Optimization of cross-coupling reaction for synthesis of antitumor drug vismodegib
2014-09-06CaoMengZhaoHuchengHuBingJiMin
Cao Meng Zhao Hucheng Hu Bing Ji Min
(School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China)
Optimization of cross-coupling reaction for synthesis of antitumor drug vismodegib
Cao Meng Zhao Hucheng Hu Bing Ji Min
(School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China)
In order to improve the yield and reduce the cost in the synthesis of antitumor drug vismodegib, the key intermediates are prepared and the Negishi reaction step is examined. The effects of different molar ratios of reactants, dosages of catalyst and time for refluxing are investigated by using single factor tests. The results demonstrate that, when the molar ratios of 2-bromopyridine, 2-chloro-N-(4-chloro-3-iodophenyl)-4-(methylsulfonyl)benzamide, zinc chloride,n-butyllithium and tetrakis(triphenyl phosphine)palladium are changed to 1.0∶0.5∶1.5∶1.1∶0.05 and the mixture is refluxed for 24 h, the production yield is improved to 72%. This reaction condition significantly enhances the synthetic efficiency, avoids consuming excessive raw materials/catalysts, and ,meanwhile, prevents a prolonged reaction time. The optimization of the proportion of reactants and the heating time is proved to be important for the efficiency and economy in cross-coupling reaction to synthesize vismodegib.
vismodegib; synthesis; cross-coupling; optimization
Tumor genesis and progress are highly relevant to the biological signaling pathways between tumor cells or between tumor cell and its extracellular matrix[1]. Among those signal pathways, the Hedgehog (Hh) pathway is a popular topic for molecular oncology research[2-5]. During embryogenesis, the Hh pathway regulates development information transmission for embryonic cells, and plays an important role in cell differentiation, organ development and body patterning[6]. On the other hand, disordered Hh signaling is also a pathological vital factor in many human diseases especially neoplasms, including basal cell carcinoma, medulloblastoma, small cell lung cancer and gastrointestinal carcinoma[7-10].
Vismodegib (Erivedge, HhAntag691 or GDC-0449) is the first marketed small molecule Hedgehog signaling pathway targeting drug. Approved by the U.S. Food and Drug Administration for treating adult’s advanced basal cell carcinoma in January, 2012, this synthetic agent has a significant Hh pathway targeting potential and notable antineoplastic effect[11]. The drug has being undergoing clinical trials for metastatic colorectal cancer, small-cell lung cancer, advanced stomach cancer, pancreatic cancer, medulloblastoma and chondrosarcoma since June 2011. As a smoothened (Smo) inhibitor, vismodegib is also proved to be effective in leukemia[12], prostate cancer[13]and hepatocellular carcinoma[14]therapies.
The vismodegib molecule is chemically constituted by a (pyridin-2-yl)-aryl structure. Generally it can be synthesized from a pyridine-2-yl derivative with a certain phenyl compound through cross-coupling reaction[11,15]. In particular, the 2-bromopyridine is first lithiumized byn-butyllithium and followed by zinc(II) salt treatment to form pyridin-2-ylzinc(II) halogenide, and then coupled with 2-chloro-N-(4-chloro-3-iodophenyl)-4-(methylsulfonyl) benzamide under a catalytic amount of Pd(PPh3)4to give the target vismodegib. This synthetic route is a typical Negishi reaction, which is a tricky process because under different conditions, the yield as well as the total cost of the product is varied.
With reactant 2-chloro-N-(4-chloro-3-iodophenyl)-4-(methylsulfonyl) benzamide synthesized and 2-bromopyridine commercially available, we examined the Negishi reaction conditions to achieve vismodegib, and analyzed the effect of different molar ratios of raw material, catalyst dosage and reaction time on the yield by using single factor tests. Through comparison of the tested reaction conditions, a favorable reacting molar ratio of compound 3/compound 2/n-BuLi/ZnCl2/catalyst was finalized as 1.0∶0.5∶1.5∶1.1∶0.05, and an optimum refluxing time of 24 h was established. Furthermore, under this peculiar condition, a relatively high yield (72%) of vismodegib was achieved.
