Hard Foulers Induced Crevice Corrosion of HSLA Steel in the Coastal Waters of the Gulf of Mannar (Bay of Bengal), India
2014-07-30PalanichamyandSubramanian
S. Palanichamy* and G. Subramanian
Offshore Platform and Marine Electrochemistry Centre, CSIR - Central Electrochemical Research Institute,New Harbour Area, Tuticorin 628004, India
1 Introduction1
The seawater is a complex corrosive system. The corrosive action of seawater is a function of various factors,such as salinity, temperature, dissolved oxygen, pH, marine local currents, and the development of biofouling, known as an important factor (LaQue, 1982). The biofouling process reflects a temporal sequence of developments of the community. The influence of corrosion processes of the initial colonisation bymarine bacteria, microalgae and other microorganisms on surfaces has been studied for a long time(Beech >amp;Sunner, 2004; Little, 2004; Videla >amp; Herrera,2005).
LaQue and Clapp (1945) have found that certain species of marine organisms accelerate corrosion. The attachment of marine organisms on the surface of metals and metal alloys could be an important contributing factor in the complex corrosion process by producing local changes in dissolved oxygen concentration, pH, and oxidation-reduction potential.Industrialization and urbanization of the cities along the coastal region over the years have made the environment more aggressive in terms of both pollution and corrosion. This fact necessitates the need for greater knowledge of the behaviour of materials in coastal seawater and in particular calls for data on their resistance.The chemical and physical properties of natural seawater that affect corrosion of metals have been well documented, (Compton, 1970; Dexter and Culberson, 1980; Schumacher, 1979). Variations in the chemistry of open ocean seawater tend to take place slowly over time periods of a few to several months. Such gradual changes may produce an equally gradual change in the corrosion rate of structural materials with season and location,but they are unlikely to produce sharp changes in either corrosion mechanism or rate (Zayedet al., 2005). On the other hand, changes that take place over periods of hours to days can occur as a result of point inputs of various chemical pollutants. These effects can lead to sharp chemical gradients over short distances on a temporary or more continuous basis,that will affect the performance of marine engineering alloys.
Long-term studies of seawater corrosion done in the early part of this century (at several locations throughout the world) have been summarized by LaQue (1949). The review(Ijsseling, 1989) on long-time materials performance in the marine environment reveal remarkable consistency in the corrosion of many engineering alloys in open ocean waters regardless of geographical location. However, LaQue (1949),Ijsseling (1989) and Palanichamyet al. (2012) have suggested that large deviations can occur locally in coastal and/or polluted areas. Studies by Palanichamy and Rajendran (2000) have indicated that local conditions of coastal water quality can differ significantly as a result of industrial pollution. The effects of pollutants introduced into the coastal waters on the corrosion of marine engineering alloys is not fully understood. Much of the marine corrosion database available in this country is irrelevant for polluted waters. In order to obtain realistic corrosion data, these effects should be taken into account.
From the 1970s and into the early 1980s, marine corrosion and marine fouling were treated as two separate processes, and several investigators ignored the role of biological constituent of seawater in metal corrosion. Over the years, difficulties in obtaining meaningful data in simulated laboratory tests (Kain and Lee, 1985) highlighted the fact that natural seawater is more aggressive than synthetic substitutes. Today, it is widely acknowledged by investigators worldwide that dissolved organics and the living components of seawater play a crucial role in marine corrosion (Baboian, 2005).
The studies mentioned above are clear indications that local water quality can differ significantly from open ocean waters which can produce anomalous corrosive behavior of the common engineering alloys. There is also some evidence in the literature that local pollution can have a strong effect on the type and extent of fouling which, in turn, can affect the performance of engineering alloys (Subramanian, 1993).Although data on the performance of engineering materials in polluted waters is rather limited, they nevertheless strongly suggest that corrosion data from open ocean environments are unlikely to be relevant for service of the alloys in coastal seawater locations that receive significant amounts of pollutants. Unfortunately, data on materials performance in Indian waters are mainly limited to open ocean conditions.Thorough scrutiny of literature reveals that there is only scant information on the corrosion and biofouling characteristics of HSLA steel in coastal seawater.High strength low alloy (HSLA) steels, are designed to provide better mechanical properties and greater resistance to corrosion than conventional carbon steels. It has been widely used in marine environments for the construction of barges, dredges, bridges, offshore structures and beams.Hence in this study an attempt has been made to investigate the relationship between the variations in the fouling assemblage and corrosion behaviour of HSLA steel,simultaneously at three different coastal locations in the Gulf of Mannar, India, which is the first of its kind in the literature of Indian waters.
2 Experimental
2.1 Test locations
Three different test locations were chosen in the Gulf of Mannar(Bayof Bengal) on the basis of available data(Palanichamy and Rajendran 2000).The coastal waters of Tuticorin (8◦8’N; 78◦13’E) are relatively polluted compared to the coastal waters of Mandapam (9◦16’N;79◦9’E). This variation may be due to the harbour and industrial activities along the Tuticorin coast. Two locations were considered in the coastal waters of Tuticorin, one in the open sea and the other in the breakwaters of the harbour. The depth of the water column in the open sea and in the harbour waters of Tuticorin was 6 m and 8 m, respectively, while it was 2 m at the Mandapam site.
2.2 Preparation of metal coupons and exposure to seawater
The HSLA steel coupons supplied by M/s. Lawrence Metal Industries, Chennai had the following composition:0.15% C; 1.40% Cr; 2.1% Ni; 0.3% Cu; 0.13% V; 0.3% Mn;0.04% P; 0.05% S; 0.7% Si; 0.8% Al, and balance Fe. The coupons of the size 150×100×2 mm were cut from a sheet,pickled in Clarke’s solution as per the ASTM recommendation (1995), washed with soap solution, dried,ground successively with metallographic emery paper of increasing fineness of up to 800 grit (Champion 1952),polished with 1, 0.5 and 0.3 mm alumina slurries (Buehler),weighed to an accuracy of 10-4g, and stored in desiccators until use. The coupons were mounted on wooden rafts using PVC washers and insulated brass bolts and nuts. The rafts were then immersed in a water depth of 1 m below the mean low tide level at the three test locations previously described.The coupons were removed after exposure for 3, 6, 9, 12, 18 and 24 months for the evaluation of corrosion and fouling.
2.3 Specimen preparation and tensile strength measurement
Standard plate-type rectangular tension test specimens with a cross sectional area of 20 mm2were prepared in triplicate as per the ASTM Test Method E8 (1985a). These specimens received the same surface preparation, mounting and immersion procedures as described above, and were removed after exposure for 12 and 24 months. The tensile strength (ultimate tensile strength) of the as-received and seawater exposed structural steel specimens were measured using a Universal Testing Machine (Universal 2001, Model No. 60). From the obtained load for each specimen, the ultimate tensile strength was calculated by dividing the maximum force carried by the specimen during the tension test by the original cross-sectional area of the specimen, and expressed as megapascals (MPa).
