硫酸盐还原菌的脱硫性能和铁还原性能
2014-08-15陈明翔周集体路达张玉
陈明翔,周集体,路达,2,张玉
(1.大连理工大学 环境学院,工业生态与环境工程教育部重点实验室,辽宁 大连 116024; 2.河北大学 化学与环境科学学院,河北 保定 071002)
随着工业的发展,化石燃料的大量燃烧所产生的二氧化硫(SO2)和一氧化氮(NO),造成了酸雨、臭氧层破坏、光化学烟雾等环境污染,并严重威胁人类的健康[1].SO2和NO一体化脱除技术,作为大气污染治理的发展趋势之一,已经得到广泛研究[2-3].目前,比较常见的烟气同时脱硫脱硝技术主要包括等离子体法、液相氧化法、光催化氧化法、络合吸收法、吸附法等[4-8].尽管这些方法有着诸多的优点,但仍有许多方面需要改进,如高能耗、高成本、存在二次污染等问题.生物法工业废气净化技术因其设备简单、投资及运行费用低、且无二次污染等优点日益受到人们的关注[1].目前,多数研究采用生物法单独脱除SO2或NO,而生物法同步脱硫脱硝尚处于实验探索阶段[3].
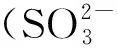
1 材料和方法
1.1 菌种
实验采用的SRB是由大连市春柳河污水处理厂的厌氧污泥经富集、驯化、反复稀释涂布而得.经16S rDNA测序和Blast分析表明,菌株与脱硫弧菌属(Desulfovibriosp.)的同源性最高,故命名为Desulfovibriosp.CMX.在GenBank上登录,其序列号为GU584224.在广东省微生物菌种保藏中心的保藏号为GIM1.765.
1.2 培养基
富集培养基:K2HPO4,0.50 g;NH4Cl,1.00 g;MgSO4·7H2O,2.00 g;Na2SO4,0.86 g;CaCl2,0.10 g;酵母浸粉,1.00 g;乳酸钠(质量分数为70%~80%),5 mL;蒸馏水,1 000 mL,pH值调至7.00±0.20.在0.1 MPa,121 ℃下灭菌20 min.在培养基使用前用滤膜过滤灭菌加入0.10 g的维生素C.
基础培养基:Na2HPO4·12H2O,5.00 g;NaH2PO4·2H2O,2.20 g;酵母浸粉,1.00 g;乳酸钠(质量分数为70%~80%),5 mL;蒸馏水,1 000 mL.在0.1 MPa,121 ℃下灭菌20 min.在培养基使用前用滤膜过滤灭菌加入0.10 g的维生素C.
1.3 菌株脱硫性能的优化
1.3.1 pH值对D.sp.CMX的脱硫性能的影响

1.3.2 不同碳源对D.sp.CMX脱硫性能的影响

1.4 菌株Fe(Ⅲ)EDTA还原能力的考察
分别将0,5,15,25 mmol/L的Fe(Ⅲ)EDTA加入100 mL基础培养基中,并将D.sp.CMX(细胞蛋白质量浓度为20.00 mg/L)分别厌氧接种于初始Fe(Ⅲ)EDTA浓度不同的培养基中,在pH=7、温度为30 ℃的条件下厌氧培养,并定期取样测定其蛋白生长量、亚铁(Fe2+)和总铁含量.
1.5 分析方法

2 结果和讨论
2.1 pH对D.sp.CMX的脱硫性能的影响



图1 pH值不同时D.sp.CMX的脱硫性能Fig.1 Sulfate reducing performances of D.sp.CMX at different pH values
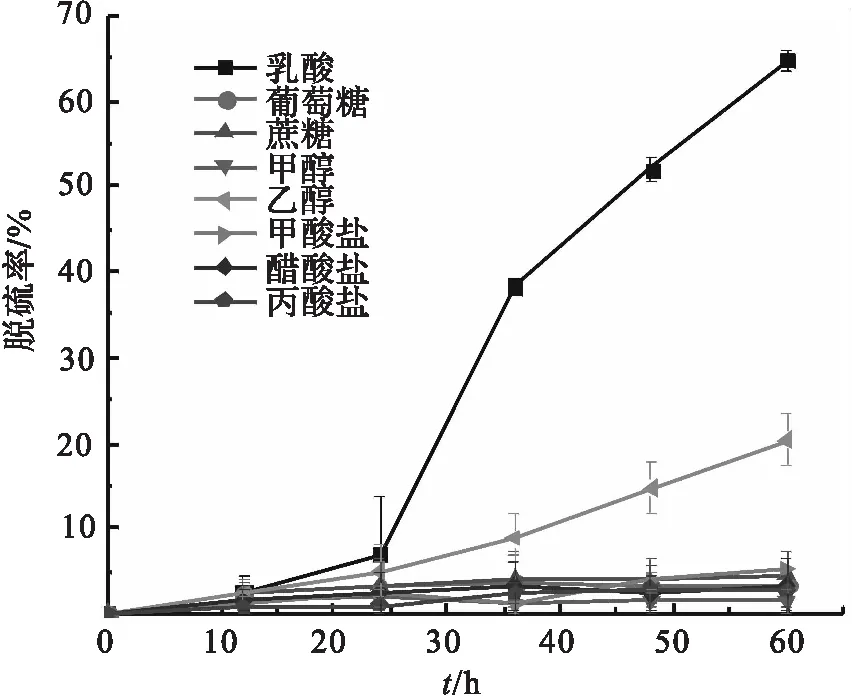
图2 不同碳源时D.sp.CMX的脱硫性能Fig.2 Sulfate reducing performances of D.sp.CMX at different carbon sources
2.2 碳源对D.sp.CMX的脱硫性能的影响
2.3 初始质量浓度对D.sp.CMX的脱硫性能的影响