1 Experimental
1.1 General
The synthesis reaction of vismodegib is shown in Fig.1. Commercial reagents were used as received. Melting points were measured in open capillaries and were uncorrected. Compounds were routinely checked by TLC with silica gel GF-254 glass plates and viewed under UV light at 254 nm.1H and13C NMR spectra were taken on a Bruker AM-300 spectrophotometer with tetramethylsilane (TMS) as an internal standard and CDCl3as solvent. Multiplicity is indicated as follows: s (singlet); d (doublet); t (triplet); m (multiplet); dd (doublet of doublets); td (triplet of doublets); br s (broad singlet), etc. and coupling constants are given in hertz. Mass spectra (MS) were obtained from Finnigan MAT-95 Spectrometry Services.
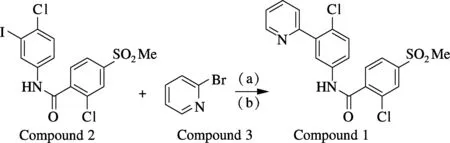
Fig.1 Synthesis of vismodegib (named as compound 1) by Negishi coupling reaction from 2-chloro-N-(4-chloro-3-iodophenyl)-4-(methylsulfonyl) benzamide (named as compound 2) and 2-bromopyridine (named as compound 3) under the conditions of (a)n-BuLi, ZnCl2, THF,-78℃ and (b) Pd(PPh3)4, THF, r.t. to reflux
1.2 2-chloro-4-(methylsulfonyl)benzoyl chloride
2-chloro-4-(methylsulfonyl)benzoic acid (compound 4, 3.0 g, 12.8 mmol) and thionyl chloride (8.2 g, 68.9 mmol) were mixed in 25 mL THF solution and heated to reflux for 2 h. After cooling to room temperature, the solvent was evaporated under reduced pressure to give 2-chloro-4-(methylsulfonyl)benzoyl chloride (compound 5) as white solid (2.9 g, 91%).
1.3 1-chloro-2-iodo-4-nitrobenzene
2-chloro-5-nitroaniline (compound 6, 10.0 g, 58.1 mmol) were dissolved in 30 mL HCl (The mass fraction is 36%), and mixed with 30 mL cold water and stirred for 20 min at 0 to 5 ℃. Later 10 mL water solution of sodium nitrite (4.2 g, 60.9 mmol) were added dropwise to the mixture, and the reaction was kept stirring for 1 h at 0 to 5 ℃, followed by adding with 10 mL water solution of potassium iodide (14.5, 87.4 mmol) dropwise at 0 to 5 ℃ for 30 min with vigorous stirring. The precipitate was filtered and washed 3 times with saturated sodium thiosulfate solution. The filtrate was dried under reduced pressure to give 1-chloro-2-iodo-4-nitrobenzene (compound 7) as yellow powder (13.7 g, 83%).1H-NMR(CDCl3, 300 MHz):δ(10-6) 8.70 (d,J2.61 Hz, 1 H), 8.16 (q, 1 H), 7.61 (d,J8.76 Hz, 1 H).
1.4 4-chloro-3-iodoaniline
Ferrous powder (5.0 g, 89.5 mmol) was added slowly to 40 mL acetic acid containing compound 7 (8.0 g, 28.3 mmol) and heated to 80 ℃ with stirring for 30 min. The reaction mixture was poured into 150 mL water, and the aqueous phase was extracted with chloroform (3 times, 50 mL). The combined organic phase was washed 3 times with saturated sodium carbonate then dried by magnesium sulfate for 12 h, and evaporated under reduced pressure to give the crude product. Further purification by silica gel flash chromatography gave 4-chloro-3-iodoaniline (compound 8) as brown solid (5.3 g, 74%).1H-NMR (CDCl3-d6, 300 MHz):δ(10-6) 7.15 (d,J8.43 Hz, 1 H), 7.16 (d,J2.82 Hz, 1 H), 6.57 (q, 1 H), 3.63 (s, 2 H).
