Sn-1,3-specif i c Interesterif i cation of Soybean Oil with Medium-chainTriacylglycerol Catalyzed by Lipozyme TL IM☆
2014-07-25HongliYangYingMuHongtaoChenChunyangSuTiankuiYang2ZhilongXiu
Hongli Yang*,Ying Mu*,Hongtao ChenChunyang SuTiankui Yang2,**,Zhilong Xiu**
Biotechnology and Bioengineering
Sn-1,3-specif i c Interesterif i cation of Soybean Oil with Medium-chain
T
riacylglycerol Catalyzed by Lipozyme TL IM☆
Hongli Yang1,*,Ying Mu1,*,Hongtao Chen1,Chunyang Su1,Tiankui Yang1,2,**,Zhilong Xiu1,**
1School of Life Science and Biotechnology,Dalian University of Technology,Dalian 116024,China2Wilmar(Shanghai)Biotechnology Research&Development Center Co.,Ltd.,Shanghai 200137,China
A R T I C L EI N F O
Article history:
Sn-1,3-specif i city
Interesterif i cation
Thermomyces lanuginose lipase
Medium-chain triacylglycerol
Structured lipids
The structured lipids are produced through sn-1,3-specif i c interesterif i cation of soybean oil with medium-chain triacylglycerol(MCT)in continuous reactions catalyzed by Thermomyces lanuginose lipase(Lipozyme TL IM). Cheap Lipozyme TL IM presents similar interesterif i cation degree(ID),sn-1,3-specif i city and residual activity asexpensiveRhizomucormieheiinbatchreactions.Inpacked-bedinteresterif i cationofsoybeanoilwith MCTcatalyzedbyLipozymeTLIM,theresidencetimehasasignif i canteffectonID,whiletemperaturehasasmalleffectat 45-70°C.The sn-1,3-specif i city of Lipozyme TL IM is not satisfactory when reaction temperature is higher than 60°C.The optimal residence time and temperature are 30-40 min and 55°C,respectively.Among the solvents, includingacetone,isopropanol,tert-butanol,and isobutanol,usedto recovertheactivity oftheusedLipozymeTL IM,acetone is the most suitable one than the other solvents.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Structured lipids(SLs)are generally triacylglycerols(TAGs)or phospholipidsthathavebeenmodif i edtochangetheirfattyacid(FA)composition and/or their positional distribution on the glycerol backbone by chemical and/or enzymatic process[1,2].For TAGs with MLM structures, longchainfattyacids(LCFAs)and/orpolyunsaturatedfattyacidsarepredominantly incorporated in position sn-2 and medium-chain fatty acids (MCFAs)in positions sn-1 and sn-3.MLM-type SLs are widely used in medical industries,since MCFA in positions sn-1 and sn-3 can be easily bioavailable and the formed 2-monoacylglycerol(MAG)is readily absorbed[3].
Long-chain TAGs(LCTs)(e.g.soybean oil and saff l ower oil)can provide energy and serve as a source of essential fatty acids when it is used inmakingfatemulsionfortotalparenteralnutritionandenteraladministration[4].However,LCFAsaremetabolizedslowlyinthebody.Mediumchain TAGs(MCT)are readily metabolized for quick energy compared to LCTs,but it lacks essential fatty acids and large doses of MCT will lead to the accumulation of ketone bodies,which is bad to the body.
Therefore,TAGs with both MCFAs and LCFAs are good for human body.Although simple physical mixtures of LCT and MCT have been administered to patients,a physical mixture is not equivalent to a SL since the physical mixture is metabolized in the old rules.Interesterif i cation of LCT and MCT is a way to overcome the problem.Lacking selectivity, the chemical interesterif i cation yields randomly distributed LCFAs and MCFAsinallthreepositionsofglycerolbackbone[5].Enzymaticinteresterif i cation would be a preferable alternative for SL production due to the advantageous of selectivity,mild reaction conditions,less byproducts and easy recovery of catalysts[6-9].
