ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
2014-07-25XiaojieJuShuoweiPiRuiXieXiaojingGuoJieyiLiuYalanYuLuJiangXiaohuaLuQianmingChenLiangyinChu
Xiaojie Ju,Shuowei Pi,Rui Xie,Xiaojing Guo,Jieyi Liu,Yalan Yu,Lu Jiang,Xiaohua Lu, Qianming Chen,Liangyin Chu*,
Materials and Product Engineering
ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
Xiaojie Ju1,Shuowei Pi1,Rui Xie1,Xiaojing Guo2,Jieyi Liu1,Yalan Yu1,Lu Jiang3,Xiaohua Lu2, Qianming Chen3,Liangyin Chu*,1
1School of Chemical Engineering,Sichuan University,Chengdu 610065,China2State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemistry and Chemical Engineering,Nanjing University of Technology,Nanjing 210009,China3State Key Laboratory of Oral Diseases,Sichuan University,Chengdu 610065,China
A R T I C L EI N F O
Article history:
Stimuli-responsive material
Phase transition behavior
Alcohol-responsive property
Ion-responsive property
In this paper,we report on the comprehensive alcohol-/ion-responsive properties of a smart copolymer poly(N-isopropylacrylamide-co-benzo-18-crown-6-acrylamide)(P(NIPAM-co-BCAm)).The orthogonal design method is adopted for experimental design.The experimental results show that alcohol can trigger the shrinking and Ba2+can induce the swelling of the P(NIPAM-co-BCAm)copolymer.According to the phase transition temperature(LCST)change results of the copolymer,the inf l uence of variables on the LCST changes weakens in the following order:alcohol concentration>alcohol species>metal ion species>BCAm concentration>ion concentration.The larger the alcohol concentration and the larger the molecular size of alcohols,the lower the LCST value;on the contrary,the more the BCAm content in the copolymer or the larger the BCAm/ion complex stabilityconstant(lgK)orthelargertheionconcentrationis,thehighertheLCSTvalue.ForaP(NIPAM-co-BCAm) copolymerwithaf i xedBCAmcontent,abinaryfunctionofionconcentrationandlgKofBCAm/ionisdevelopedto precisely predict the LCST values of the copolymer in different metal ion solutions.The results provide valuable information for fabricating artif i cial biomimetic G-protein-gated inwardly rectifying potassium(GIRK)channels that are activated by alcohol and inhibited by Ba2+.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Metalionsplayimportantrolesinthelifeactivities.Manymetalions are essential metabolism components and cofactors for various biological processes,including oxidative phosphorylation,gene regulation and free-radical homeostasis[1].For example,G-protein-gated inwardly rectifying potassium(GIRK)channels,which are widely distributed in the brain,have important functions in inhibitory regulation of neuronal excitability[2,3].Ontheotherhand,alcoholscouldmodulatepotassium channels and affect various functions of the central nervous system. Aryal et al.reported that the open and close functions of such GIRK channels play a key role in the function of the brain,and ethanol can activate GIRK channels and Ba2+can inhibit the channels[4].So,the competition and corporation between alcohol molecules and metal ions are important for life systems.If artif i cial materials could be developedtomimictheactionbehaviorsofGIRKchannelsinresponsetoboth alcohol molecules and metal ions in the opposite directions,it could be possible to artif i cially achieve biomimetic GIRK channels that are very important for biomedical therapies.
Stimuli-responsive smart materials are attracting ever-increasing attention due to their dramatic response to environmental stimuli such as mild change of temperature[5,6],pH[7],and magnetic f i eld [8,9].In recent years,a series of ion-recognition responsive materials, which are mainly composed of poly(N-isopropylacrylamide-co-benzo-18-crown-6-acrylamide)(P(NIPAM-co-BCAm))copolymers,have been developed for various applications with different conf i gurations such as microcapsules[10,11],hydrogels[12-14]and membranes [15-20].It has been reported that poly(N-isopropylacrylamide) (PNIPAM)polymers demonstrate interesting coil-to-globule-to-coil phenomena in alcohol-water mixtures in response to alcohol concentration[21-23].IfethanolcantriggertheshrinkingandBa2+caninduce theswellingofP(NIPAM-co-BCAm)copolymersproperly,itisfeasibleto fabricate artif i cial biomimetic GIRK channels by immobilizing the P(NIPAM-co-BCAm)copolymers as functional gates in artif i cial channels.However,comprehensive dual ion-recognition and alcoholresponsive properties of P(NIPAM-co-BCAm)have not been systematically investigated up to now,i.e.,understanding of the competition and corporation effects between alcohol molecules and metal ions on the phase transition of P(NIPAM-co-BCAm)is still lacking.