1.5 2-chloro-N-(4-chloro-3-iodophenyl)-4-(methylsulfonyl)benzamide
Compound 8 (3.1 g, 12.3 mmol) was dissolved in 25 mL THF and added dropwise to 25 mL THF solution containing compound 5 (2.9 g, 11.5 mmol) at 0 to 5 ℃ under stirring. Then 2 mL TEA was added dropwise to the mixture, and kept stirring at room temperature for 4 h. The reaction mixture was poured into 150 mL water, and the precipitate was filtered. The filtrate was washed with water and dried to give 2-chloro-N-(4-chloro-3-iodophenyl)-4-(methylsulfonyl)benzamide (compound 2) as light yellow solid (5.4 g, 89%).1H-NMR (CDCl3, 300 MHz):δ(10-6) 8.20 (s, 1 H), 8.02 (s, 1 H), 7.90 (s, 3H), 7.62 (s, 1 H), 7.46 (s, 1 H), 3.09 (s, 3 H).
1.6 2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide
The manipulated different amounts ofn-BuLi, ZnCl2, compound 2 and Pd(PPh3)4, as well as the refluxing time are listed in Tab.1. A 30 mL THF solution of 2-bromopyridine (5 g, 31.9 mmol) was added dropwise withn-BuLi in cyclohexane solution at -78 ℃ with stirring. The reaction mixture turned dark and was kept stirring for 40 min. Then 40 mL THF solution of ZnCl2was injected slowly to the mixture at -78 ℃. After stirring for 20 min, the reaction was allowed to slowly reach room temperature, and then added drop wise to a mixture of compound 2 and Pd(PPh3)4in 30 mL THF under nitrogen protection. The reaction was then slowly heated to reflux and kept stirring and after a certain time poured into 400 mL water. Extraction with ethyl acetate (3 times, 50 mL) and evaporation of the solvent gave the crude product, which was purified by column chromatography on silica gel to give compound 1.1H-NMR (DMSO-d6, 500 MHz):δ(10-6) 10.90 (s, 1 H), 8.70 (d,J4.4, 1 H), 8.13 (d,J1.55, 1 H), 8.01 (q, 2 H), 7.92 (m, 2 H), 7.74 (q, 1 H), 7.69 (d,J7.9, 1 H), 7.58 (d,J8.7, 1 H), 7.44 (M, 1 H), 3.34 (s, 3 H). TOF-MS m/z: 419.1 [M-1]+, 421.1 [M+1]+.
2 Results and Discussion
Utilizing compound 4 and compound 6 as starting materials, we successfully synthesize the essential intermediate compound 2 (see Fig.2). At the beginning, compound 4 is chlorinated to give benzoyl chloride compound 5 (91%). Compound 6 is converted to compound 7 by the diazo-reaction in 83% yield. Compound 7 is reduced to compound 8 (74%). Then benzoyl chloride compound 5 is reacted with compound 8 to give compound 2 under TEA in 89% yield. By coupling compound 2 and compound 3 (commercially available), target compound 1 is achieved through the Negishi reaction. While in this step with different reaction conditions, the yield of compound 1 is varied obviously. To approach a favorable synthesis condition, single factor experiments are conducted to analyze the Negishi reaction (see Tab.1).

Tab.1 Condition analysis of the Negishi-coupling reaction of the synthesis of compound 1
*Under the optimal condition, the cost and efficiency are also taken into consideration.
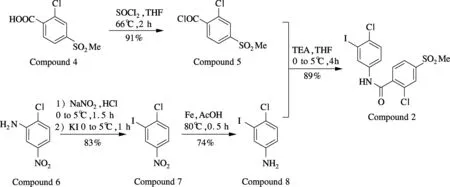
Fig.2 Synthesis of 2-chloro-N-(4-chloro-3-iodophenyl)-4-(methylsulfonyl)benzamide (compound 2)
We first examine how the ratio ofn-BuLi influences the reaction. The results display that a higher amount ofn-BuLi can increase the conversion rate of compound 2, but too muchn-BuLi will bring a disadvantage because this active organolithium reagent can easily cause side reactions (see Fig.3).