ρ/(g·L-1)图3 初始的质量浓度不同时D.sp.CMX的脱硫性能Fig.3 Sulfate reducing performances of D.sp.CMX at different sulfate concentrations
2.4 D.sp.CMX的铁还原能力的考察

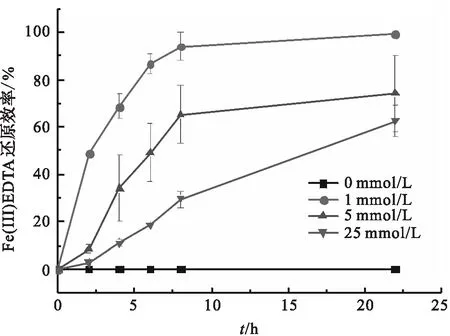
图4 D.sp.CMX的铁还原能力Fig.4 Ferric reducing capacity of D.sp.CMX

图5 D.sp.CMX在Fe(Ⅲ)EDTA中的生长情况Fig.5 Growth of D.sp.CMX in Fe(Ⅲ)EDTA solution
SRB可以直接将Fe(Ⅲ)、尤其是水溶态的Fe(Ⅲ)生物还原,但是,又有研究表明,25 mmol/L的Fe(Ⅲ)EDTA不是一个合适的电子受体,大部分的异养铁还原微生物无法还原25 mmol/L的Fe(Ⅲ)EDTA[23-25].而在本实验中,菌株D.sp.CMX在25 mmol/L的Fe(Ⅲ)EDTA溶液中,也可以发生异养的铁还原.随着Fe(Ⅲ)EDTA浓度的升高,D.sp.CMX在22 h时的蛋白浓度反而降低,这可能是由络合剂对SRB的毒性导致的,已有研究证明,EDTA、柠檬酸都对SRB的活性有一定的抑制作用[26].
3 结论
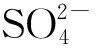
参 考 文 献:
[1] 谢志荣,魏在山,曾贵华,等.生物法同时脱硫脱硝试验研究[J].环境工程学报,2009,3(9):1648-1652.
XIE Zhirong,WEI Zaishan,ZENG Guihua,et al.Simultaneous desulfurization and denitrification by a biological method[J].Chinese Journal of Environmental Engineering,2009,3(9):1648-1652.
[2] 赵荣志,梁宝瑞,宋存义,等.密相半干法低温同时脱硫脱硝影响因素[J].环境工程学报,2013,7(12):4945-4950.
ZHAO Rongzhi,LIANG Baorui,SONG Cunyi,et al.Influencing factors during simultaneous removal of NOxand SO2in semi-dry dense bed at low temperature[J].Chinese Journal of Environmental Engineering,2013,7(12):4945-4950.
[3] 徐姣,张卫江,田桂林.生物法同步脱除SO2和NO的实验研究[J].化学工程,2010,38(3):61-64.
XU Jiao,ZHANG Weijiang,TIAN Guilin.Experimental study on biological simultaneous removal of SO2-containing and NO-containing waste gases[J].Chemical Engineering(China),2010,38(3):61-64.
[4] ZHAO Yi,GUO Tianxiang,CHEN Zhouyan,et al.Simultaneous removal of SO2and NO using M/NaClO2complex absorbent[J].Chem Eng J,2010,160(1):42-47.
[5] YUAN Yuan,ZHANG Junying,LI Hailong,et al.Simultaneous removal of SO2,NO and mercury using TiO2-aluminum silicate fiber by phohotocatalysis[J].Chem Eng J,2012,192:21-28.
[6] HUANG Liwei,DANG Yongxia.Removal of SO2and NOx by pulsed corona combined with in situ Ca(OH)2absorption[J].Chinese J Chem Eng,2011,19(3):518-522.
[7] MENENDEZ J A,ARENILLAS A,FIDALGO B,et a1.Microwave heating processes involving carbon materials[J].Fuel Process Technol,2010,91(1):1-8.
[8] 曲兵,刘盛余,徐园园,等.鼓泡反应器中液相络合催化同时脱硫脱硝的研究[J].环境工程学报,2012,6(2):555-560.
QU Bing,LIU Shengyu,XU Yuanyuan,et al.Simultaneous desulfurization and denitrification by complex catalysis in bubbing reactor[J].Chinese Journal of Environmental Engineering,2012,6(2):555-560.
[9] CHEN Mingxiang,ZHANG Yu,ZHOU Jiti,et al.Sulfate removal by Desulfovibrio sp.CMX in chelate scrubbing solutions for NO removal[J].Bioresour Technol,2013,143:455-460.
[10] DONG Xiyang,ZHANG Yu,ZHOU Jiti,et al.Fe(II)EDTA-NO reduction coupled with Fe(II)EDTA oxidation by a nitrate-and Fe(II)-reducing bacterium[J].Bioresour Technol,2013,138:339-344.
[11] BRADFORD M M.A rapid and sensitive method for the quantification of microquantities of protein utilizing the principle of protein-dye binding[J].Anal Biochem,1976,72:248-254.