In this study,we report on a systematical investigation of comprehensive alcohol-/ion-responsive properties of P(NIPAM-co-BCAm) copolymers for the f i rst time.Schematic illustration of thermoresponsive,ion-recognition and alcohol-responsive properties of P(NIPAM-co-BCAm)copolymers[Fig.1(a)]is illustrated in Fig.1.The thermo-responsive property is derived from the PNIPAM units.The P(NIPAM-co-BCAm)copolymer chain would change from coil to globule when the temperature increases across the lower critical solution temperature(LCST)[Fig.1(b)][24].The BCAm units act as ion-recognition sensors.The P(NIPAM-co-BCAm)copolymer chain could change from globule to coil isothermally when the BCAm units recognize special metal ions[Fig.1(c)][25].The alcohol-responsive property is also from the PNIPAM units.The P(NIPAM-co-BCAm) copolymer demonstrates a coil-to-globule transition in alcohol-water mixtures with increasing alcohol concentration when the alcohol concentration is lower than the upper critical response concentration [Fig.1(d)][21-23].To investigate the comprehensive and cooperative effects of alcohol species,alcohol concentration,metal ion species,ion concentration and BCAm content on the phase transition behaviors of P(NIPAM-co-BCAm)copolymers,the orthogonal design method is adopted to design the experiments.The orthogonal experimental design allows for the necessary data collection to determine the sequence of effect degree of independent variables on the performance [26-28].Besides,the orthogonal experimental method reduces effectively the number of experimental runs.In this study,there are f i ve factors and four different levels for each factor;therefore,45(i.e.,1024)experimental runs would be carried out if single factor experiments are adopted,but the orthogonal experiments can decrease theexperimentalrunsto42(i.e.,16).Thefactorsandlevelsintheexperiments are carefully designed according to the orthogonal design table L16(45)by referring to the relevant solution environment for GIRK channels[4],in order to provide valuable information for achieving biomimetic GIRK channels with artif i cial P(NIPAM-co-BCAm)gates.
2.Experimental
2.1.Materials
N-isopropylacrylamide(NIPAM,provided by Kohjin Co.,Japan)was purif i ed by recrystallization with a hexane/acetone mixture(50/50, by volume).Benzo-18-crown-6 acrylamide(BCAm)was synthesized according to reported procedures[29,30].2,2′-Azoisobutyronitrile (AIBN,Shanghai Fourth Reagent Factory)was recrystallized with ethanol.To minimize salting-out effects,nitrate was chosen as the model salt[31].All the solvents and other chemicals were of analytical grade and were used as received.Deionized water(Milli-Q,Milli-Pore, 18.2 MΩ,25°C)was used throughout the experiments.

Fig.1.Schematic illustration of the chemical structure(a)and thermo-responsive(b),ion-recognition(c)and alcohol-responsive(d)properties of P(NIPAM-co-BCAm)copolymers.
2.2.Synthesis of P(NIPAM-co-BCAm)copolymers
A series of P(NIPAM-co-BCAm)copolymers with different molar ratios of NIPAM and BCAm monomers were synthesized by free-radical polymerization using AIBN as initiator.The monomers and initiator were dissolved in tetrahydrofuran(THF)as reaction solution and the total monomer concentration was 0.3 mol·L−1.The molar ratio of AIBN to total monomers was 1%,and that of BCAm to total monomers varied from 4%to 13%.The polymerization reaction was carried out under N2atmosphere at 60°C for 24 h.After polymerization,an excess of ethyl ether was added to the solution to precipitate the copolymers. The copolymers were purif i ed twice by reprecipitation with ethyl ether from THF,and dried under vacuum at 40°C for 48 h.
A PNIPAM homopolymer,which served as the reference,was also prepared and purif i ed using the similar protocol without addition of BCAm.
The formations of prepared P(NIPAM-co-BCAm)copolymers were determined by a FT-IR spectrometer(IR Prestige-21,Shimadzu)in the range of 4000-400 cm−1with KBr disc technique.The compositions of P(NIPAM-co-BCAm)copolymers were determined by1H NMR spectroscopy(Bruker-400,Bruker)operating at 400 MHz and using deuterium oxide as solvent.The molecular masses of the copolymers were determined by gel permeation chromatography(GPC,Waters-2410,Waters).
2.3.Characterizations of phase transition behaviors of polymers
To scientif i cally design the experiments on the phase transition behaviors of P(NIPAM-co-BCAm)copolymers with different BCAm contents in various ion solutions,the orthogonal experimental design was adopted[26-28].Alcohol species,alcohol concentration,metal ion species,ion concentration and BCAm content in copolymers were selected as the main independent variables(factors)in this study,and each variable(factor)was tested at four levels as shown in Table 1. The level order for each factor was arranged at random according to the orthogonal experimental design method.The order of the inf l uence degree of f i ve factors on the phase transition behaviors of P(NIPAM-co-BCAm)copolymers could be sorted out upon the orthogonal experimental method.
TomeasurethephasetransitionbehaviorofP(NIPAM-co-BCAm)copolymers,thecopolymers were dissolved in aqueous solutionscontaining different alcohols and metal ions as arranged in the orthogonal design.Then,the temperature-dependent transmittance changes of copolymer solutions were measured using a UV-visible spectrophotometer(at 500 nm,Shimadzu UV-1700,Japan)equipped with a temperature-controlled cell(Shimadzu TCC-240A).The temperature, at which the transmittance of copolymer solution decreased to half of its initial value,was recorded as the corresponding LCST value of the P(NIPAM-co-BCAm)copolymers.