2.4 Characterization of fouling and corrosion
The biomass i.e. fouling load formed on metal coupons over the study period was scrapped and dried to a constant weight at 100◦C and weighed to an accuracy of 10-4g. The fouling load was expressed as kg m-2and each value represents the mean of the triplicate coupons. The fouling characteristics of the HSLA steel were also studied in terms of the numbers of settled organisms, species-wise occurrence of macrofouling organisms and community development patterns. Fouling organisms were identified to genus and species levels following previous records from the study regions (Palanichamyet al., 2012). After scraping the fouling load, the corrosion products were removed using recommended pickling solutions, i.e. Clarke’s solution. The corrosion rates were determined by the gravimetric method and expressed as mmpy and each value was the mean of the triplicate coupons. The size and depth of the crevices were measured using a high-resolution microscope (ASTM 1985b;Metals Handbook 1987). Digital images of the coupons were obtained before and after the removal of the fouling and corrosion products after exposure for 12 and 24 months.
2.5 Seawater analysis
Subsurface seawater was collected from the three test locations on a quarterly basis during the 24 month study period using a Hydro-Bios (Kiel) water sampler. Analyses were carried out following the standard procedures outlined by Strickland and Parsons (1972) which included general physico-chemical parameters, dissolved nutrients and major ions. Heavy metals in the water samples were extracted following the APDC-MIBK pre-concentration procedure(Brookset al., 1967) and estimated on an atomic absorption spectrometer (GBC 932 Plus).
2.6 Statistical analyses
One way ANOVA was performed by using the Mircocal ORIGIN software to analyze statistical significance among selected water quality parameters for the three test locations.One way ANOVA was performed for evaluating the statistical significance of corrosion rates, fouling load and loss in ultimate tensile strength among the three test locations after 12 and 24 months of exposure. The linear regression fit and ANOVA were computed for all the mean values of the fouling load and corrosion rate data obtained for the entire study period.
3 Results
3.1 Characteristics of seawater
The mean values of physicochemical parameters of seawaters of 1) Open sea - Tuticorin, 2) Harbour - Tuticorin and 3) Mandapam are presented in Table 1. In general the values of the water quality parameters were found to be normal, and did not show much seasonal variation during the study period. Hence the mean values of the eight quarters are taken into consideration. The values of heavy metals like copper, cadmium and mercury were found to be statistically higher in the Tuticorin waters when compared to that of the Mandapam waters (p·>lt;·0.001). Among the different parameters, nitrate, silicate and ammonia were higher at Tuticorin (both in the open sea and harbour locations) than at Mandapam on a statistically significant basis (p>lt; 0.001).
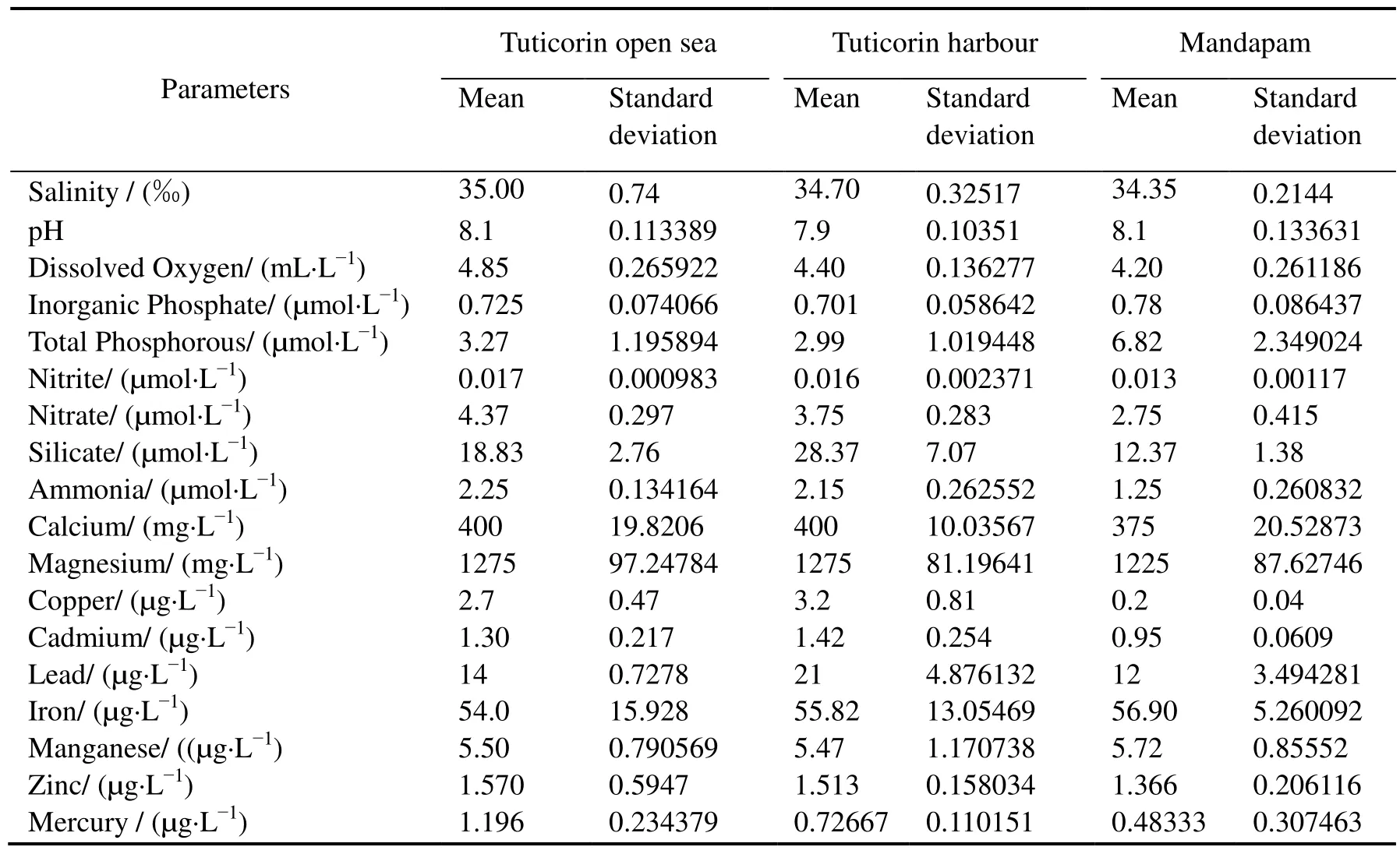
Table 1 Seawater quality parameters for the three locations showing the mean values (n=8) and their SDs
3.2 Biofouling community development pattern