However,the enzyme-catalyzed process is expensive for industrial production.One way to lower the production cost is using lipase with relativelylowprice.ThesilicagranulatedThermomyceslanuginoselipase (Lipozyme TL IM,Novozymes A/S)is known for its low price and is commonly applied in interesterif i cation[10,11].However,in the transesterif i cation reaction between glyceride and acyl donor catalyzed byLipozymeTLIM,theacylmigrationofacylgroupfrompositionsn-1,3 to position sn-2 is usually observed,which means that sn-1,3-specif i ty of Lipozyme TL IM is not good in that system[12].A preliminary experimentshowedthatthesn-1,3-specif i tyofLipozymeTLIMisdifferent in different reaction systems.In order to obtain MLM-type SLs with high yield in interesterif i cation of LCT and MCT,the sn-1,3-specif i ty of Lipozyme TL IMshould beinvestigated.Inaddition,for the interesterif ication of TAG with another TAG,there is no report on the sn-1,3-specif i city of Lipozyme TL IM,since it is not easy to determine the changeofsn-2positionalfattyacidsbetweenTAGs.Inthisstudy,asimple method is used to evaluate sn-1,3-specif i ty of Lipozyme TL IM.
On the other hand,although the operational costs could be lowered if the biocatalyst is immobilized in a stable state and theprocess is optimized[13],the immobilized enzyme is still liable to be deactivated in the reaction process.The stability of enzyme is a key for practical applications.Some efforts have been made to overcome this problemandimprovethelipaseactivity[14].Inthisstudy,washingbysolventsis usedtoregenerateusedLipozymeTLIMtoreducetheoperational costs.
In the present study,we test the sn-1,3-specif i ty,reaction time and lipase stability of cheap Lipozyme TL IM by comparing with that of Rhizomucor miehei(Lipozyme RM IM),which has strict sn-1,3-specif i ty [15-17].In TAG-TAG reaction system,the effect of f l ow rate and temperature on its sn-1,3-specif i ty is investigated.MLM-type SLs are produced by interesterif i cation of soybean oil with MCT in solventfree media in a continuous packed-bed reactor,with Lipozyme TL IM as catalyst.The effects of f l ow rate,temperature,and lipase stability on the interesterif i cation degree are also studied.The used Lipozyme TL IM is regenerated by solvents acetone,isopropanol,tert-butanol,and isobutanol.
2.Materials and Methods

Fig.1.HPLC chromatograms before(A)and after(B)enzymatic interesterif i cation in a batch reactor[enzyme load:15%(based on substrate mass);incubation:temperature at 50°C in an orbital shaking water at 200 r·min−1].
2.1.Materials
Soybean oil was purchased from a local grocery store(Dalian, China).MCT containing 60%(by mol)caprylic acid(C8:0)and 40%(by mol)capric acid(C10:0),and fatty acid methyl ester(FAME)standards for gas chromatography(GC)analysis were donated by Wilmar Biotechnology Research&Development Center Co.,Ltd(Shanghai, China).The FA mole composition(%)of the soybean oil was:12.1 of palmitic acid(C16:0),4.4 of stearic acid(C18:0),25.4 of oleic acid (C18:1),51.8 of linoleic acid(C18:2),5.6 of linolenic acid(C18:3),0.4 of arachidonic acid(C20:0),and 0.3 of behenic acid(C22:0).The immobilized thermostable lipases from Lipozyme TL IM and Lipozyme RM IM were obtained from Novozymes(China).Other reagents were of analytical grades.
2.2.Batch reaction
About 20 g of the oil blend(soybean oil/MCT,mass ratio 50:50)in a 50-ml round bottomed f l ask,in solvent-free system,was used for lipase-catalyzed interesterif i cation.Initial lipase(15%based on total substrates mass)without additional water was used.
The reactionmixture was incubatedinanorbital shakingwater bath at stirring rate of 200 r·min−1at 50°C.Samples of 300 μl were withdrawn from the reaction system at 0.5,1,2,4,6,and 8 h.All samples were stored at−20°C prior to analysis.All reactions were performed in duplicate.
2.3.Packed bed reactor
The reactor is a jacketed glass column(1.15 cm i.d.×20 cm length) packed with certain quality of Lipozyme TL IM.The operation process and the set-up of the column are similar to those in previous reports [18,19].The substrate was soybean oil and MCT of 50:50(mass ratio). Samples were taken regularly during the reaction process to examine the stability of Lipozyme TL IM under certain conditions.