3.Results and Discussion
3.1.Composition characterizations of P(NIPAM-co-BCAm)copolymers
By comparing the FT-IR spectra of the BCAm monomer,PNIPAM homopolymer and P(NIPAM-co-BCAm)copolymers,the copolymerization of NIPAM and BCAm is conf i rmed.Specif i cally,the appearance of the followingcharacteristicpeaksintheFT-IRspectraofP(NIPAM-co-BCAm)copolymerssuggestsasuccessfulcopolymerization:(1)astrong1516cm−1band for C=C skeletal stretching vibration of the phenyl ring,(2)a 1231 cm−1band for C-O asymmetric stretching vibration in Ar-O-R, and(3)a 1053 cm−1band for C-O symmetric stretching vibration in Ar-O-R.Corresponding bands also appear in the FT-IR spectrum of the BCAm monomer.Furthermore,the characteristic double peaks at 1388 and 1366 cm−1for the isopropyl group of NIPAM appear in both FT-IR spectra of PNIPAM and P(NIPAM-co-BCAm)polymers.These feature peakssuggestasuccessfulfabricationofP(NIPAM-co-BCAm)copolymers.
The actual BCAm contents in the P(NIPAM-co-BCAm)copolymers, which are calculated by comparing the integral of 6.7-7.3(Ar-H,3H) to 1.03-1.05[(CH)3NH-,6H]from the1H NMR results,are 4.1%(by mol),8.4%(by mol)and 11.2%(by mol).
TheweightaveragemolecularmassesoftheP(NIPAM-co-BCAm)copolymers(Mw)are in the range 2260-5220 as determined by GPC data, and the magnitudes of the molecular masses are independent of the composition of copolymers.
3.2.Analysis of orthogonal experimental data
There are f i ve factors and four levels for each factor in the experiments,andatotalof16experimentalrunsaredesignedaccording to the orthogonal design table L16(45)(Table 2).The temperaturedependent transmittance changes of copolymer solutions are shown in Fig.2.Obviously,all of the copolymer solutions undergo an abrupt change in transmittance when the temperature changes across the corresponding LCST value.The measured LCST values from Fig.2 are also summarized in Table 2.
The average LCST value of each factor at each level is calculated (symbolically indicated as kij,where i represents a factor and j represents a level).Take the factor A(actual BCAm content)as an example, thef i rstlevel(0%,bymol)isarrangedin1#,2#,3#and4#experimental runs and the corresponding LCST values are 32.5,29.2,18.4 and 8.6 respectively,so the average LCST value of factor A at level-1 is: Another example,for the factor B(metal ion species),the third level (K+)is arranged in 3#,7#,11#and 15#experimental runs and the corresponding LCST values are 18.4,34.0,29.1 and 34.0 respectively, so the average LCST value of factor B at level-3 is:
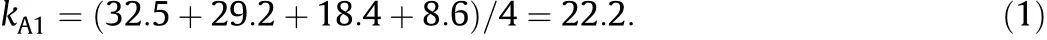
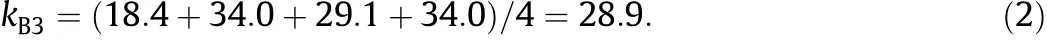
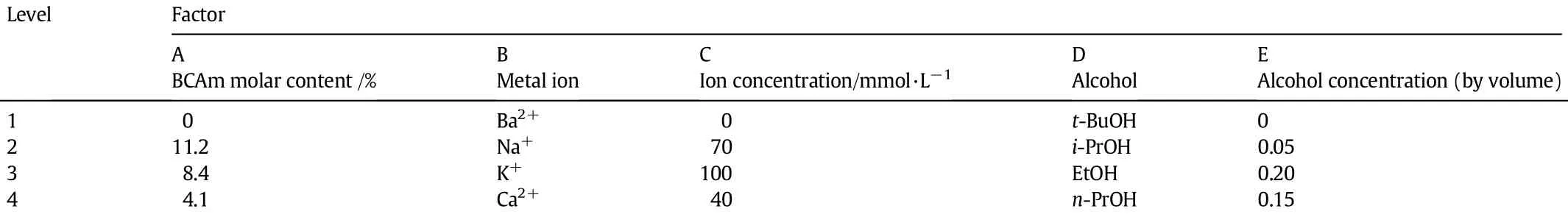
Table 1Experimental variables:Factors and levels
The“Largest kijgap”is def i ned as the difference between the maximum and minimum values of kijfor each factor i,which means the degree of change in the LCST value when the factor i varies among the four levels.The larger the“Largest kijgap”is,the larger the LCST value change.For example,the“Largest kBjgap”of factor B(metal ion species) (kB,max−kB,min)is 11.0,which indicates that the average LCST value changes 11.0°C when the metal ion species varies among Ba2+,K+, Ca2+and Na+.So,the parameter“Largest kijgap”presents the signif icance or inf l uence degree of the variables(factors)on the LCST values intheorthogonalexperiments.The“Largestkijgap”valuesoffactorA(actual BCAm content),factor B(metal ion species),factor C(ion concentration),factor D(alcohol species)and factor E(alcohol concentration)are 6.9,11.0,6.6,11.2 and 17.9,respectively.So,the inf l uence degree of the f i ve factors on the LCST value in order from large to small is listed as follows:alcohol concentration>alcohol species>metal ion species>BCAm content>ion concentration.That is,the inf l uence of alcohols on the LCST shift is larger than that of metal ions and BCAm units.The alcohol-responsive sites in the P(NIPAM-co-BCAm)copolymer are the NIPAM units,and the ion-recognition sites are the BCAm units.In the orthogonal experiments,the molar ratio of BCAm units is at most 11.2%,i.e.,theNIPAMunits aremuchmorethanBCAm unitsintheP(NIPAM-co-BCAm)copolymers.Inotherwords,thealcohol-responsivesitesaremuch more than the ion-recognition sites in the P(NIPAM-co-BCAm)copolymers.So,the inf l uence degree of alcohols on the phase transition behaviorsofP(NIPAM-co-BCAm)ismoresignif i cantthanthatofmetalionsand BCAm units.