The patterns of fouling after 12 and 24 months of exposure are illustrated in Figs. 1a and 2a for the Tuticorin open sea, Figs. 3a and 4a for Tuticorin harbour and Fig.s 5a and 6a for Mandapam. Fouling at the three tests locations was composed of a variety of organisms such as algae,barnacles bryozoans, polychaete worms, simple and compound ascidians, and oysters. Oysters were particularly dominant in the Tuticorin open sea, while barnacles were the major foulants in the Tuticorin harbour and Mandapam.However at Mandapam the ascidian dominated as the secondary foulant. With regard to the fouling organisms, the surface of the 3 and 6 months exposed coupons in the Tuticorin open sea were characterized by oysters, barnacles,algal mat, and polychaetes. At 9 months of exposure the ascidian fouling was recorded, while the bryozoan fouling was observed at 12 months of exposure and thereafter the composition of the fouling assemblages did not vary till the end of the 24 months exposure period. In the Tuticorin open sea the surface of the coupons from 12 months onwards was fully covered by oysters on which the other fouling organisms appeared as secondary foulants. At Tuticorin harbour the surface of the 3 months exposed coupons was characterized by barnacles, polychaetes, algal mat, and the incidence of ascidians fouling was recorded on the surface of the 6 months exposed coupons. The occurrence of bryozoans fouling was recorded at 12 months of exposure.At Mandapam, the fouling assemblage at 3 and 6 months of exposed coupons was comprised of barnacles, algal mat,polychaetes and ascidians. The occurrence of oyster and bryozoan fouling was recorded at 9 months of exposure.Incidents of sponge fouling could be noticed only at 12 months of exposure. Oyster fouling at Tuticorin (both open sea and harbour) was contributed mainly by Crassostrea cristagalli, while that at Mandapam was represented exclusively by Crassostrea madrasensis. The barnacles,Balanus amphitrite and Balanus reticulatus accounted for a major proportion of the fouling at Tuticorin harbour and Mandapam, while Balanus tintinnabulum additionally occurred in the Tuticorin open sea. Algal fouling was found throughout the study period and the number of species increased with time. The algal fouling was composed predominantly of Ulva lactuca, Caulerpa racemosa,Turbinaria ornata,Hypnea muciformes,Ceramium cruciatum and Gracilaria edulis throughout the study period at all of the test locations. Among the three locations, algal fouling was found to be relatively higher at Mandapam.

Fig. 1 HSLA steel exposed for 12 months at Open sea-Tuticorin.Scale bar = 25 mm on both images
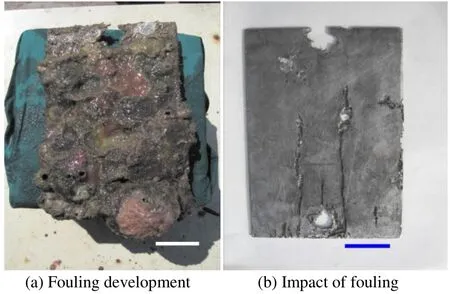
Fig. 2 HSLA steel exposed for 24 months at Open sea-Tuticorin.Scale bar = 25 mm on both images
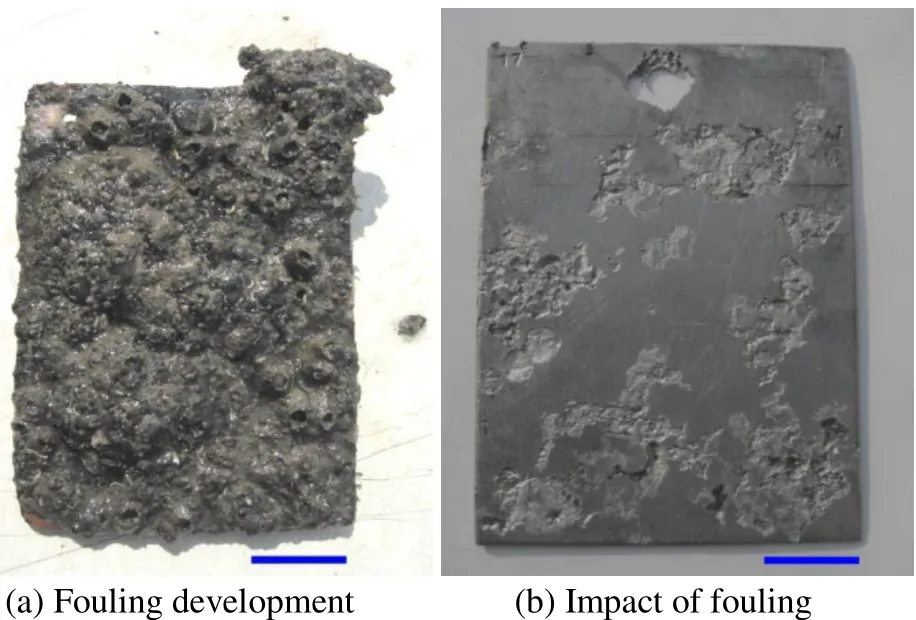
Fig. 3 HSLA steel exposed for 12 months at Harbour-Tuticorin.Scale bar = 25 mm on both images
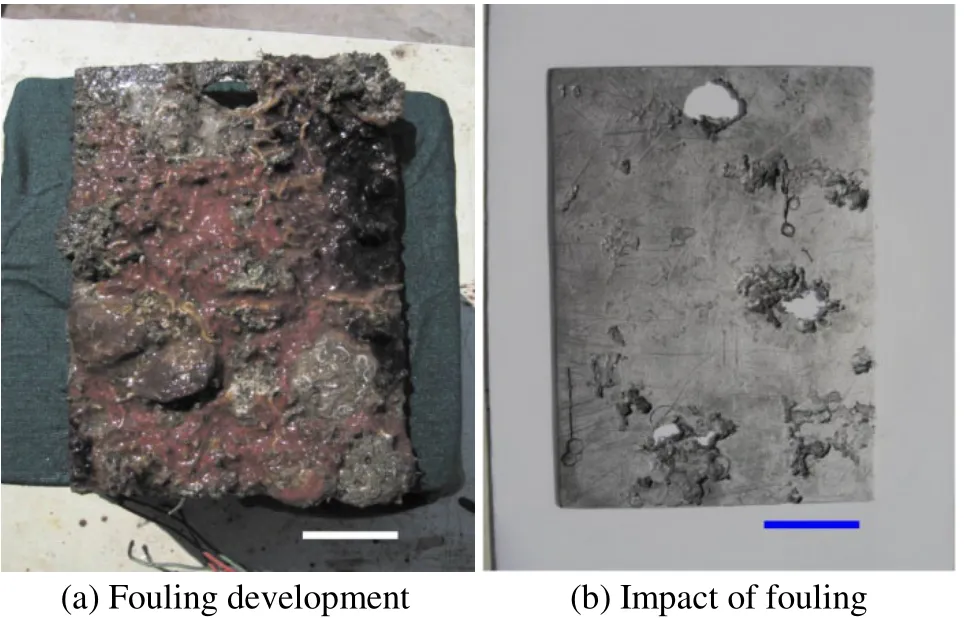
Fig. 4 HSLA steel exposed for 24 months at Harbour-Tuticorin.Scale bar = 25 mm on both images

Fig. 5 HSLA steel exposed for 12 months at Mandapam.Scale bar = 25 mm on both images

Fig. 6 HSLA steel exposed for 24 months at Mandapam.Scale bar = 25 mm on both images
3.3 Fouling load
The fouling load on the coupons at the three test locations over a period of 24 months is shown in Fig. 7. The fouling load values at both locations in Tuticorin were identical until 9 months of exposure and thereafter, when it increased drastically in the Tuticorin open sea with a maximum value at 18 months. At Tuticorin harbour, although the fouling loadexhibited an increasing trend, there was a slight drop at 18 months and attained the maximum value at 24 months coinciding with the value of the Tuticorin open sea for the same exposure period. However, the difference in the fouling load was larger for the Tuticorin open sea when compared to the Tuticorin harbour particularly at 12 and 18 months of exposure.
