Functional regeneration of the brain: white matter matters
2015-01-18TengGuan,JimingKong
Functional regeneration of the brain: white matter matters
In the human brain, white matter makes up about 50% of the brain volume and consumes 43.8% of the brain’s total energy budget for resting potential maintenance (Harris and Attwell, 2012). Composed of primarily myelinated axons, the white matter is the “highways and subways of the brain”connecting one region to another and traffcking in and out of the grey matter. In the myelinated nerve fbers, layers of myelin sheaths wrap around each axon to provide protection and insulation to the axon and to allow rapid conduction of action potentials. Myelin establishment and maintenance is considered a crucial requirement for fully functional connections between neurons in the central nerve system (CNS).
Myelin biogenesis and oligodendrocyte precursors:Myelin in CNS is formed by oligodendrocytes, which arise from oligodendrocyte precursor cells (OPCs). During embryonic development, OPCs are generated from progenitor zones in the telencephalon in developmental succession from ventral to dorsal. Compared with the restricted localization in early development, OPCs are found widely distributed in the adult CNS, accounting for 5—8% of the total cell population. Under physiological conditions, adult OPCs persist in substantial numbers, are relatively quiescent but retain regenerative capacities to maintain white matter homeostasis. In response to neuronal activity, OPCs proliferate, migrate and make contact with axons (Figure 1), and then differentiate into mature oligodendrocytes to generate myelin (Gibson et al., 2014). It is unclear how the neuronal signals facilitate initial axon-OPC matching. Nevertheless, the processes of OPCs are filopodia-like but become stabilized after their initial axon contact. Using live imaging in zebrafsh, Czopka et al. (2013) show that the time window for active myelin sheath generation is short and restricted, relative to the life of individual oligodendrocyte. In the human brain, such a OPCs based myelinated fber microstructure alteration is a highly “plastic” and dynamic process for optimal cognitive and behavioral functions.
Remyelination in neurological disorders:Disruption of CNS myelin occurs in numerous pathological conditions, including demyelinating neurodegenerative diseases, cerebral ischemia, and psychiatric disorders. Oligodendrocyte death and subsequent axonal demyelination is a hallmark feature of white matter pathology. On the other hand, demyelination often suffices to cause functional deficits of the nervous system. Remyelination is therefore a key to restore such neurological defcits. Cell transplantation studies reveal that mature oligodendrocytes lose their capability to remyelinate new axons. In response to myelin loss, OPCs are capable of generating new oligodendrocytes to remyelinate axons. At early disease stages, myelin injury and oligodendrocyte loss may trigger OPCs to differentiate into myelinating oligodendrocytes. With disease progression, however, remyelination may eventually fail due to exhaustion of OPCs.
Arguably, structural and functional integrity of myelin represents a vulnerable element of the complex CNS system and is thus implicated in a broad range of neurological diseases. In our laboratory, we focus on white matter repair following cerebral ischemia. Subcortical white matter stroke accounts for approximately 30% of all stroke subtypes, and white matter injury is a component of most classes of stroke damage. In common with multiple sclerosis, traumatic brain injury and spinal cord injury, white matter stroke can trigger the endogenous, albeit limited, repair capacity through OPCs. We aim to elucidate the pathophysiology of white matter stroke and try to develop therapies designed specifically to attenuate oligodendrocyte damage and to enhance functional remyelination. Given the central role of oligodendrocyte in ischemic white matter injury, we have investigated vulnerability of oligodendrocytes and their precursor cells, and reported that pre-myelinating OPCs are the most vulnerable cells in the brain to hypoxic or ischemic stimuli (Li et al., 2013). OPCs are also more sensitive to oxidative stress and inflammatory injury than mature oligodendrocytes as well (Thorburne and Juurlink, 1996). Our recent work reveals that OPCs require extraordinarily high energy supply in order to differentiate into myelinating oligodendrocytes and synthesize myelin lipid, an initial step for myelin repair. Because of its low tolerance for energy failure during OPCsmaturation, a mild episode of ischemic attack might be fatal. At worst-case scenarios, repeated episodes of minor ischemic attack can eventually exhaust the OPCs pool and disrupt intrinsic reparative capacities. We found that the ischemia-induced death of OPCs was through the Bcl-2/E1B-19K-interacting protein 3 (BNIP3) cell death pathway (Li et al., 2013). Identifcation of such molecular cell death pathway may help develop therapeutic strategies for protecting OPCs against ischemic attacks.