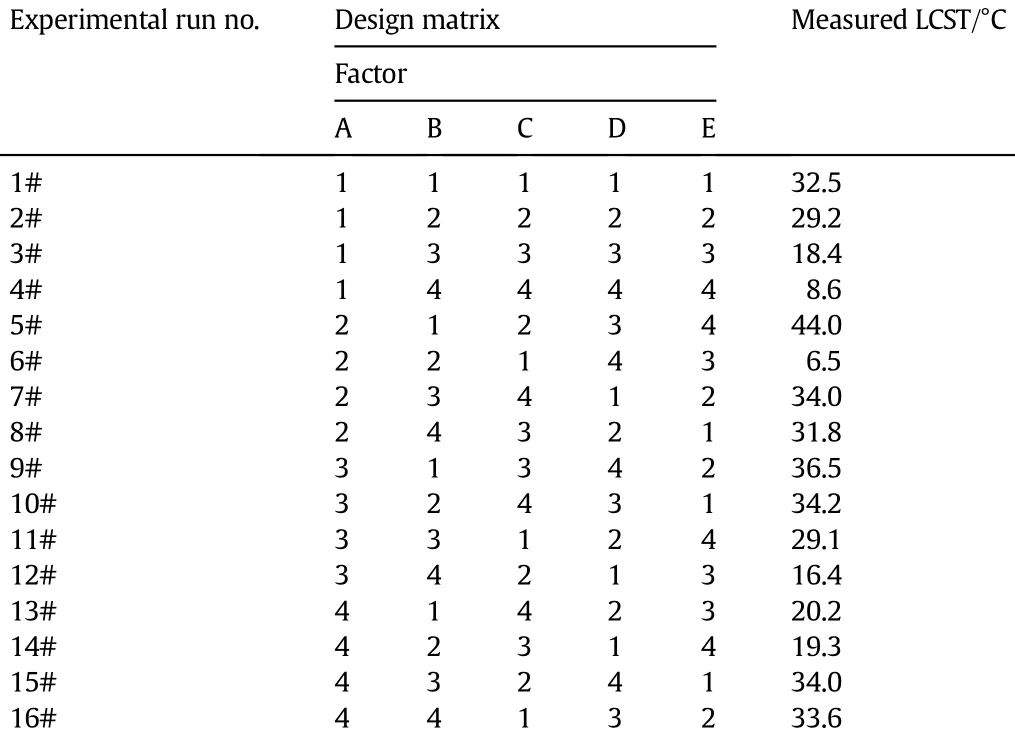
Table 2Orthogonal design matrix&measured LCST

Fig.3.The change of average LCST value of P(NIPAM-co-BCAm)copolymers with increasing the alcohol concentration.

Fig.2.Temperature-dependent transmittance change of P(NIPAM-co-BCAm)copolymer solutions in the orthogonal experiment.
Fig.3 shows the effect of alcohol concentration on the phase transition of P(NIPAM-co-BCAm)copolymers.The average LCST value of the copolymer decreases signif i cantly when the volume fraction of alcohol increases from 0 to 0.2.This phenomenon is due to the competition between the copolymer chain and alcohol molecules to form hydrogenbonds with water molecules.The amide groups of the P(NIPAM-co-BCAm)copolymer can form hydrogen-bonds with water molecules in pure water and maintain a hydration shell around the copolymer chain, which results in a coil state of the P(NIPAM-co-BCAm)copolymer.On the other hand,the alcohol molecules can also form hydrogen-bonds with water molecules in an alcohol-water mixture[32,33].Therefore, when the P(NIPAM-co-BCAm)copolymer is dissolved in an alcoholwater mixture with low alcohol concentration,a part of the hydrationshellaroundthecopolymerchainisdestroyedduetothecompetitionbetween the copolymer and alcohol molecules.Then,the damage quickly propagates all throughout the entire hydration structure,leading to a collapse of the dehydrated copolymer chain structure.Therefore,with the increasing alcohol concentration,the LCST value of the P(NIPAM-co-BCAm)copolymer shifts to a lower value.