The mean fouling load values were significantly different between the Tuticorin harbour and the Mandapam test locations, for 12 months (F= 8.88182,p= 0.04073) and 24 months (F=124.16046,p=3.69163E−4); between Mandapam and the Tuticorin open sea, for 12 months (F= 28.82644,p=0.00581) and 24 months (F=56.12437,p=0.0017).However the mean fouling load values were not statistically significant between the Tuticorin open sea and the Tuticorin harbour for 12 (F=5.90399,p=0.072) and 24 (F=0,p=1)months.

Fig. 7 The fouling load on HSLA steel exposed to seawater at three test locations over a period of 24 months
3.4 Quantitative fouling community development
The quantitative fouling community development on HSLA steel exposed to seawater for 3, 6, 9, 12,18 and 24 months is given in Table 2.The numerical density and surface coverage data reveal that oyster fouling was dominant in the Tuticorin open sea throughout the study period, but barnacle fouling dominated up to 12 months at Tuticorin harbour and up to 9 months at Mandapam.Dense fouling by compound ascidians was observed for 12 months and 18 months of exposure at Mandapam and at 24 months for Tuticorin harbor. The maximum density of the bryozoan fouling occurred at Mandapam after exposure for 9 months,whereas it was sparse in the Tuticorin waters at the 12 and 18 months exposure periods.
3.5 Corrosion rates
The corrosion rates of the HSLA steel coupons exposed to seawater at the three test locations over a period of 24 months are shown in Fig. 8. The rates of corrosion progressively decreased with immersion time at all three test locations, although the initial rates differed among the locations. However, in the Tuticorin open sea, the corrosion rates were higher when compared to the other two locations throughout the study period. The mean corrosion rates were significantly different between the Tuticorin open sea and the Tuticorin harbour test locations, for 12 months (F=91.3325,p= 6.69648E−4) and 24 months (F= 45.17453,p= 0.00255); between Mandapam and Tuticorin open sea, for 12 months (F= 64.03442,p= 0.00132) and 24 months (F=24.75289,p= 0.00762). However the mean corrosion rate values were not statistically significant between the Tuticorin harbour and Mandapam for 12 (F= 5.24819,p=0.08377) and 24(F= 5.56621,p= 0.07772) months.
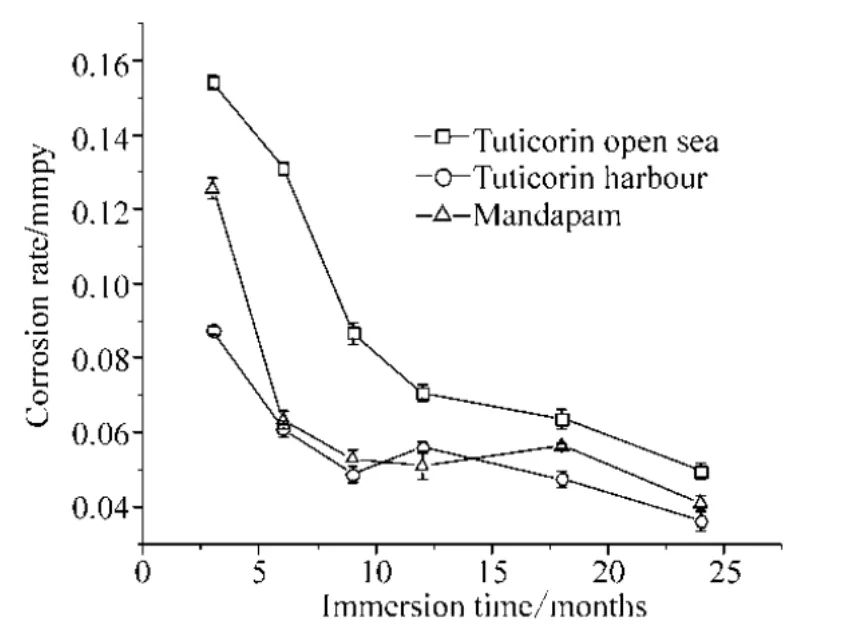
Fig.8 The corrosion rates of HSLA steel exposed to seawater at three test locations over a period of 24 months
3.6 Impact of fouling on the surface of HSLA steel
The surface of the coupons was characterized by crevices beneath hard foulers attached in the Tuticorin harbour and Mandapam, whereas in the Tuticorin open sea the coupons experienced tunneling corrosion besides the crevices. Such attacks are illustrated in Figs. 1b and 2b for the Tuticorin open sea, Figs. 3b and 4b are for the Tuticorin harbour and Figs. 5b and 6b are for Mandapam. The crevice density and percentage area of attack with time of immersion at the three test locations are summarized in Fig. 9. The percentage area of the crevice attack was relatively higher at Tuticorin harbour and Mandapam than in the Tuticorin open sea,while the crevice density approached more or less identical proportion in the Tuticorin open sea and Mandapam except for the 6 and 9 months period. The extent of the crevice attack was markedly higher at Tuticorin harbour from 12 months onwards.