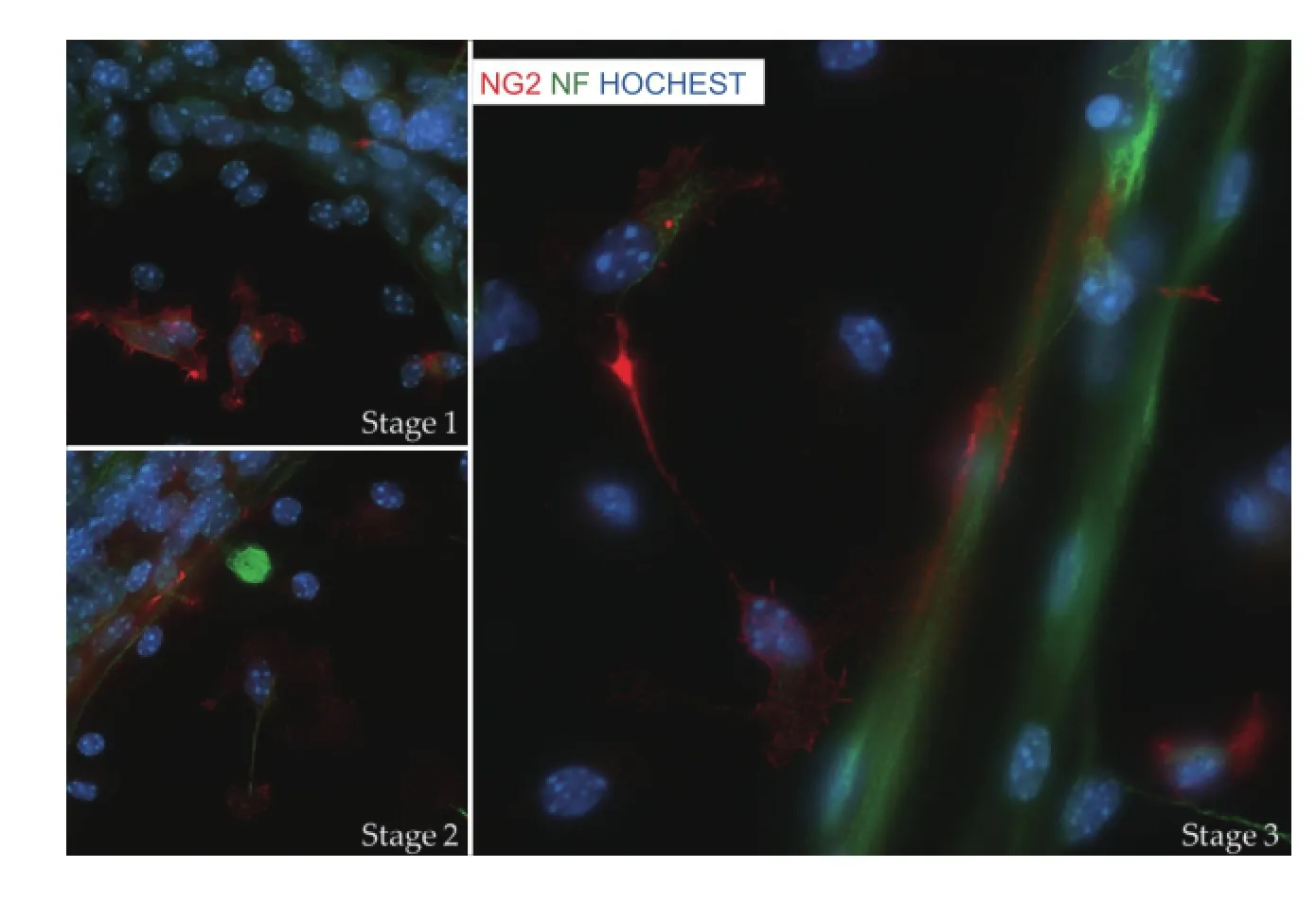
Figure 1 Early stages of myelination.
Strategies for white matter repair:White matter stroke is known to affect other cellular elements in the affected area. In stroke acute phase, infammatory signals from microglia/ macrophage enable OPCs to proliferate and divide. Macrophage-derived OPCs recruitment is consider as beneficial for remyelination. A switch from M1 to M2 microglia/macrophage activation is critical for OPCs to differentiate into new myelin-forming cells. Persistent M1 activation is the major cause of remyelination failure. In addition, extracellular matrix degradation, reactive astrogliosis, and axonal injury are considered obstacles for OPCs to migrate into the injury site and successfully differentiate into myelinating oligodendrocyte. Strategies to overcome such obstacles may provide effective remyelination therapies. Preclinical research efforts in this regard over the last decade have focused on two major approaches: 1) stimulating endogenous repair process and 2) transplanting exogenous myelinating precursors. Both approaches have strong scientifc rationales for oligodendrocyte-based remyelination. The endogenous approaches are aiming at the OPC differentiation pathway (Keough and Yong, 2013). These strategies include intrinsic (i.e., Notch, Wnt and RXR signaling pathways) and extrinsic (i.e., Semaphorins and LINGO-1) cellular mechanisms. Boosting the endogenous OPC proliferation and overcoming lesion-associated inhibitory effects are attractive although the remyelinated fibers tend to show thin and pale myelin sheaths with shorter internodes. Drug-based enhancement of remyelination could help functionally restore the myelin sheaths to some extent, but the outcome is not yet satisfactory (Brugarolas and Popko, 2014).