Fig.4showstheeffectofalcoholspeciesontheaverageLCSTvalueof the P(NIPAM-co-BCAm)copolymer.The average LCST value is the smallest in n-PrOH solution and is the largest in EtOH solution.There are two competitive actions to inf l uence the average LCST value.One is the effect of hydrogen-bond donating capacities of alcohols,and another is the effect of hydrated clathrate structure formed between alcohol molecules and water molecules in alcohol-water mixtures. The hydrogen-bonds between alcohol molecules and the copolymer chain can prevent the copolymer from collapsing.However,the alcohol molecule could remove water molecules from the hydrated copolymer chains to form hydrated clathrate structure,which promotes the dehydration and collapse of thecopolymer chain.Firstly,thehydrogen-bond donatingcapacities ofaliphatic alcohols areof thesame orderof magnitude,and the hydrogen-bond accepting capacities of these alcohol molecules are similar[34].Moreover,t-BuOH and i-PrOH are much weaker hydrogen bond donors than n-alcohol.Secondly,the number of water molecules required to form a stable hydrated clathrate structurearoundanalcoholmoleculeisrelatedtothenumberof carbon atoms in the alcohol molecule[35]and the length of the main chain of the alcohol molecule[21].The alcohol molecule with more carbon atoms and a longer main chain could remove more water molecules from the hydrated copolymer chains,which promotes the dehydration and collapse of the copolymer chain.
Fig.5 shows the effect of metal ion species on the phase transitions of the P(NIPAM-co-BCAm)copolymer.The average LCST value in Ba2+solution is the largest and the second is in K+solution,but there are nearly no differences in Na+and Ca2+solutions.These phenomena are caused by the formation of“host-guest”complexes of BCAm units with specif i c metal ions.In aqueous solution,BCAm units and water molecules competitively bind with metal ions.Although the ion hydration capacity would enhance with decreasing the ion radius and/or increasing the ion charge,the desolvation energy of the metal ion has to be compensated by the complexation of crown ether with ion[36-38]. The order of the complex stability constant,lgK,of 18-crown-6 with metal ions is Ba2+>K+>Na+>Ca2+[39].So,the BCAm units exhibit higher ion selectivities towards Ba2+and K+over Na+and Ca2+,which shows a good correlation with the LCST shift in different ion solutions. WhentheP(NIPAM-co-BCAm)copolymerisdissolvedintoBa2+solution, the side chains of the copolymer bearing stable pendent BCAm/Ba2+complexes would be charged.The electrostatic repulsion among the charged BCAm/Ba2+groups would make the side chains repulse each other,which would counteract the shrinkage and aggregation of P(NIPAM-co-BCAm)chains induced by the increase of temperature.In addition,the charged BCAm/Ba2+groups would cause the increase of osmotic pressure within the copolymer chains.In order to achieve the osmotic pressure equilibrium,water molecules must diffuse into the copolymer[40,41],which also makes the copolymer stretch more.As a result,the LCST shift in Ba2+solution is the largest.
Fig.6 shows the effect of BCAm content in the copolymer on the average LCST value of the P(NIPAM-co-BCAm)copolymer.The average LCST value increases with the increasing molar ratio of BCAm units in the copolymer at f i rst,and then levels off when the BCAm content continues to increase.As the BCAm content increases,more BCAm/Mn+complexes would form and the electrostatic repulsion would increase, therefore the LCST value of the P(NIPAM-co-BCAm)copolymer increases.When the BCAm content is high enough,the amount of BCAm/Mn+complexes would be saturated;therefore,the LCST value would not change anymore.

Fig.4.The average LCST values of P(NIPAM-co-BCAm)copolymers in different solutions with various alcohol species.

Fig.5.The average LCST values of P(NIPAM-co-BCAm)copolymers in different solutions with various metal ion species.

Fig.6.ThechangeofaverageLCSTvaluesofP(NIPAM-co-BCAm)copolymerswithincreasing the BCAm content.
3.3.Effect of metal ion concentration on the LCST
The inf l uence degree of metal ion concentration on the LCST change of the P(NIPAM-co-BCAm)copolymer in the orthogonal experiments is the smallest as mentioned above.That is to say,the effect of metal ion concentration on the phase transition behavior of the copolymer in the orthogonal experiments is the most insignif i cant.To get more accurate results,theeffectof metalionconcentrationon theLCST shiftof the P(NIPAM-co-BCAm)copolymer is studied specially.A P(NIPAM-co-BCAm)copolymer with actual BCAm content of 18.5%(by mol)is synthesized to experimentally investigate the effect of Ba2+concentration on the LCST shift of the copolymer.Fig.7 shows the temperaturedependent transmittance changes of the copolymer in Ba2+solutions. Obviously,the LCST shifts to a higher value as the Ba2+concentration increases,and the LCST increases quickly when the Ba2+concentration is below 50 mmol·L−1.The electrostatic repulsion among the charged BCAm/Ba2+complexes would be stronger if the solution could provide more Ba2+to form BCAm/Ba2+complexes.Whentheconcentration increases more than 50 mmol·L−1,there are excessive Ba2+for BCAm units,so the LCST does not shift too much anymore.At the same time, asthesaltconcentrationincreases,thesalting-outeffectbecomescorrespondingly stronger[31],which also hinders theLCST from shiftingto a higher value.