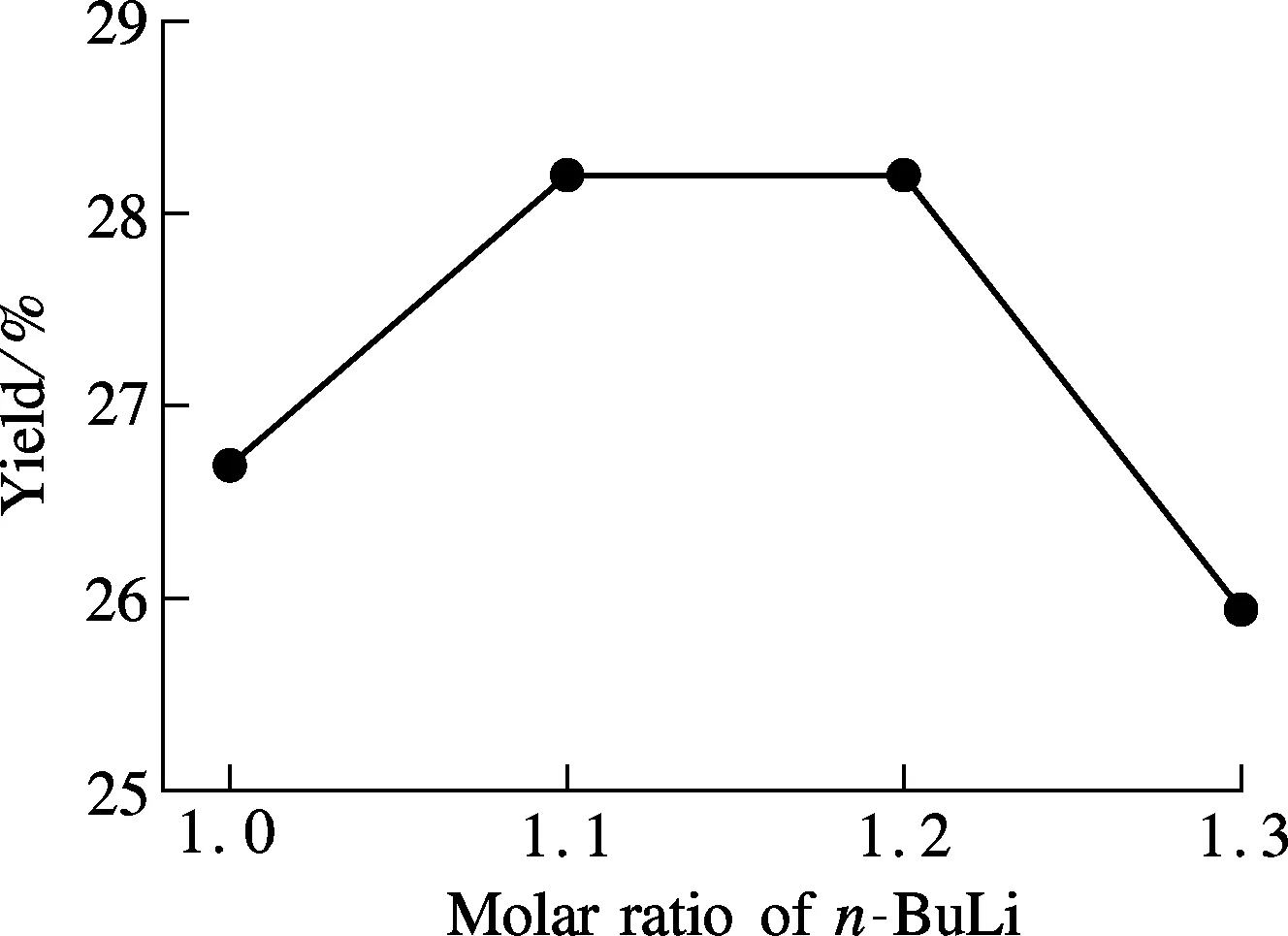
Fig.3 Investigation into the role of reactantn-BuLi
The effect of ZnCl2is also investigated. Under the conditions of various amounts of ZnCl2, the data of the synthetic yield indicate that a higher molar ratio of ZnCl2/compound 3 can result in a better outcome of the product; but when the ratio exceeds 1.5∶1.0, the improvement becomes non-significant (see Fig.4).