2.4.Analysis of TAG compositions
The TAG compositions were analyzed by reversed-phase highperformance liquid chromatography(RP-HPLC).The temperature of Kromasil ODS-2 column(250 mm×4.6 mm i.d.,particle size 5 μm) was set at 30°C.The mobile phase was a binary solvent system of acetonitrile and dichloromethane.A linear gradient of dichloromethane from 30%to 70%over 40 min was applied at a f l ow rate of 1 ml·min−1and reversed to the initial acetonitrile for equilibration of the system. The detector was ELSD 3300(Alltech,USA).And the nitrogen f l ow rate was 1.7 L·min−1.The lipid samples were dissolved in chloroform (1 mg·ml−1),and 10 μl was injected with double measurements.The TAGs were expressed in terms of the relative area percentages of the total TAGs after normalization.The peaks of TAGs were identif i ed by TAG standards and equivalent carbon number(ECN).After comparing the prof i les before and after reaction,the changes in the amount of ECN (24,26 and 28,see Fig.1)can be used to monitor the degree of reaction. The interesterif i cation degree(ID)of reaction is def i ned as

where PEand P0are the area percentage of ECN(24,26 and 28)of the blend before and after reaction,respectively.
2.5.Evaluation of acyl migration at position sn-2 of TAG
Insoybeanoil,palmitate(C16:0)ismainlyconf i nedtopositionssn-1 and sn-3,while linoleate(C18:2)concentrating at position sn-2 is generally greater than those at positions sn-1 and sn-3[20].There are no C16:0 and C18:2 in MCT.The change of C16:0 and C18:2 at position sn-2 of TAG can be used to evaluate the acyl migration(sn-1,3-specif i city)at position sn-2 of TAG in the interesterif i cation.
The fatty acid(FFA)compositions at position sn-2 of TAGs were determinedaccordingto theIUPACstandard method[21].Thescraped-off band containing sn-2 MAGs was used for the analysis of FFA compositions by GC.In the enzymatic interesterif i cation of soybean oil with MCT,the change(molar percentage)of C16:0 and C18:2 at position sn-2 was chosen as the representation of sn-1,3-specif i city in this study while the other peaks in GC were ignored.
2.6.Methylation and analysis
Fattyacidmethylesters(FAMEs)oftheisolatedTAGswereprepared through transesterif i cation catalyzed by NaOMe in methanol.2 ml of hexane and 3 ml of sodium methylate solution[30%(mass)in methanol]were added in the tube,and mixed by shaking for 5 min.Centrifugation was carried out at 3000 r·min−1for 2 min.The upper layer containing methyl esters was decanted to GC-vials for GC analysis.Anhydrous sodium sulfate was added to dry the mixture.
The FAME was analyzed by GC with a 2014 series chromatograph (Shimadzu,Japan),equipped with a f l ame-ionization detector and a fused-silica capillary column(30 m×0.25 mm i.d.,0.25 μm f i lmthickness;Institute of Chemical Physics,Dalian).The carrier gas was nitrogen with a f l ow rate of 30 ml·min−1.The injector was used in split mode with a ratio of 1:20.Oven temperature was programmed from 80°C(1 min)to 160°C at a rate of 40°C·min−1,increased to 210°C at a rate of 10°C·min−1and held for 10 min.The injector and detector temperatures were 260°C and 310°C,respectively.The FAMEs were identif i ed by comparison of their retention time with standards from Sigma Chemical(St.Louis,MO).Three measurements were conducted for all analysis.The averages were used for result evaluation.
2.7.Determination of diacylglycerol(DAG)content and free fatty acids The DAG content was determined by the method of Yang et al.[22]. The free fatty acids were determined with AOCS method Cd 3d-63 [23].
2.8.Regeneration of deactivated lipase
The deactivatedlipase wasregenerated withdifferentsolvents(acetone,isopropanol,tert-butanol or isobutanol)when its activity was lower than a set value,def i ned as 45%of the original ID.The regeneration was carried out as follows.The deactivated lipase was discharged fromthepackedbedreactorandsubmergedinasmalldosageofsolvent for 70 min.The volume in every measurement was about 10 ml of solvent for 1 g of enzyme.The solvent was removed by vacuum f i ltration and then immersion with 10 ml of soybean oil for 1 g of enzyme at 45 °C for 9.5 h.The enzyme was immersed in the solvent again for 20 min to remove the residual soybean oil and the solvent was removed by vacuum f i ltration.The recovered lipase was packed in the reactor again.