Fig.8 shows theLCST changesof theP(NIPAM-co-BCAm)copolymer in different metal ion solutions,in which the data in Ba2+solutions are plotted from Fig.7 and those in K+and Na+solutions are cited from Ref.[25].Interestingly,there exists a good linear relation between the LCSTand lg(Cion)for these three kinds ofmetalions.More interestingly, there exists a good linear relation between the slopes and intercepts of these lines withthevalueof lgK,asshowninFig.9.From Figs.8 to 9,the LCST of the P(NIPAM-co-BCAm)copolymer can be calculated by using a binary function of ion concentration and lgK as follows:

Fig.7.Temperature-dependenttransmittancechangeofthesolutionsofP(NIPAM-co-BCAm) copolymer containing 18.5%(by mol)BCAm with increasing the Ba2+concentration.
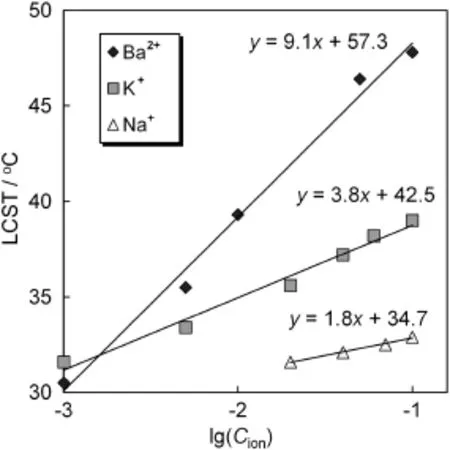
Fig.8.The LCST change of P(NIPAM-co-BCAm)copolymer in different metal ion solutions with different ion concentrations.
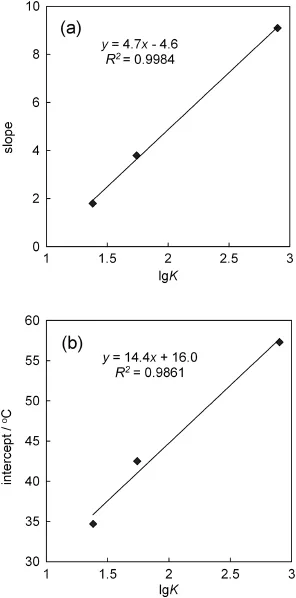
Fig.9.The slopes(a)and intercepts(b)of thelines inFig.8 asthefunction of the complex stability constant lgK.
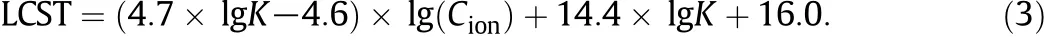
Eq.(3)indicates that,if the ion species(lgK is related to the ion species)and the ion concentration are known,the LCST of the P(NIPAM-co-BCAm)copolymer can be predicted.Fig.10 illustrates the prediction of LCST values of the P(NIPAM-co-BCAm)copolymer in different metal ion conditions,and the average relative error is 2.7% for all experimental data points.Obviously,the experimental data f i t in well with the calculated data from Eq.(3).
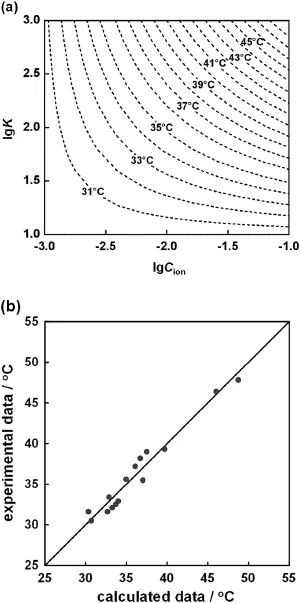
Fig.10.(a)Prediction of LCST values of P(NIPAM-co-BCAm)copolymer in different metal ion conditions,inwhich the dotted lines are calculatedfrom Eq.(3)and(b)parity plot for the calculated and experimental LCST data.
4.Conclusions
In this study,the comprehensive alcohol-/ion-responsive properties of theP(NIPAM-co-BCAm)copolymerhave been systematically investigated using the orthogonal design method.The comprehensive and cooperative effects of alcohol species,alcohol concentration,metal ion species,ion concentration and BCAm content in the copolymer on the phase transition behaviors of the P(NIPAM-co-BCAm)copolymer are presented.It is found that alcohols make the LCST of the P(NIPAM-co-BCAm)copolymershifttoalowervalue,whilespecif i cmetalions(especiallyBa2+)bringtheoppositeeffect.Suchoppositeresponseproperties of the P(NIPAM-co-BCAm)copolymer to alcohol and Ba2+are quite similar to the action behaviors of GIRK channels.According to the inf l uence on the LCST change,the inf l uence of variables is in the following order:alcohol concentration>alcohol species>metal ion species>BCAm content>ion concentration.The larger the alcohol concentration or the larger the molecular size of alcohol molecules is, the lower the LCST shift.On the contrary,the more the BCAm content in the copolymer is,the higher the LCST shift.The larger the complex stability constant lgK of BCAm with metal ion or the larger the metal ion concentration is,the higher the LCST shift.For P(NIPAM-co-BCAm) copolymers with f i xed BCAm contents,the LCST values of the P(NIPAM-co-BCAm)copolymers in different metal ion solutions can be predicted by a binary function of the ion concentration and the complex stability constant(lgK)of BCAm with metal ion.The results in this study provide valuable information for achieving biomimetic GIRK channels with P(NIPAM-co-BCAm)copolymers as artif i cial gating materials.