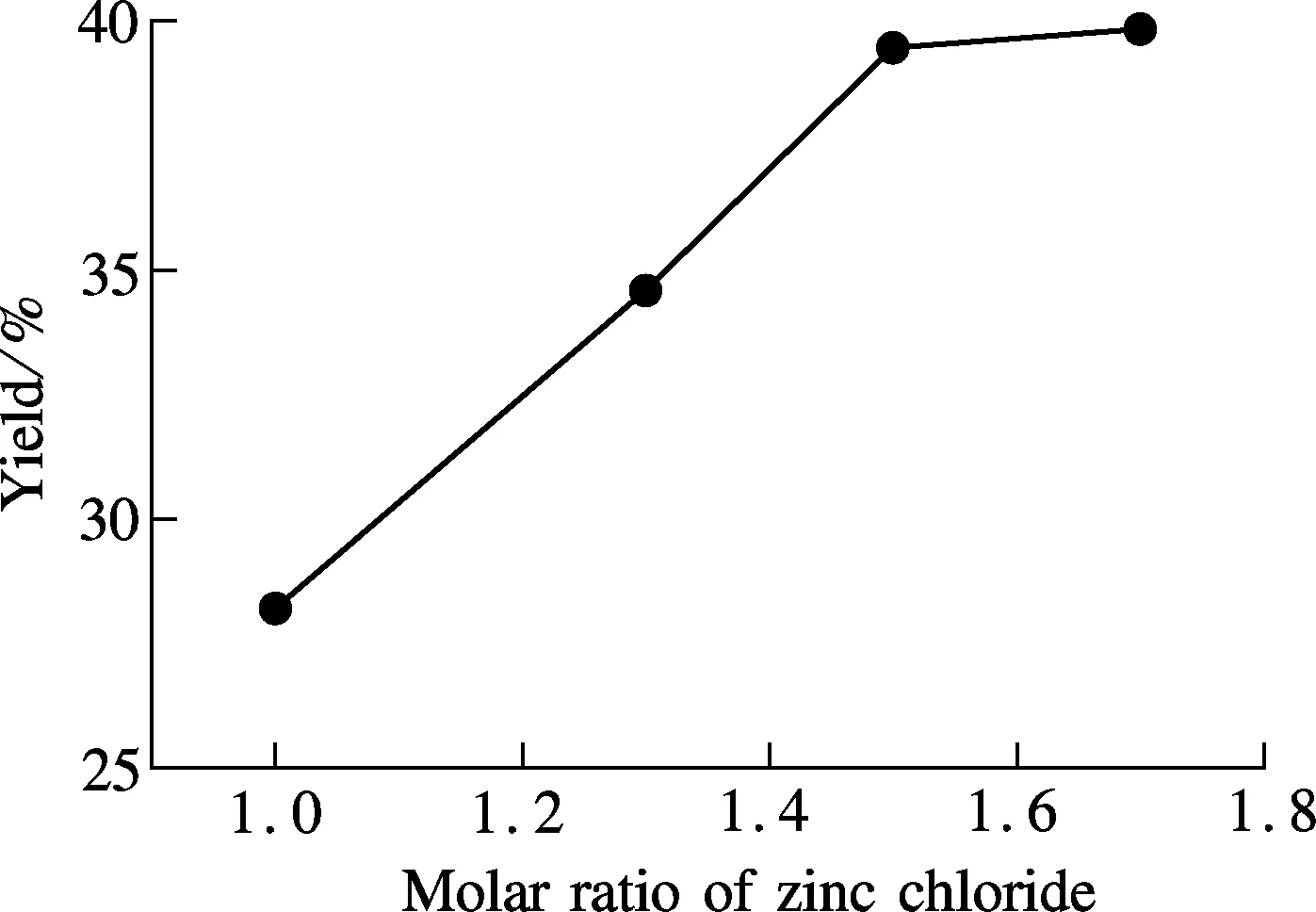
Fig.4 Effect of ZnCl2on the yield of final product compound 1
In order to increase the conversion rate of compound 2, we attempt to decrease the compound 2/compound 3 ratio and find that it can hardly further increase compound 2 utilization when the ratio is lower than 0.5∶1.0 (see Fig.5).

Fig.5 Effect of the molar ratio of compound 2 on transformation rate
In addition, the regulation of the catalyst ratio demonstrates that, the Pd(PPh3)4/compound 3 ratio at 0.05∶1.00 is adequate to enhance the yield while higher catalyst dosage shows no noticeable improvement (see Fig.6).