Fig.2.ComparisonofID(a)andsn-1,3-specif i city(b)withLipozymeRMIMandLipozyme TL IM in batch reactions(reaction conditions:see Fig.1).
3.Results and Discussion
3.1.Comparison of Lipozyme TL IM and Lipozyme RM IM in batch reaction
3.1.1.Effect of reaction time on interesterif i cation degree
For interesterif i cation with soybean oil and MCT using commercial immobilizedlipases,thehighcostoflipaseisarestrictivefactorforcommercial applications.In the market,Lipozyme RM IM is much more expensive than Lipozyme TL IM.In this study,the IDs of Lipozyme RM IM and Lipozyme TL IM with soybean oil and MCT in batch reactions are compared in Fig.2(a),showing that Lipozyme TL IM has a similar activity to Lipozyme RM IM under the same reaction condition,which is in agreement with previous report[24].
3.1.2.Sn-1,3-specif i city of Lipozyme TL IM
AlthoughLipozymeTLIMisspecif i cforpositionssn-1andsn-3ofSLs in some reactions,there is no report on the sn-1,3 positional specif i city of Lipozyme TL IM in the TAG-TAG exchange systems.In this study,the changeofC16:0andC18:2atpositionsn-2ofTAGisusedtocharacterize the sn-1,3-specif i city of Lipozyme TL IM.Fig.2b shows that the time courses of C16:0 and C18:2 at position sn-2 are different.As reaction time increases,the C16:0 at sn-2 position increases,while C18:2 decreases.The increment of C16:0 is 6.32%(the difference between the lowest value and the highest value)catalyzed by Lipozyme TL IM, which is higher than that by Lipozyme RM IM(2.70%).The decrement of C18:2 is 6.90%catalyzed by Lipozyme TL IM,which is also higher than that of Lipozyme RM IM(4.10%).Lipozyme RM IM is a lipase with a strict sn-1,3-specif i city in many reactions.The results indicate that the change of C16:0 and C18:2 at position sn-2 of Lipozyme TL IM is more than that of Lipozyme RM IM.The sn-1,3 positional specif i city of Lipozyme TL IM is a little lower than that of Lipozyme RM IM.
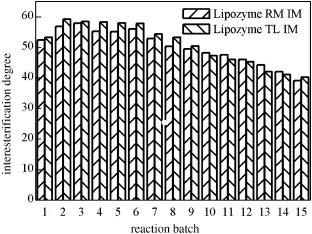
Fig.3.Comparison of stability of Lipozyme RM IM and Lipozyme TL IM in batch reactions (reaction conditions:see Fig.1).
3.1.3.Reaction batch on the residual activity
In order to make Lipozyme TL IM more suitable for commercial processes,the catalytic activity and operational stability,which is important in industrial applications,should be evaluated.After each batch, the lipase was f i ltered,recovered and the next batch was carried out with fresh substrate.The activity is assessed by ID.Fig.3 shows the stabilityofLipozymeTLIM compared with LipozymeRM IM for 15 batches undertheoptimalconditions,withonebatchfor12h.TheLipozymeRM IM reaches the maximum ID of 57.89%in batch 3 and Lipozyme TL IM reaches 59.18%in batch 2.Lipozyme TL IM has similar IDs to Lipozyme RM IM in the same reaction batch,while the price of former is only1/7 of that of later.Thus the cheaper Lipozyme TL IM can be used as biocatalyst for the interesterif i cation of soybean oil and MCT in commercial applications.

Fig.4.Effect of residence time on ID(a)and sn-1,3-specif i city(b)of Lipozyme TL IM in continuous reaction(reaction temperature:50°C).