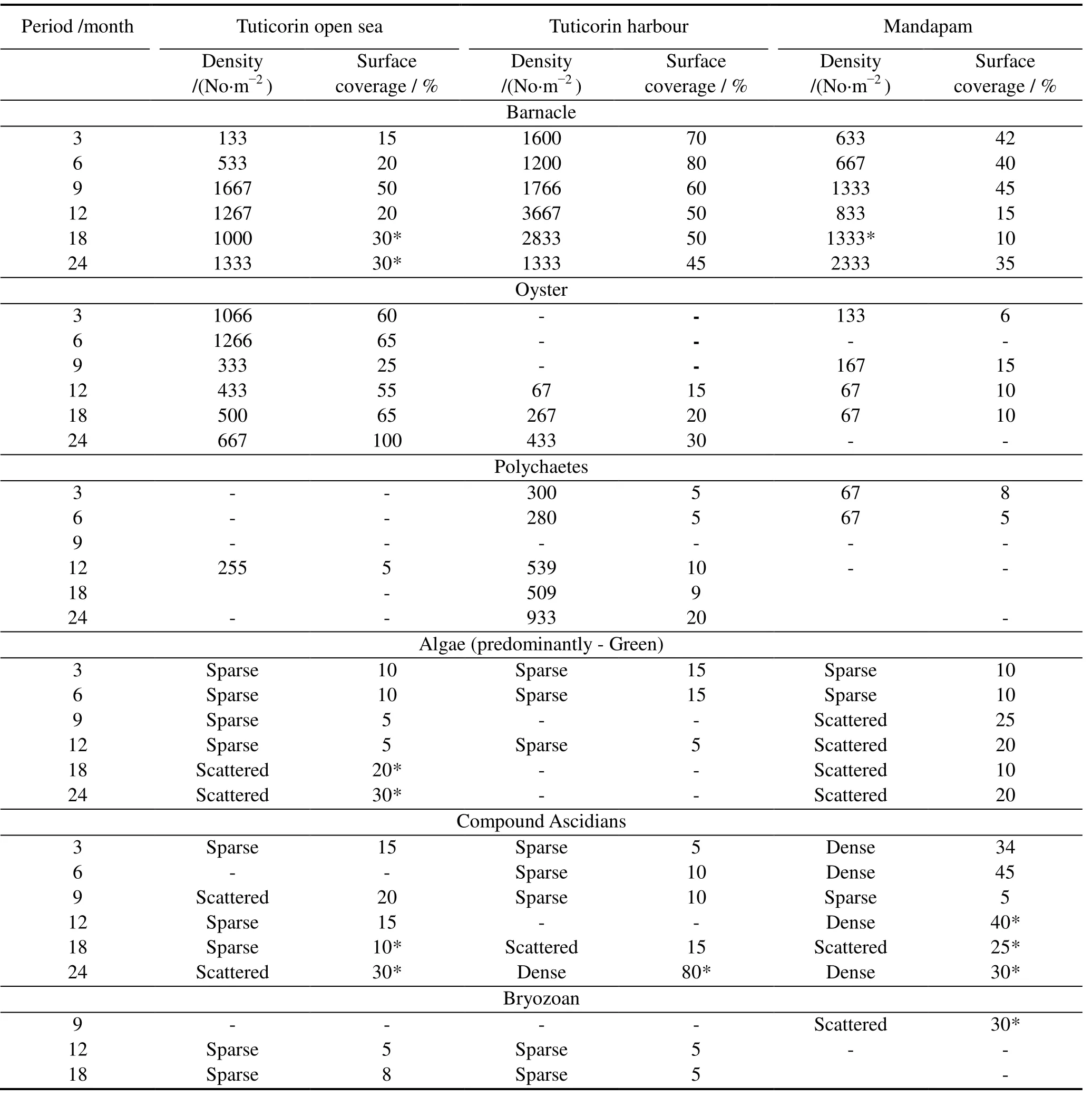
Table 2 Quantification of fouling community development pattern on HSLA steel exposed to seawater over a period of 24 months
3.7 Loss in tensile strength of HSLA steel
The tensile strengths (ultimate tensile strengths) of both as received and seawater exposed HSLA steel, are shown in Fig.10. In general, the percentage loss of tensile strength increased with time at all the test locations. The loss in the ultimate tensile strength of the HSLA steel was 67.5% and 77.2% for 12 months and 24 months in the Tuticorin open sea,60.5% and 68.3% for 12 months and 24 months at Tuticorin harbour and 35% and 43.3% for 12 months and 24 months at Mandapam, respectively. The mean tensile strength values were significantly different between any two test locations for 12 and 24 months. Thus, the ANOVA values were statistically significant between the Tuticorin open sea and Tuticorin harbour (for 12 months,F=14.91176,p=0.01812 and for 24 months,F=20.94231,p=0.01021); Tuticorin harbour and Mandapam (for 12 months,F=122.51131,p=3.78903×10−4and for 24 months,F=162.16875,p=2.19064×10−4), and Mandapam and Tuticorin open sea (for 12 months,F=269.46626,p=8.06257×10−5and for 24 months,F=366.36923,p=4.38987×10−5).
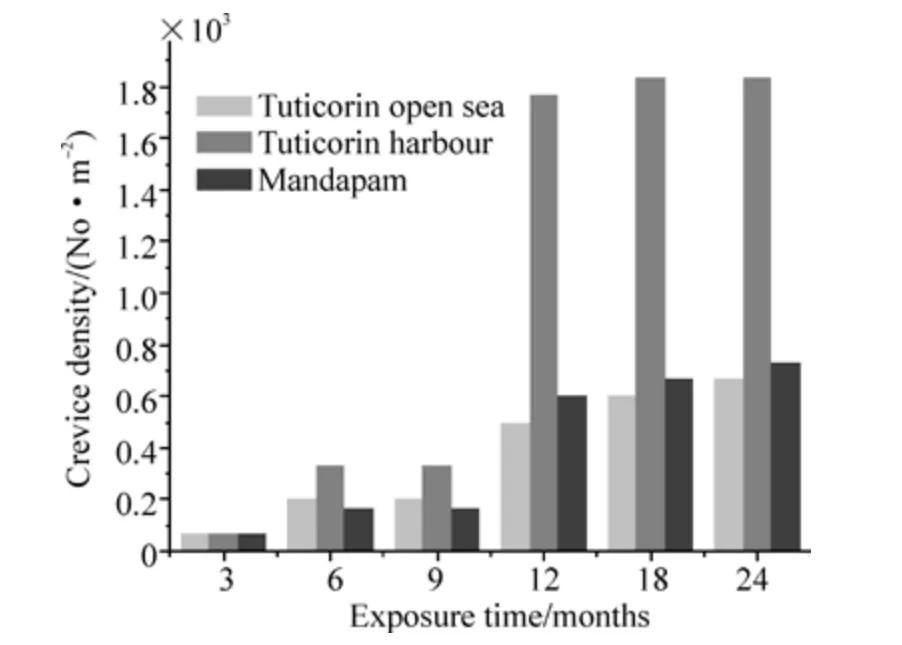
Fig.9a Percentage area of attack on HSLA steel with time of immersion at the three test locations
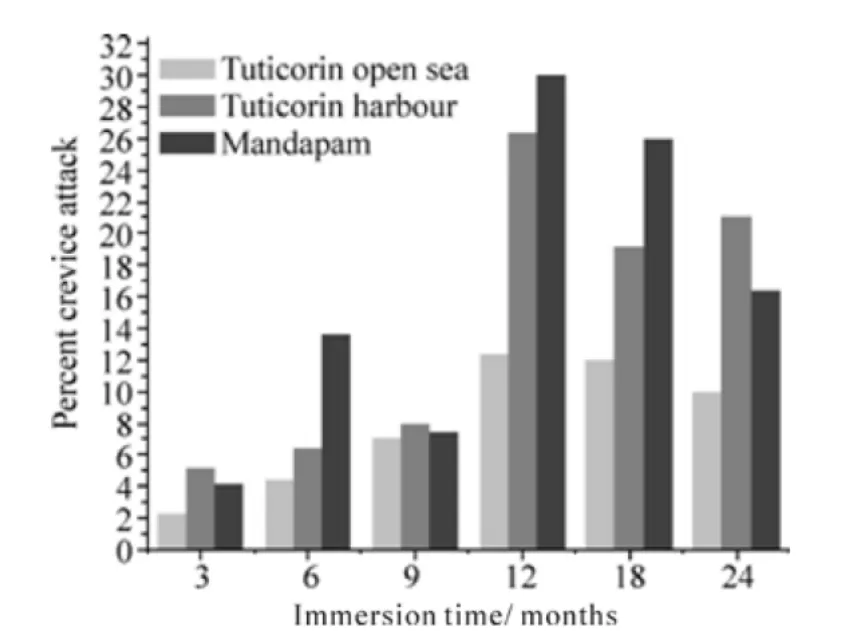
Fig. 9b Crevice density on HSLA steel with time of immersion at the three test locations
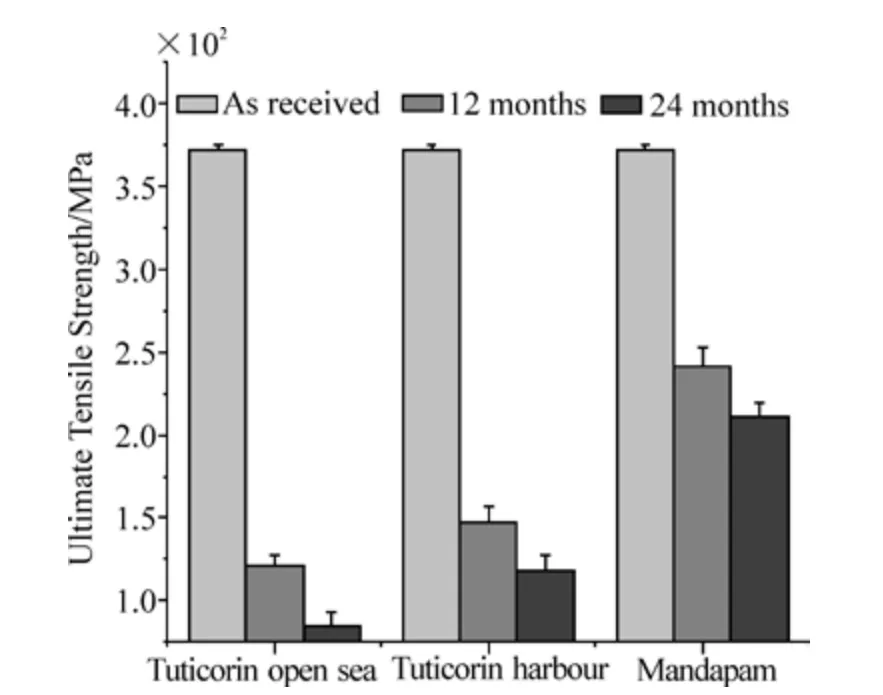
Fig. 10 Loss in ultimate tensile strength of HSLA steel exposed to natural seawater for 12 and 24 months
4 Discussion
One of the unique and troublesome aspects of ocean engineering systems and materials in seawater is the occurrence of marine fouling. Fouling on marine installations/structures induces corrosion, impairs the functioning of moving parts, increases the drag and creates several other problems (Callow 1999). The growth of fouling organisms is strongly influenced by the local environment and it can greatly alter corrosion processes that are occurring on structural members (Melchers 2007).Oyster fouling was predominant in the Tuticorin open sea with a maximum dimension of 70×60×20 mm and being that they are hard shelled organisms adds to more biofouling load. This could be the reason for the higher fouling load recorded in the Tuticorin open sea. The occurrence of the barnacleBalanus tintinnabulumwas another cause of the higher biomass in the Tuticorin waters. The relatively low amount of fouling at Mandapam was presumably due to the smaller types of organisms occurring in these waters. It must also be added that the broyzoan fouling observed after exposure for 9 months at Mandapam resulted in no further development in the fouling community up to the end of the study period.