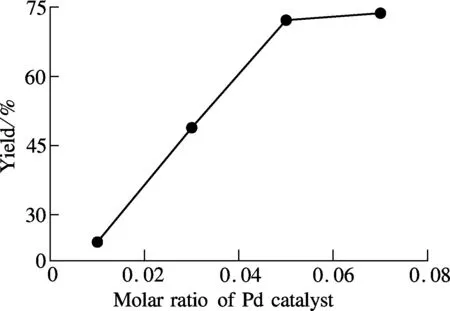
Fig.6 Effect of Pd catalyst on the yield of final compound 1
A sufficient refluxing time is also an important factor for the reaction efficiency. We examine the yield of compound 1 using different refluxing times for reaction. The results reveal that, when the reacton mixture is heated for 24 h, a relatively high yield of compound 1 is achieved (see Fig.7). Longer reaction time cannot further improve the yield.
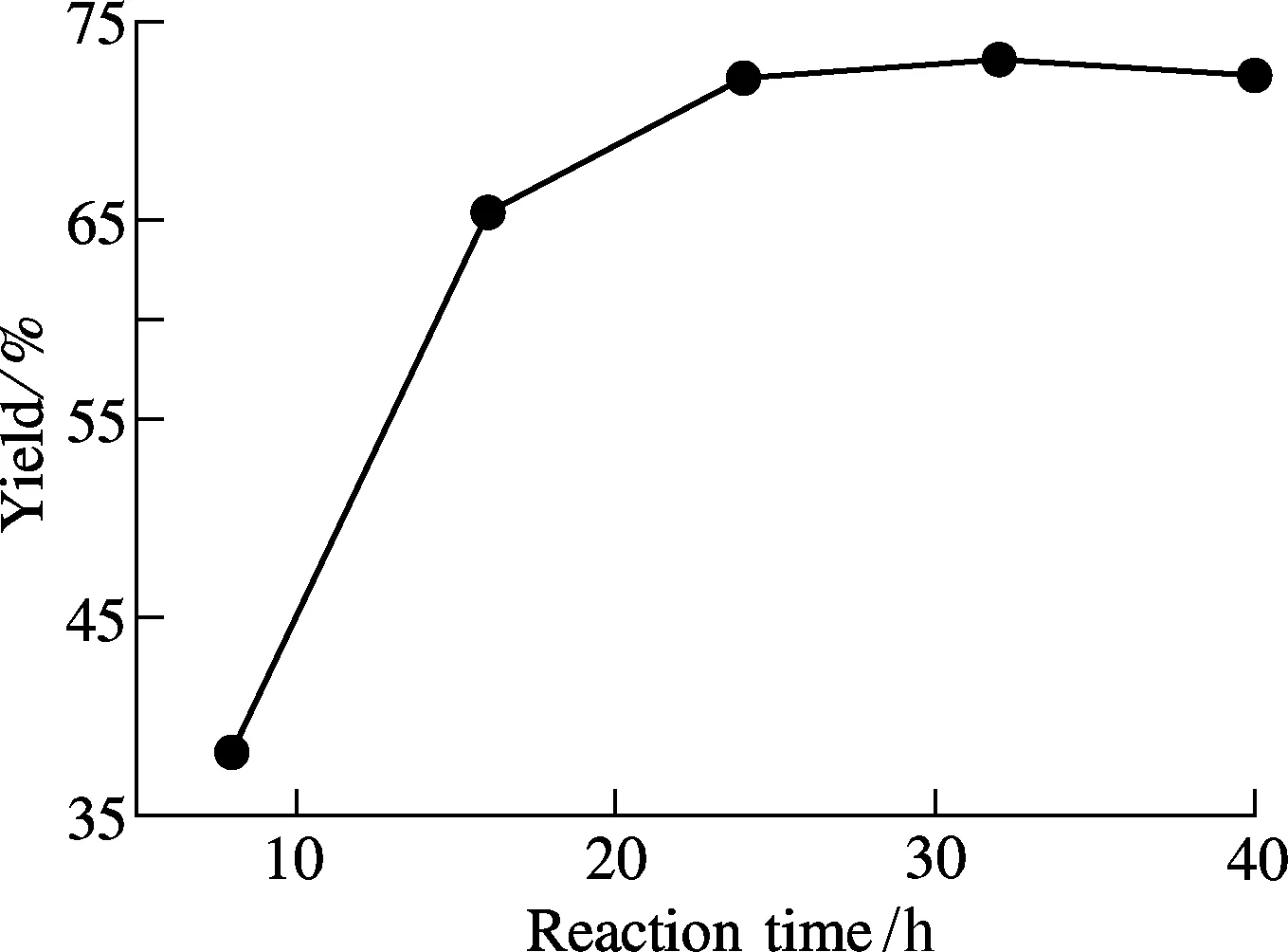
Fig.7 Effect of reaction time on the yield of compound 1
Based on the above findings, a molar ratio of 1.0∶0.5∶1.5∶1.1∶0.05 (compound 3/compound 2/n-BuLi/ZnCl2/catalyst) and a refluxing time of 24 h is found to be the optimal reaction condition. Under this condition, the yield of compound 1 is 72%, which is higher than literature reported[11,16-17].
