicated in axon growth signaling, while the PI3 kinase (PI3K)-AKT-mTOR pathway has been predominantly linked to anti-apoptotic and anabolic signaling. Both of these aspects co-operate to optimize neuronal development and function. Blocking of RAF kinase signaling is sufficient to block neurotrophin-induced axon growth in embryonic dorsal root ganglion (DRG) neurons bothin vitroandin vivo(Markus et al., 2002; Zhong et al., 2007), and in the absence of nerve growth factor (NGF)/tropomyosin receptor kinase A (TrkA) signaling, activation of RAF signaling strongly promotes axon elongation of embryonic sensory neurons in culture (Markus et al., 2002). We have further embarked on a series of studies of the effects of elevated neuronal RAF signaling in promoting axon growth and regenerationin vivo. As recently reported (O’Donovan et al., 2014), the activation of B-RAF, by itself, can drive axon growth in models of both fetal development and adult post-injury regeneration. The peripheral axons of nociceptive sensory neurons depend on NGF/TrkA signaling for cutaneous innervation. Conditional knock-in expression of a kinase activated (ka) B-RAF (B-RAFV600E) in these neurons allowed for full elongation and innervation of the epidermis in embryos lacking TrkA signaling. Even more excitingly, activation of B-RAF enabled re-innervation of the dorsal horn of the spinal cord by regenerating sensory fbers in adult mice after dorsal root crush injury (Figure 1). The underlying mechanism seems unrelated to that invoked by preconditioning peripheral lesion because the transcription factor activating transcription factor 3 (ATF3) was not elevated by kaB-RAF expression. Furthermore, we observed robust regenerative growth of large numbers of retinal axons in crushed adult optic nerve, with some regenerating fbers reaching and crossing the optic chiasm (Figure 2).

Figure 2 Signaling molecules linked to axon regeneration.
The ability of kaB-RAF expressing neurons to overcome inhibitory molecules expressed in the injured tissue was foreshadowed in our embryonic experiments, where we observed a dramatic overgrowth of kaB-RAF-expressing central nociceptive axons in the dorsal horn of the spinal cord, extending substantially into normally ‘forbidden’ deeper layers. Repulsive surface molecules including Semaphorins and Plexins in these areas usually act to keep nociceptor nerve endings confned to the most superfcial layer of the dorsal horn (Yoshida et al., 2006). The adult central nervous system expresses abundant growth inhibitory cues. Spinal cord injury (SCI) further upregulates inhibitory signals in the lesion site (Sharma et al., 2012). It will be interesting to see whether B-RAF activation can enable substantial axon regeneration in SCI scenarios as it does in the optic nerve.
B-RAF promotes axon elongationviathe canonical downstream Ser/Thr kinase effectors MEK1 and MEK2. The RAF-MEK-ERK cascade is a well-studied pathway that regulates and modulates numerous cellular processes, including axonal transport, local protein synthesis and gene expression patterns. Useful targets to promote axon regeneration are likely to be found among transcription factors or epigenetic mechanisms, which typically increase or restrict the expression of groups of functionally linked genes, such as genes involved in axon extension. We found that both nerve growth factor (NGF) and increased B-RAF signaling increase the binding activity of Egr family transcription factors (Zhong et al., 2007). The Egrs are immediate early genes known to be required for NGF-induced axon growth (Levkovitz et al., 2001). Regarding epigenetic regulation, activated B-RAF-dependent DNA de-methylation and ectopic induction of a neuronal differentiation marker microtubule-associated protein 2 (MAP2) has been shown in non-neuronal cells (Maddodi et al., 2010), however role of DNA methylation status in axon extension awaits further study.
From a druggability point of view, it is likely to be easier to inhibit intracellular growth-inhibitory pathways than to directly activate growth-promoting pathways such as B-RAF signaling. Several growth inhibitory signaling molecules have already been identified, in particular phosphatase and tensin homolog (PTEN), suppressor of cytokine signaling 3 (SOCS3) and krüppel-like factor 4 (KLF4) discussed below. But there certainly are more to be discovered, in particular among the phosphatases. As Ser/Thr kinases, the RAFs and MEKs are subject to negative regulation by phosphatases. In non-neuronal cells, protein phosphatase 2A (PP2A), PH domain and leucine rich repeat protein phosphatase 1/2 (PHLPP1/2), dual specificity phosphatase 5 (DUSP5) and other phosphatases have been shown to antagonize MAP kinase pathway signaling in various contexts; their function in neurons remains to be tested. The phosphatase DUSP6 has recently been implicated in downregulation of ERK activity in sensory neurons (Finelli et al., 2013). Interestingly these authors found that NGF itself,viathe transcription factor Smad1, increases DUSP6 expression, resulting in negative feedback regulation of NGF -MAP kinase signaling. Elevated expression of phosphatases dampening MAP kinase signaling may be one cause of the reduced growth competency in mature CNS neurons.
The most dramatic optic nerve axon regeneration was seen in mice carrying both the conditional kaB-RAF and the PTEN loss-of-function alleles. PTEN is a phosphatase that antagonizes PI3K-AKT signaling. PTEN deletion results in increased activity of PI3K-AKT-mTOR signaling,i.e., it activates the second major, anabolic growth-associated intracellular signaling pathway that is typically engaged by growth factor tyrosine kinase receptors such as TrkA.