This was evident from the biomass load which remained more or less constant between 12 and 24 months of exposure (Fig. 7). This observation supports the view of Sutherland (1976) that bryozoan fouling in general inhibits other macrofouling species. Bryozoans are known to produce secondary metabolites as a defense system(Faulkner, 2001) in respect to competition for space and predation (Lopaniket al., 2004). In general, the patterns and characteristics of the fouling recorded in the present study were in close agreement with the observations by earlier investigators of the Mandapam (Eashwaret al., 1990) and Tuticorin (Maruthamuthuet al., 1990 and Palanichamyet al.,2012) waters.
The quality and quantity of the fouling assemblage at a given site or environment depends on its biotic and abiotic conditions (Swami and Udayakumar, 2010). The mean fouling load values were significantly different between the Tuticorin harbour and Mandapam test locations, for 12 months and 24 months; between Mandapam and the Tuticorin open sea, for 12 months. This could be due to the variations in the fouling assemblage and abiotic conditions between the Mandapam and Tuticorin waters. Further the levels of heavy metals like copper, cadmium and mercury were found to be statistically higher at Tuticorin waters when compared to that at Mandapam water, but they did not inhibit the fouling growth at Tuticorin waters.
As the rates of corrosion showed a declining trend with time at all three test locations, it could be inferred that fouling provided a shielding effect. The steady decrease in the corrosion rate values of the HSLA steel coupons with exposure periods and the concomitant increase in the oyster fouling (with maximum coverage) in the Tuticorin open sea implies the shielding effect of fouling assemblages, which resulted in a reduction in O2diffusion and utilization of available O2for respiration (Ravindran and Pillai, 1984;Littleet al., 2008). Large differences in the oxygen concentration at the metal surface may be created by the larger (macro) fouling organisms. It is likely, therefore, that local oxygen concentration cells may be created when there are gaps in fouling layers or between two adjacent species leading to corrosion of the metal under the area covered by the organism. Algae by virtue of its photosynthetic activity accelerates corrosion (Subramanian, 1993) and also putrefaction of algae under the dense mat of the fouling assemblage are capable of producing pH levels as low as 2(Subramanian, 1993), leading to uneven corrosion.