[1]A.Sigel,H.Sigel,Metal Ions in Biological Systems,vol.39,Marcel Dekker,New York, 2002.
[2]T.Kobayashi,K.Ikeda,H.Kojima,H.Niki,R.Yano,T.Yoshioka,T.Kumanishi,Ethanol opens G-protein-activated inwardly rectifying K+channels,Nat.Neurosci.2(1999) 1091-1097.
[3]C.Lüscher,P.A.Slesinger,Emerging roles for G protein-gated inwardly rectifying potassium(GIRK)channels in health and disease,Nat.Rev.Neurosci.11(2010) 301-315.
[4]P.Aryal,H.Dvir,S.Choe,P.A.Slesinger,A discrete alcohol pocket involved in GIRK channel activation,Nat.Neurosci.12(2009)988-995.
[5]L.Y.Chu,J.H.Zhu,W.M.Chen,T.Niitsuma,T.Yamaguchi,S.Nakao,Effect of graft yield on the thermo-responsive permeability through porous membranes with plasmagrafted poly(N-isopropylacrylamide)gates,Chin.J.Chem.Eng.11(2003)269-275.
[6]X.Lin,X.J.Ju,R.Xie,M.Y.Jiang,J.Wei,L.Y.Chu,Halloysite nanotube composited thermo-responsive hydrogel system for controlled-release,Chin.J.Chem.Eng.21 (2013)991-998.
[7]L.Y.Chu,R.Xie,X.J.Ju,Stimuli-responsive membranes:smart tools for controllable mass-transfer and separation processes,Chin.J.Chem.Eng.19(2011)891-903.
[8]W.Wang,L.Liu,X.J.Ju,D.Zerrouki,R.Xie,L.H.Yang,L.Y.Chu,A novel thermo-induced self-bursting microcapsule with magnetic-targeting property,ChemPhysChem 10 (2009)2405-2409.
[9]A.Xia,J.H.Hu,C.C.Wang,D.L.Jiang,Synthesis of magnetic microspheres with controllable structure via polymerization-triggered self-positioning of nanocrystals, Small 3(2007)1811-1817.
[10]L.Y.Chu,T.Yamaguchi,S.Nakao,A molecular recognition microcapsule for environmental stimuli-responsive controlled-release,Adv.Mater.14(2002)386-389.
[11]S.W.Pi,X.J.Ju,H.G.Wu,R.Xie,L.Y.Chu,Smart responsive microcapsules capable of recognizing heavy metal ions,J.Colloid Interface Sci.349(2010)512-518.
[12]X.J.Ju,L.Y.Chu,L.Liu,P.Mi,Y.M.Lee,A novel thermo-responsive hydrogel with ionrecognition property through supramolecular host-guest complexation,J.Phys. Chem.B 112(2008)1112-1118.
[13]X.J.Ju,L.Liu,R.Xie,C.H.Niu,L.Y.Chu,Dual thermo-responsive and ion-recognizable monodisperse microspheres,Polymer 50(2009)922-929.
[14]X.J.Ju,S.B.Zhang,M.Y.Zhou,R.Xie,L.Yang,L.Y.Chu,Novel heavy-metal adsorption material:ion-recognition P(NIPAM-co-BCAm)hydrogels for removal of lead(II) ions,J.Hazard.Mater.167(2009)114-118.
[15]T.Yamaguchi,T.Ito,T.Sato,T.Shinbo,S.Nakao,Development of a fast response molecular recognition ion gating membrane,J.Am.Chem.Soc.121(1999)4078-4079.
[16]T.Ito,T.Hioki,T.Yamaguchi,T.Shinbo,S.Nakao,S.Kimura,Development of a molecular recognition ion gating membrane and estimation of its pore size control,J. Am.Chem.Soc.124(2002)7840-7846.
[17]T.Ito,Y.Sato,T.Yamaguchi,S.Nakao,Response mechanism of a molecular recognition ion gating membrane,Macromolecules 37(2004)3407-3414.
[18]T.Ito,T.Yamaguchi,Osmotic pressure control in response to a specif i c ion signal at physiological temperature using a molecular recognition ion gating membrane,J. Am.Chem.Soc.126(2004)6202-6203.
[19]T.Ito,T.Yamaguchi,Controlled release of model drugs through a molecular recognition ion gating membrane in response to a specif i c ion signal,Langmuir 22(2006) 3945-3949.
[20]T.Ito,T.Yamaguchi,Nonlinear self-excited oscillation of a synthetic ion-channelinspired membrane,Angew.Chem.Int.Edit.45(2006)5630-5633.