Combinatory approaches such as the co-activation of RAF and PI3K are likely to be required to achieve suffcient axon regeneration that could enable axons to re-innervate their target tissues. Cytokine signaling has long been implicated in peripheral axon regeneration (Zhong et al., 1999). Cytokines through the gp130 receptor activate the Janus kinases (JAKs), which in turn trigger STAT family transcription factors. The combination of enhanced JAK-STAT signaling,viadeletion of its negative regulator SOCS3, with PTEN deletion has been reported to further enhance optic nerve regeneration over PTEN deletion alone (Sun et al., 2011). The growth-promoting transcription factor STAT3 directly interacts with the inhibitory transcription factor KLF4, and deletion of KLF4 by itself enabled some axon regeneration in the optic nerve (Moore et al., 2009), dependent on Janus Kinase (JAK) activity (Qin et al., 2013). In addition, a study combining PTEN deletion with elevation of intraretinal cAMP activity and infammation of the eye reported for the frst time a partial recovery of specifc visual functions (de Lima et al., 2012). While still subject to detailed verifcation, these results are exciting.
Our own initial gene expression profiling study (un-published) indicates that B-RAF activates a genetic program that is distinct from those seen after PTEN or SOCS3 deletion. KLF4 expression was not affected by B-RAF activation. Therefore, activation of RAF signaling in combination with any of these paradigms is likely to further strengthen CNS axon regenerative growth. Figure 2 is an abbreviated schematic of the intracellular signaling mediators discussed here that have been shown to, or may be, involved in the regulation of mammalian CNS axon regeneration.
Finally, the combination of activated intracellular growth pathways with neutralization of extracellular growth inhibitory molecules needs further investigation. Degradation of the extracellular matrix component chondroitin sulfate proteoglycan (CSPG) has been shown to enhance axonal sprouting and regeneration. Myelin-associated axon growth inhibitory molecules have also been intensively studied over the past decade. Whether axonal sprouting or generation can be further promoted by a combination of B-RAF activation and removal of the inhibitory signals is currently under investigation.
Activation of B-RAF kinase or its growth-promoting downstream signaling mechanisms may also be helpful in clinical conditions besides traumatic nerve injury. Many neurodegenerative disorders involve axon damage and die-back, glaucoma and amyotrophic lateral sclerosis (ALS) being well-characterized examples. Activation of intracellular axon growth signals may render axons more resilient to metabolic stress in a degenerative environment. Given its effcacy in crushed retinal axons, it will be interesting to test the effects of B-RAF activation in rodent models of glaucoma, where degeneration of retinal axons at the level of the optic nerve head eventually leads to the death of retinal ganglion neurons, with concomitant irreversible loss of vision.
While we are still far from being able to reverse the consequences of spinal cord and other CNS injuries, real progress has been made in the recent years, especially in the regard of the activation of intracellular signaling pathways to drive CNS axon regeneration. This approach, in combination with others, may eventually lead us to meaningful regeneration and functional recovery following traumatic injury and degenerative lesions to the CNS.
I gratefully acknowledge funding from the National Eye Institute (R01EY022409), the Craig H. Neilsen Foundation (296098), the Wings for Life Foundation (WFL-US-028/14), the New York State Spinal Cord Injury Research Trust Fund, and the Burke Foundation. All former and current members of the Zhong laboratory as well as our collaborators are acknowledged for their contribution and support to the work which forms the basis of this article. Annette Markus is acknowledged for discussion and special support. I sincerely apologize to all those colleagues whose important work is not cited due to space constraints.
Jian Zhong*
Burke Medical Research Institute, Brain and Mind Research Institute, Weill Medical College of Cornell University, White Plains, NY, USA
*Correspondence to: Jian Zhong, Ph.D., jiz2010@med.cornell.edu.
Accepted:2015-01-16
de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L (2012) Fulllength axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A 109:9149-9154.
Finelli MJ, Murphy KJ, Chen L, Zou H (2013) Differential phosphorylation of Smad1 integrates BMP and neurotrophin pathways through Erk/Dusp in axon development. Cell Rep 3:1592-1606.
Levkovitz Y, O’Donovan KJ, Baraban JM (2001) Blockade of NGF-induced neurite outgrowth by a dominant-negative inhibitor of the egr family of transcription regulatory factors. J Neurosci 21:45-52.
Maddodi N, Bhat KM, Devi S, Zhang SC, Setaluri V (2010) Oncogenic BRAFV600E induces expression of neuronal differentiation marker MAP2 in melanoma cells by promoter demethylation and down-regulation of transcription repressor HES1. J Biol Chem 285:242-254.
Markus A, Zhong J, Snider WD (2002) Raf and akt mediate distinct aspects of sensory axon growth. Neuron 35:65-76.
Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL (2009) KLF family members regulate intrinsic axon regeneration ability. Science 326:298-301.
O’Donovan KJ, Ma K, Guo H, Wang C, Sun F, Han SB, Kim H, Wong JK, Charron J, Zou H, Son YJ, He Z, Zhong J (2014) B-RAF kinase drives developmental axon growth and promotes axon regeneration in the injured mature CNS. J Exp Med 211:801-814.
Qin S, Zou Y, Zhang CL (2013) Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat Commun 4:2633.
Sharma K, Selzer ME, Li S (2012) Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol 237:370-378.
Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z (2011) Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480:372-375.
Yoshida Y, Han B, Mendelsohn M, Jessell TM (2006) PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron 52:775-788.
Zhong J, Dietzel ID, Wahle P, Kopf M, Heumann R (1999) Sensory impairments and delayed regeneration of sensory axons in interleukin-6-defcient mice. J Neurosci 19:4305-4313.
Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD (2007) Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci 10:598-607.
10.4103/1673-5374.153670 http∶//www.nrronline.org/
Zhong J (2015) RAFting the rapids of axon regeneration signaling. Neural Regen Res 10(3)∶341-343.
杂志排行
中国神经再生研究(英文版)的其它文章
- TAM receptors: two pathways to regulate adult neurogenesis
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters
- Empowering sonic hedgehog to rescue brain cells after ischemic stroke