[12] 彭艳平,余水静,赖和劲.硫酸盐还原菌生长条件优化及驯化试验研究[J].江西理工大学学报,2013,34(3):6-10.
PENG Yanping,YU Shuijing,LAI Hejin.The domestication and optimization of sulfate reducting bacteria growth conditions[J].Journal of Jiangxi University of Science and Technology,2013,34(3):6-10.
[13] BAYOUMY M A E,BEWTRA J K,ALI H I,et al.Sulfide production by sulfate reducing bacteria with lactate as feed in an upflow anaerobic fixed film reactor[J].Water Air Soil Pollut,1999,112:67-84.
[14] 马岩岩,徐建平.脱硫微生物培养条件及脱硫性能研究[J].安徽工程科技学院学报,2010,25(2):33-35.
MA Yanyan,XU Jianping.On culture condition and desulfurization performance of desulfurization strain[J].Journal of Anhui University of Technology and Science:Natural Science,2010,25(2):33-35.
[15] SMUL A,GOETHALS L,VERSTRAETE W.Effect of COD to sulphate ratio and temperature in expanded-granular-sludge-blanket reactors for sulphate reduction[J].Process Biochem,1999,34:407-416.
[16] VAN DER MAAS P,VAN DER BRINK P,UTOMO S,et al.NO removal in continuous BioDeNOxreactors:Fe(II)EDTA2-regeneration,biomass growth,and EDTA degradation[J].Biotechnol Bioeng,2006,94:575-584.
[17] 徐姣.生物法脱除工业废气中SO2和NO的研究[D].天津:天津大学,2008.
[18] LIAMLEAM W,ANNACHHATRE A P.Electron donors for biological sulfate reduction[J].Biotechnol Adv,2007,25:452-463.
[19] 姬玉欣,马春,金仁村,等.硫酸盐生物还原中电子供体的选择[J].化工进展,2011,30(12):2593-2600.
JI Yuxin,MA Chun,JIN Rencun,et al.Selection of electron donors for biological sulfate reduction[J].Chemical Industry and Engineering Progress,2011,30(12):2593-2600.
[20] 蒋永荣,周亶,容翠娟,等.高效硫酸盐还原菌的分离及特性研究[J].环境科学与技术,2009,32(11):13-17.
JIANG Yongrong,ZHOU Dan,RONG Cuijuan,et al.Isolation of high efficient sulfate-reducing bacteria and its biological desulfurization capability[J].Environmental Science and Technology,2009,32(11):13-17.
[21] OYEKOLA O O,VANHILLE R P,HARRISON S T L.Kinetic analysis of biological sulphate reduction using lactate as carbon source and electron donor:Effect of sulphate concentration[J].Chem Eng Sci,2010,65:4771-4781.
[22] DEMMINK J F,BEENACKERS A A C M.Gas desulfurization with ferric chelates of EDTA and HEDTA:new model for the oxidative absorption of hydrogen sulfide[J].Ind Eng Chem Res,1998,37:1444-1453.
[23] COLEMAN M L,HEDRICK D B,LOVLEY D R,et al.Reduction of Fe(Ⅲ)in sediments by sulphate-reducing bacteria[J].Nat,1993,361:436-438.
[24] LOVLEY D R,RODEN E E,PHILLIPS E J P,et al.Enzymatic iron and uranium reduction by sulphate-reducing bacteria[J].Mar Geol,1993,113:41-53.
[25] FINNERAN K T,FORBUSH H M,VANPRAAGH C G,et al.Desulfitobacterium metallireducens sp.nov.,an anaerobic bacterium that couples growth to the reduction of metals and humic acids[J].J Syst Evol Microbiol,2002,52:1929-1935.
[26] ASBELL M A,EAGON R G.Role of multivalent cations in the organization,structure and assembly of the cell wall of Pseudomonas aeruginosa[J].J Bacteriol,1966,92,380-387.