White matter repair would potentially benefit from oligodendrocyte replacement therapy. Preclinical studies show that human CNS neural stem cells, a neural precursor population isolated from human fetal brain, form myelin around the damaged nerve axons and restore lost motor function in a shiverer mouse model (Uchida et al., 2012). Encouraged by the preclinical data, this group has initiated a phase I clinical trial to evaluate the safety and potential effcacy of the cell replacement therapy (Gupta et al., 2012). An alternative cell source — platelet derived growth factor responsive neural precursors (PRPs) may be of choice for clinical cell transplantation (Plemel et al., 2011). Compared to embryonic stem cells, PRPs tend to have a limited fate to myelinate surrounding axons in the lesion environment. Preclinical studies indicate that the OPCs markers platelet-derived growth factor receptor (PDGFRα) and neural/glial antigen 2 (NG2) were expressed in 35.0 ± 3.2% and 34.5 ± 3.7% of the transplanted PRPs, respectively. Another 31.5 ± 3.3% of PRPs co-labeled with CC1, a marker of mature oligodendrocytes. Percentage of PRP-derived astrocytes was quite low (8.9 ± 2.2%) and no transplanted PRP-derived cells displayed a neuronal phenotype. This group confrmed that transplanted PRPs successfully formed mature myelin sheathsin vivo. Implanting PRPs into Shiverer mice confrmed the ability of PRP-derived cells to produce compact myelin sheaths with normal periodicity. However, this group points out that despite clear integration and myelination, the PRPs fail to promote functional recovery in spinal cord injury model. There are certainly concerns associated with the cell replacement therapies such as safety, immune rejection and effective method for cell delivery.
In conclusion, functional restoration of the brain relies not only on reestablishment of the neural networks, but also on appropriate remyelination of the axons. Since oligodendrocytes are the most vulnerable cells in the brain, strategies to protect oligodendrocytes and enhance remyelination shall help restore the brain functions and reduce demyelination-associated post-injury symptoms such as depression and dementia.
Teng Guan, Jiming Kong*
Department of Human Anatomy and Cell Science, University of Manitoba, 745 Bannatyne Avenue, Winnipeg, MB R3E 0J9, Canada
*Correspondence to: Jiming Kong, Ph.D., Jiming.Kong@umanitoba.ca.
Accepted:2015-02-25
Brugarolas P, Popko B (2014) Remyelination therapy goes to trial for multiple sclerosis. Neurol Neuroimmunol Neuroinfamm 1:e26.
Czopka T, Ffrench-Constant C, Lyons DA (2013) Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell 25:599-609.
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304.
Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, Perry R, Farrell J, Jeremy RJ, Ulman M, Huhn SL, Barkovich AJ, Rowitch DH (2012) Neural stem cell engraftment and myelination in the human brain. Sci Transl Med 4:155ra137.
Harris JJ, Attwell D (2012) The energetics of CNS white matter. J Neurosci 32:356-371.
Keough MB, Yong VW (2013) Remyelination therapy for multiple sclerosis. Neurotherapeutics 10:44-54.
Li C, Guan T, Chen X, Li W, Cai Q, Niu J, Xiao L, Kong J (2013) BNIP3 mediates pre-myelinating oligodendrocyte cell death in hypoxia and ischemia. J Neurochem 127:426-433.
Plemel JR, Chojnacki A, Sparling JS, Liu J, Plunet W, Duncan GJ, Park SE, Weiss S, Tetzlaff W (2011) Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia 59:1891-1910.
Thorburne SK, Juurlink BH (1996) Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67:1014-1022.
Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X, Nguyen T, Cummings BJ, Anderson AJ, Tamaki SJ, Tsukamoto A, Weissman IL, Matsumoto SG, Sherman LS, Kroenke CD, Back SA (2012) Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med 4:155ra136.
10.4103/1673-5374.153675 http∶//www.nrronline.org/
Guan T, Kong J (2015) Functional regeneration of the brain∶ white matter matters. Neural Regen Res 10(3)∶355-356.
杂志排行
中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- TAM receptors: two pathways to regulate adult neurogenesis
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Empowering sonic hedgehog to rescue brain cells after ischemic stroke