3.2.Continuous reaction in packed bed reactor
3.2.1.Effect of residence time on ID and sn-1,3-specif i city
Fig.4(a)shows that theeffect of residence time on theIDfor continuousinteresterif i cationcatalyzedbyLipozymeTLIMinapackedbedreactor.As the residence time increases,the ID increases from 39.14%to 56.18%.Lower f l ow rate results in higher ID.For residence time more than 40 min,the improvement on ID is insignif i cant.Therefore,40 min was selected as reaction time for further experiments.The DAG and FFA contents in the same reaction are also shown in the f i gure, which decrease as the residence time increases.The results are not in agreement with those of Xu et al.[18].For the reason of the difference, further study is needed.
Fig.4(b)shows the effect of residence time on sn-1,3-specif i city of Lipozyme TL IM.As the residence time increases,the C16:0 at position sn-2 increases from 12.32%to 22.40%and C18:2 decreases from 60.07%to 42.62%.The change of C16:0 and C18:2 at sn-2 is little with the residence time between 20 and 40 min,while it is obvious for the residence time more than 40 min.This suggests that Lipozyme TL IM has good sn-1,3-specif i city in short reaction time(<40 min).Therefore, 30-40 min was selected for optimal time for further reaction in this study.

Fig.5.Effectof reaction temperature on ID(a)and sn-1,3-specif i city(b)with Lipozyme TL IM in a continuous reaction(f l ow rate:0.426 ml·min−1;residence time:33.42 min).
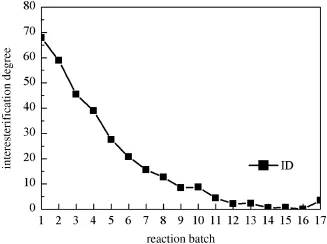
Fig.6.Operational stability of Lipozyme TL IM in a continuous reaction in packed bed reactor(f l ow rate,0.426 ml·min−1;residence time,33.42 min;reaction temperature, 55°C).
3.2.2.Effect of temperature on ID and sn-1,3-specif i city
Reaction temperature should be the primary consideration for continuous production of SLs[25].Fig.5(a)shows the effect of reaction temperature on the ID for continuous interesterif i cation catalyzed by Lipozyme TL IM in the packed bed reactor.In the temperature range of 45-70°C,the ID increases slowly and the effect of reactiontemperature is not signif i cant.The results are consistent with those of Xu et al.and the DAG and FFA contents of product at different temperatures are similar to previous results[26].
Higher temperature favors acyl migration[27].Fig.5(b)indicates the effect of temperature on sn-1,3-specif i city of Lipozyme TL IM.As the temperature increases,the change of C16:0 and C18:2 at sn-2 is not signif i cantly different from the original.For temperatures higher than 60°C,the change is obvious.This proves that sn-1,3-specif i city of Lipozyme TL IM could be achieved in the interesterif i cation of soybean oil with MCT at mildtemperatures.The suitable temperature of interesterif i cation should be determined by considering the reaction rate,acyl migration and economic cost.
3.2.3.Operational stability and lipase regeneration
Although enzymatic interesterif i cation is advantageous over chemical interesterif i cation,the lipase-catalyzed interesterif i cation reaction is not commonly used in the commercial production.The major problem is the cost of lipase.The operational stability of lipase in a packed bed is important for reducing the cost.The ID is used to assess of the lipase activity.Fig.6 shows the stability of Lipozyme TL IM in 12 batches,each for 12 h,under the optimal conditions. The ID decreases gradually and maintains at very low level,with ID close to 0 in the 12th cycles.The result is consistent with previous report[12].The reason may be that the silica granulated Lipozyme TL IM is not tightly adsorbed on the support and the lipase is off during the reaction.Du et al.also reported that the inactivation of enzyme is related to the immobilization material[28].And hydrophilic support produced poor adsorption of lipase and resulted in low interesterif ication rate[29].
For industrial applications,the regeneration of deactivated Lipozyme TL IM should be investigated.In this study,solvents with different values of lg P(logarithm of partition coeff i cient in octanol-water two-phase system)are used to wash the deactivated Lipozyme TL IM to recover the activity of lipase.lg P values of acetone,isopropanol,tert-butanol and isobutanol are−0.24,0.13,0.37 and 0.60,respectively.Fig.7 shows the treatment results with different solvents to regenerate the activity of Lipozyme TL IM after each batch of interesterif i cation in the packed bed reactor.In the f i rst cycle,the ID treated by tert-butanol is the highest,but it diminishes more drastically than that treated by acetone and isopropanol. With the treatment of acetone,higher ID is achieved in four treatments compared to the other solvents,probably because hydrophilic acetone could remove poisonous components(e.g.glycerol,DAG and others)absorbed on the hydrophilic support material of the enzyme to maintain the active sites of lipase.