3 Conclusion
In the medical chemistry scientific research and large scale production in industrial manufacture, an optimal synthetic technology is always crucial and necessary to achieve the expected target chemical molecule. A proper preparative condition may not only enhance the production yield but simplify the reaction procedure, and meanwhile, lower the cost. Vismodegib is a popular compound because it is the first Hedgehog signaling pathway targeting agent approved by FDA, and it is proved potent in a remarkable number of human tumors. The bottleneck of its synthetic route is the cross-coupling reaction, requiring ultra-low temperature, anhydrous and anoxybiotic conditions, expensive catalyst, as well as tedious handling procedure, all of which make the reaction efficiency very variable. The reported transformation rate of compound 1 synthesized from compound 2 is around 60%. In the present report we examined the reaction condition carefully using single factor analysis, and attempted to illuminate the relationship between each manipulatable condition and the yield of compound 1. By separately regulating the amount of ZnCl2,n-BuLi, compound 2, catalyst and refluxing time, our results demonstrate that the patterns of each reaction condition in influencing the final product are predictable and that when compound 3/compound 2/ZnCl2/n-BuLi/catalyst molar ratio is 1.0∶0.5∶1.5∶1.1∶0.05 with the refluxing time of 24 h, the yield of compound 1 can be as high as 72%, suggesting a significant improvement.
Our study indicates that, the optimized reactant/catalyst molar ratio along with the appropriate refluxing time does not consume excessive raw materials and catalysts and, in the meantime, avoids a prolonged reaction time; thus, it indeed increases the efficiency and reduces the cost. The results will also be beneficial to industrial scale-up, helpful for making the preparation process easier to handle and more economical. Furthermore, the factors we analyzed and summarized may also be applicable in the optimization of other similar reactions.
[1]Hanahan D, Weinberg R A. Hallmarks of cancer: the next generation [J].Cell, 2011, 144(5): 646-674.
[2]Carney T J, Ingham P W. Drugging Hedgehog: signaling the pathway to translation [J].BMCBiology, 2013, 11(1): 37.
[3]Coni S, Infante P, Gulino A. Control of stem cells and cancer stem cells by Hedgehog signaling: pharmacologic clues from pathway dissection [J].BiochemicalPharmacology, 2013, 85(5): 623-628.
[4]Lasky J L, Nakano I. Overview of brain tumor stem cells—implications for treatment [J].CurrentSignalTransductionTherapy, 2013, 8(1): 45-54.
[5]Szkandera J, Kiesslich T, Haybaeck J, et al. Hedgehog signaling pathway in ovarian cancer [J].InternationalJournalofMolecularSciences, 2013, 14(1):1179-1196.
[6]Varjosalo M, Taipale J. Hedgehog: functions and mechanisms [J].Genes&Development, 2008, 22(18): 2454-2472.
[7]Epstein E H. Basal cell carcinomas: attack of the hedgehog [J].NatureReviewsCancer, 2008, 8(10): 743-754.
[8]Taipale J, Beachy P A. The Hedgehog and Wnt signalling pathways in cancer [J].Nature, 2001, 411(6835): 349-354.