HSLA steel experienced relatively higher corrosion throughout the study period at the Tuticorin open sea than the other two locations, indicating the aggressiveness of the environment. The severity of the environment of the Tuticorin open sea is substantiated that the 12 and 24 months corrosion rate values of the HSLA steel are 35%-46% (12 months) and 19%-28% (24 months) higher than the corresponding values of the other two locations. The Tuticorin open sea location is situated below the offshore platform of CECRI. This site adjoins the south breakwater road whose slope is reinforced by boulders. During the southwest monsoon period (April through October), the site is characterized by severe wave actions which produce turbulence, splashing and swirling. The impact of the physical conditions on enhancing oyster fouling has been discussed earlier by Chellam (1978). Hence, it is presumed that the prevailing conditions would have favoured the settlement and growth of oysters. The deep longitudinal crevice attacks experienced in the Tuticorin Open sea are thought to be formed owing to the differential aeration cells between the oysters’ settlement in a longitudinal fashion.This implies that localized corrosion beneath the marine hard fouling organisms can lead to significant loss of metal.
Crevice corrosion behaviour, depth of the crevice and the width of the crevice of HSLA is mainly characterized by the type of algae species, size and density of the barnacles and oysters settlement during the particular exposure period. The width of the crevice is mainly influenced by the size of the barnacle/oyster shell on the HSLA steel surface(Subramanian, 1993). According to LaQue (1972) dead organisms cause local corrosion on metal surfaces immersed in seawater leading to crevices and pits. While Ruimuet al.(1984) reported that crevice corrosion would occur beneath the dead barnacles. Furthermore, Eashwaret al. (1992)proposed a model of barnacle induced crevice corrosion. In the present study, such crevices were also recorded beneath the barnacles particularly at Tuticorin harbour and Mandapam, where the barnacles were the predominant fouling species with a higher numerical density. The percentage area of the crevice attack was relatively higher at Tuticorin harbour and Mandapam than in the Tuticorin open sea. This can be explained by the fact that at both Tuticorin harbour and Mandapam the numerical density of the barnacles was higher than in the Tuticorin open sea, where oyster fouling was predominant with a larger dimension and a lesser numerical density.
The crevice corrosion caused by the hard foulers in this work was possibly due to a difference in oxygen levels between the surface underneath the hard shells and the uncovered surface area adjacent to the organisms (Pipe,1981). Though the percentage area of the crevice attack was relatively higher at Tuticorin harbour and Mandapam than in the Tuticorin open sea, the loss in ultimate tensile strength was highest in the Tuticorin open sea. This could be due to the deep longitudinal crevice attacks experienced in the open sea. The longitudinal crevices are thought to be formed owing to the differential aeration cells between the oysters attached in a longitudinal fashion. This implies that localized corrosion beneath the marine hard fouling organisms can lead to significant loss of mechanical strength.Therefore, the loss of mechanical strength due to marine fouling should serve as an important tool in the selection of materials for marine applications.
The corrosion rates of the present study were found to be 3-6 times lower than that reported by Subramanian (1993)for the Mandapam waters. On the contrary, the loss in ultimate tensile strength is much higher in the present study as compared with earlier work by Subramanian (1993). In general, HSLA steel undergoes uniform corrosion, but in the present study crevice corrosion is predominant at all the three test locations. This could be due to the variation in the composition of the material, especially with the presence of higher amounts of chromium, nickel and aluminium. The observed trend is also reflected by the Regression analysis findings (Fig. 11) that the corrosion rate and fouling load data for all six exposure periods at the three test locations(18 sets in all) shows a moderate negative correlation (R=−0.32857), which is statistically less significant (p=0.18311).
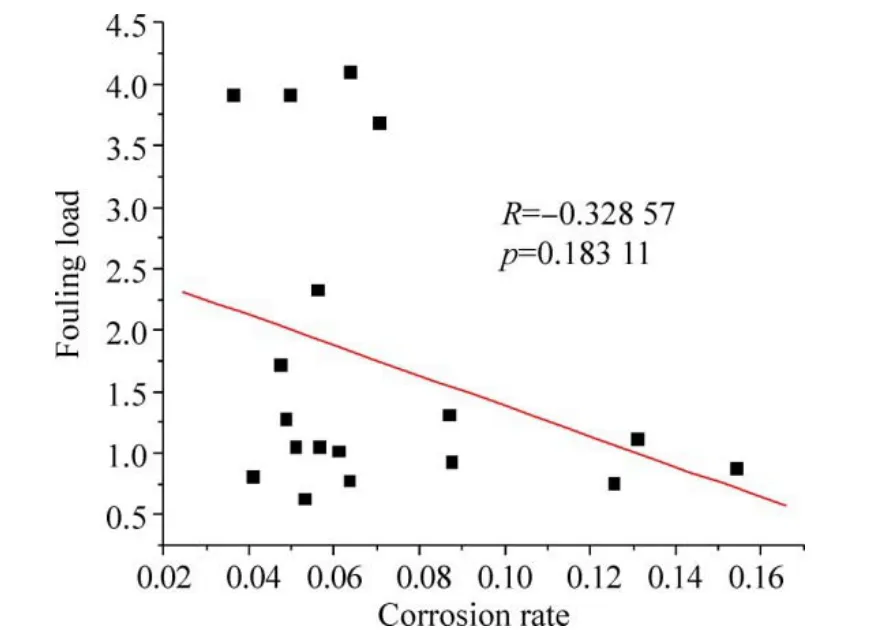
Fig. 11 Regression analysis for all the corrosion rate and fouling load data at the three test locations
5 Conclusion
Oyster fouling was predominant in the Tuticorin open sea,while barnacles were the major foulants at Tuticorin harbour and Mandapam. The fouling load in the Tuticorin waters was higher when compared to the Mandapam waters. The corrosion rates showed a decreasing trendwith time at all the three test locations. In the Tuticorin open sea, the corrosion rates were higher when compared to the other two locations throughout the study period. The surface of the coupons was characterized by crevices beneath hard foulers at Tuticorin harbour and Mandapam, whereas in the Tuticorin open sea the coupons experienced crevices of a tunneling nature. The percentage loss inultimate tensile strength increased with time at all the test locations and was at maximum in the Tuticorin open sea. The presence of relatively higher amounts of chromium, nickel and aluminium in the HSLA steel matrix is believed to be the causative factor for the crevice corrosion. As a result, the HSLA steel of this composition is not suitable for seawater applications.
Acknowledgements
The authors wish to thank the Director, Central Electrochemical Research Institute, Karaikudi for the encouragements. The enthusiastic technical service rendered by Mr. P. Ganapathy, Senior Technical Officer, CECRI Unit,Tuticorin is profoundly acknowledged. Dr. M. Eashwar,Scientist-in-Charge, CECRI Unit, Mandapam is thankfully acknowledged for extending the laboratory facilities to carry out the studies at Mandapam test location. The authors also wish to thank the Ministry of Environment and Forests, New Delhi for the financial support (Project Grant No.19-26/2003-RE).
Annual Book of ASTM Standards, Section 3, 03.01. (1985a).ASTM International, West Conshohocken, PA, 152-175.
Annual Book of ASTM Standards, Section 3, 03.02. (1985b).ASTM International, West Conshohocken, PA, 267-276.