[21]H.M.Crowther,B.Vincent,Swelling behavior of poly-N-isopropylacrylamide microgel particles in alcoholic solutions,Colloid Polym.Sci.276(1998)46-51.
[22]K.Mukae,M.Sakurai,S.Sawamura,Swelling of poly(N-isopropylacrylamide)gels in water-alcohol(C1-C4)mixed solvents,J.Phys.Chem.97(1993)737-741.
[23]F.M.Winnik,H.Ringsdorf,J.Venzmer,Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide),Macromolecules 23(1990)2415-2416.
[24]M.Heskins,J.E.Guillet,Solution properties of poly(N-isopropylacrylamide),J. Macromol.Sci.Chem.A2(1968)1441-1455.
[25]M.Irie,Y.Misumi,T.Tanaka,Stimuli-responsive polymers:chemical induced reversible phase separation of an aqueous solution of poly(N-isopropylacrylamide)with pendent crown ether groups,Polymer 34(1993)4531-4535.
[26]L.Y.Chu,W.M.Chen,X.Z.Lee,Effect of structural modif i cation on hydrocyclone performance,Sep.Purif.Technol.21(2000)71-86.
[27]C.B.C.Raj,H.L.Quen,Advanced oxidation processes for waste water treatment: optimization of UV/H2O2process through a statistical technique,Chem.Eng.Sci.60 (2005)5308-5311.
[28]D.P.Zhang,Y.L.Luo,Applied Probability Statistics,Higher Education Press,Beijing, 2000.(in Chinese).
[29]R.Ungrao,B.El-Haj,J.Smid,Substituent effects on the stability of cation complexes of 4′-substituted monobenzo crown ethers,J.Am.Chem.Soc.98(1976)5198-5202.
[30]K.Yagi,J.A.Ruiz,M.C.Sanchez,Cation binding properties of polymethacrylamide derivatives of crown ethers,Makromol.Chem.Rapid Commun.1(1988)263-268.
[31]H.Inomata,S.Goto,S.Otake,S.Saito,Effect of additives on phase transition of N-isopropylacrylamide gels,Langmuir 8(1992)687-690.
[32]G.Onori,Adiabatic compressibility and structure of aqueous solutions of ethyl alcohol,J.Chem.Phys.89(1988)4325-4332.
[33]A.K.Soper,J.L.Finney,Hydration of methanol in aqueous solution,Phys.Rev.Lett.71 (1993)4346-4349.
[34]M.K.Chantooni,I.M.Kolthoff,Resolution of acid strength in tert-butyl alcohol and isopropyl alcohol of substituted benzoic acids,phenols,and aliphatic carboxylic acids,Anal.Chem.51(1979)133-140.
[35]G.Onori,Structural properties of aqueous mixtures of monohydric alcohols from near-infrared absorption spectra,Chem.Phys.Lett.154(1989)213-216.
[36]L.X.Dang,Mechanism and thermodynamics of ion selectivity in aqueous solutions of 18-crown-6 ether:a molecular dynamics study,J.Am.Chem.Soc.117(1995) 6954-6960.
[37]X.J.Guo,Y.D.Zhu,M.J.Wei,X.M.Wu,L.H.Lu,X.H.Lu,Theoretical study of hydration effects on the selectivity of 18-crown-6 between K+and Na+,Chin.J.Chem.Eng.19 (2011)212-216.
[38]P.M.Wang,R.M.Izatt,S.E.Gillespie,J.L.Oscarson,X.X.Zhang,C.Wang,J.D.Lamb, Thermodynamics of the interaction of 18-crown-6 with K+,Ti+,Ba2+,Sr2+and Pb2+from 323.15 to 398.15 K,J.Chem.Soc.Faraday Trans.91(1995)4207-4213.
[39]R.M.Izatt,K.Pawlak,J.S.Bradshaw,R.L.Bruening,Thermodynamic and kinetic data for macrocycle interactions with cations and anions,Chem.Rev.91(1991) 1721-2085.
[40]S.Hirotsu,Y.Hirokawa,T.Tanaka,Volume-phase transitions of ionized N-isopropylacrylamide gels,J.Chem.Phys.87(1987)1392-1395.
[41]P.W.Zhu,D.H.Napper,Coil-to-globule type transitions and swelling of poly(N-isopropylacrylamide)and poly(acrylamide)at latex interfaces in alcohol-water mixtures,J.Colloid Interface Sci.177(1996)343-352.
12 November 2012
☆Supported by the National Natural Science Foundation of China(21136006),the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201163),and the National High Technology Research and Development Program (2012AA021403).
*Corresponding author.
E-mail address:chuly@scu.edu.cn(L.Chu).
http://dx.doi.org/10.1016/j.cjche.2014.06.021
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 24 March 2013
Accepted 24 April 2013
Available online 30 June 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- Oxygen Gasif i cation of Municipal Solid Waste in a Fixed-bed Gasif i er☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- Sn-1,3-specif i c Interesterif i cation of Soybean Oil with Medium-chainTriacylglycerol Catalyzed by Lipozyme TL IM☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