[9]Watkins D N, Berman D M, Burkholder S G, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer [J].Nature, 2003, 422(6929): 313-317.
[10]Berman D M, Karhadkar S S, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours [J].Nature, 2003, 425(6960): 846-851.
[11]Robarge K D, Brunton S A, Castanedo G M, et al. GDC-0449—a potent inhibitor of the hedgehog pathway [J].Bioorganic&MedicinalChemistryLetters, 2009, 19(19): 5576-5581.
[12]Okabe S, Tauchi T, Tanaka Y, et al. Effects of the hedgehog inhibitor GDC-0449, alone or in combination with dasatinib, on BCR-ABL-positive leukemia cells [J].StemCellsandDevelopment, 2012, 21(16): 2939-2948.
[13]Karlou M, Lu J F, Wu G L, et al. Hedgehog signaling inhibition by the small molecule smoothened inhibitor GDC-0449 in the bone forming prostate cancer xenograft MDA PCa 118b [J].Prostate, 2012, 72(15): 1638-1647.
[14]Philips G M, Chan I S, Swiderska M, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer [J].PLoSOne, 2011, 6(9): e23943.
[15]Gunzner J L, Sutherlin D, Stanley M S, et al. Pyridyl inhibitors of Hedgehog signalling [P]. US Patent, 07888364. 2011-02-15.
[16]Bao L, Castanedo G, Dina M S, et al. New pyridyl compound useful for treating e.g. inflammatory bowel disease, asthma, rheumatoid arthritis, psoriasis [P]. World Patent, WO2009126863-A2. 2009-10-15.
[17]Gunzner J, Sutherlin D, Stanley M, et al. New pyridyl compounds are inhibitors of hedgehog signaling pathway useful for treatment of cancer in mammal e.g. basal cell carcinoma, medullablastoma, pancreatic adenocarcinoma, small-cell lung carcinoma, breast carcinoma [P]. World Patent, WO2006028958-A2. 2006-03-16.
合成抗肿瘤药物vismodegib的交叉偶联反应工艺优化
曹 萌 赵虎城 胡 兵 吉 民
(东南大学生物科学与医学工程学院,南京 210096)
为了提高抗肿瘤药物vismodegib合成反应的产率并控制反应成本, 制备了合成目标化合物所需的中间体, 并对Negishi偶联反应条件进行了考察. 通过单因素分析法, 研究了不同的摩尔比、催化剂用量和回流时间对反应的影响. 结果表明, 在反应混合物2-溴吡啶、2-氯-N-(4-氯-3-碘苯基)-4-(甲磺酰)苯甲酰胺、氯化锌、正丁基锂和四(三苯基膦)钯的摩尔比为1.0∶0.5∶1.5∶1.1∶0.05及回流时间为24 h的条件下, 反应收率提高到了72%. 该反应条件明显提高了合成效率, 避免了原料/催化剂的过多消耗, 同时避免了过长的反应时间.反应体系配比和反应时间的优化在合成vismodegib的过程中对提高效率和经济性有重要作用.
vismodegib; 合成; 交叉偶联; 优化
TQ463
s:The National Basic Research Program of China (973 Program)(No.2011CB933503), China Postdoctoral Science Foundation (No.2013M541592).
:Cao Meng, Zhao Hucheng, Hu Bing, et al. Optimization of cross-coupling reaction for synthesis of antitumor drug vismodegib[J].Journal of Southeast University (English Edition),2014,30(1):72-76.
10.3969/j.issn.1003-7985.2014.01.014
10.3969/j.issn.1003-7985.2014.01.014
Received 2013-09-10.
Biographies:Cao Meng (1983—), male, doctor; Ji Min (corresponding author), female, doctor, professor, jimin@seu.edu.cn.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Wavelet transform and gradient direction based feature extraction method for off-line handwritten Tibetan letter recognition
- Analyses of unified congestion measures for interrupted traffic flow on urban roads
- Conditional autoregressive negative binomial model for analysis of crash count using Bayesian methods
- Design and analysis of traffic incident detection based on random forest
- Biodegradation of microcystin-RR and-LR by an indigenous bacterial strain MC-LTH11 isolated from Lake Taihu
- Inverse kinematic deriving and actuator control of Delta robot using symbolic computation technology