Annual Book of ASTM Standards, Section 3, 03.02. (1995). ASTM International, West Conshohocken, PA, 12.
Baboian R(Ed.) (2005). Corrosion tests and standards: application and interpretation.West Conshohocken,PA, ASTM International,367.
Beech IB, Sunner J (2004). Biocorrosion: towards understanding interactions between biofilms and metals.Curr.Opin.Biotechnol,15, 181-186.
Brooks RR, Presley BJ, Kaplan IR (1967). APDC-MIBK extraction system for the determination of trace elements in saline waters by atomic absorption.Talanta, 14, 806-816.
Callow ME (1999). The status and future of biocides in marine biofouling prevention.Proceedings on Recent Advances in Marine Biofouling, 3, (Oxford and IBH Publishing Co., New Delhi), 109-126.
Champion FA (1952). (Ed.).Corrosion testing procedures. London,UK, Chapman and Hall, 36-52.
Chellam A (1978). Growth of pearl oyster Pinctada fucata in the pearl culture farm at Veppalodai, Indian.J. Fish. 25, 77-83.
Compton KG (1970). The unique properties of seawater as a corrosive medium.Corrosion, 26, 448-449.
Dexter SC, Culberson CH (1980). Global variability of natural seawater.Mater. Perform., 19, 16-28.
Eashwar M, Subramanian G, Chandrasekaran P (1990). Marine fouling and corrosion studies in the coastal waters of Mandapam, India.Bull.Electrochem., 6, 699-702.
Eashwar M, Subramanian G, Chandrasekaran P,Balaskrishnan K(1992). A mechanism for barnacle induced crevice corrosion in stainless steel.Corrosion, 48, 608-612.
Faulkner DJ (2001). Marine natural products. Nat Prod Rep 18,1-49.Ijsseling, F.P., 1989. General guidelines for corrosion testing of materials for marine applications.Brit. Corros. J., 24,55-78.
Kain RM, Lee TS (1985). Recent developments in test methods for investigating crevice corrosion. In: Haynes GS, Baboian R,editors. Laboratory corrosion tests and standards.West Conshohocken(PA),ASTM International, 299-323.
LaQue FL, Clapp WF (1945). Relationships between corrosion and fouling of copper-nickel alloys in sea water.Electrochemical Society, Trans., 87, 103-125.
LaQue FL (1949).Behaviour of metals and alloys in seawater.Corrosion handbook. Uhlig, H.H., (Ed.), New York, John Wiley and Sons, 383-430.
LaQue FL (1972). Corrosion and fouling.Proceedings of the Third International Congress on Marine Corrosion and Fouling, NBS,Gaithersburg, Maryland, USA, 2-13.
LaQue FL (1982). Topics for research in marine corrosion.Mater.Perform., 21, 13-18.
Little B (2004). Recent development in microbiologically influenced corrosion: a series of paradoxes.12th Congress on Marine Corrosion and Fouling, Southampton, UK, 27- 30.
Little BJ, Jason SL, Ray RI (2008). The influence of marine microfouling on the corrosion behaviour of passive materials and copper alloys. Report No.NRL/PP/7303-07-7139, 9. Naval Research Laboratory, Stennis Space Center (MS).
Lopanik N, Gustafson KR, Lindquist N, (2004). Structure of bryostatin 20: a symbiont produced chemical defense for larvae of the host bryozoan, Bugula neritina.J Nat. Prod., 67,1412-1414.
Maruthamuthu S, Eashwar M, Manickam ST, Ambalavanan S,Venkatachari G, Balakrishnan K (1990). Marine fouling on test panels and in-service structural steel in Tuticorin harbour.Indian J. Mar. Sci., 19, 68-70.
Melchers RE (2007). Effects of water pollution on the immersion corrosion of mild and low alloy steels.Corros. Sci., 49,3149-3167.
Metals Handbook., 9th edition. (1987). ASM International, Metals Park, OH, 231-236.
Palanichamy S, Rajendran A (2000). Heavy metal concentrations in seawater and sediments of Gulf of Mannar and Palk Bay.Indian J. Mar. Sci., 29, 116-119.
Palanichamy S, Subramanian G, Eashwar M (2012). Corrosion behaviour and biofouling characteristics of structural steel in the coastal waters of the Gulf of Mannar (Bay of Bengal), India.Biofouling, 28, 441-451.
Pipe A (1981). North Sea fouling organisms and their potential effects on the corrosion of North Sea structures. In: Lewis, JR.,Mercer, AD., (Eds.),Marine corrosion on offshore structures,London, UK, Society of Chemical Industry, 13.
Ravindran K, Gopalakrishnan Pillai A (1984). Observations on the interrelation of marine corrosion and fouling in a tropical environment.Proceedings of Sixth International Congress on Marine Corrosion and Fouling, National Technical University of Athens, Athens, Greece, 159-170.
Ruimu L, Dekai L, Xuebao H, Jiancheng Z (1984).Proceedings of Sixth International Congress on Marine Corrosion and Fouling,
National Technical University of Athens, Athens, Greece, 443.
Schumacher M (Ed.) (1979). Seawater corrosion handbook. Park Ridge, NJ, Noyes Data Corporation, 494.
Strickland JDH, Parsons TR (1972). A practical handbook of seawater analysis. Ottawa, Canada, Fisheries Research Board of Canada, 310.
Subramanian, G (1993). Studies on the electrochemical behaviour of ferrous materials in natural seawater, PhD thesis, Madurai Kamaraj University, Madurai (India).
Sutherland JP (1976). Life histories and the dynamics of fouling communities. In: Costlow,J.D., (Ed.), The ecology of fouling communities, Annapolis (MD), United States Naval Institute Press, 137-153.
Swami BS, Udayakumar M (2010). Seasonal influence on settlement, distribution and diversity of fouling organisms at Mumbai harbour.Indian J. Mar. Sci., 39, 57-67.
Videla HA, Herrera LZ (2005). Microbiologically influenced corrosion: looking to the future.Int. Microbiol., 8, 169-180.
Zayed A, Guedes Soares C, Garbatov Y, Wang G (2005).Environmental factors affecting the time dependant corrosion wastage of marine structures.Proceedings of Eleventh International Congress of the International Maritime Association of the Mediterranean, London, UK, Taylor >amp;Francis Group, 589-598.
杂志排行
Journal of Marine Science and Application的其它文章
- An Approximate Method for the Surge Response of the Tension Leg Platform
- CFD Simulation of the Vertical Motion Characteristics of the Moonpool Fluid for the Truss Spar
- Dynamic Coupled Analysis of the Floating Platform Using the Asynchronous Coupling Algorithm
- Numerical Investigation of Mooring Line Damping and the Drag Coefficients of Studless Chain Links
- Floating Production Platforms and their Applications in the Development of Oil and Gas Fields in the South China Sea
- Bucket Group Effect of the Composite Multi-bucket Structure