Fig.7.Comparison of reutilization treatments of Lipozyme TL IM after reactions(reaction conditions:see Fig.6).
4.Conclusions
Lipozyme TL IM-catalyzed interesterif i cation of soybean oil with MCT is suitable to produce MLM-type SLs in solvent-free system.The satisfactory ID and sn-1,3-specif i city could be achieved under mild conditions with cheap Lipozyme TL IM.The treatment of deactivated lipase by acetone could signif i cantly improve the life time of Lipozyme TL IM and reduce the production cost in interesterif i cation.
[1]H.T.Osborn,C.C.Akoh,Structured lipids-novel fats with medical,nutraceutical,and food applications,Compr.Rev.Food Sci.F.3(2002)110-120.
[2]F.Hamam,Biologyand Biotechnology ofModif i edOils,(Ph.D.Thesis)2007.(Canada).
[3]B.Chen,H.Zhang,L.Cheong,T.Tan,X.Xu,Enzymatic production of ABA-type structured lipids containing omega-3 and medium-chain FAs:Effects of different acyl donors on the acyl migration rate,Food Bioprocess.Tech.5(2010)541-547.
[4]J.H.Lee,K.T.Lee,Structured lipids production,Handbook of Functional Lipids,CRC Press,United State,2006,pp.489-512.
[5]C.C.Akoh,B.D.Min,Structured lipids,Food Lipids,2nd edn,Marcel Dekker,New York,2002,pp.857-858.
[6]H.Zhang,X.Xu,H.Mu,J.Nilsson,J.Adler-Nissen,C.E.Høy,Lipozyme IM-catalyzed interesterif i cation for the production of margarine fats in a 1 kg scale stirred tank reactor,Eur.J.Lipid Sci.Tech.102(2000)411-418.
[7]L.Deng,X.Wang,F.Wang,J.Liu,P.Wang,T.Tan,Synthesis of wax eaters by lipasecatalyzed esterif i cation with immobilized lipase from Candida sp,Chin.J.Chem.Eng. 19(2011)978-982.
[8]C.Yin,C.Zhang,Ming Gao,Enzyme-catalyzed synthesis of vitamin E succinate using a chemically modif i ed Novozym-435,Chin.J.Chem.Eng.19(2011)135-139.
[9]Z.Li,L.Deng,J.Liu,X.Guo,Z.Yang,T.Tan,Enzymatic synthesis of fatty acid methyl esters from crude rice bran oil with immobilized Candida sp.99-125,Chin.J.Chem. Eng.18(2010)870-875.
[10]B.Mahiran,A.K.Mohd,M.Rosfarizan,B.A.Arbakariya,Optimization and kinetic study on the synthesis of palm oil ester using Lipozyme TL IM,J.Mol.Catal.B Enzym.85(2013)214-219.
[11]Y.Wang,H.Wu,M.H.Zong,Improvement of biodiesel production by lipozyme TL IM-catalyzed methanolysis using response surface methodology and acyl migration enhancer,Bioresour.Technol.99(2008)7232-7237.
[12]T.H.Rønne,T.Yang,H.Mu,C.Jacobsen,X.Xu,Enzymatic interesterif i cation of butterfat with rapeseed oil in a continuous packed bed reactor,J.Agric.Food Chem.53 (2005)5617-5624.
[13]E.Séverac,O.Galy,F.Turon,P.Monsan,A.Marty,Continuous lipase-catalyzed production of esters from crude high-oleic sunf l ower oil,Bioresour.Technol.102(2011) 4954-4961.
[14]J.W.Chen,W.T.Wu,Regeneration of immobilized Candida antarctica Lipase for transesterif i cation,J.Biosci.Bioeng.95(2003)466-469.
[15]L.Seong-Koon,A.Norlelawati,L.Z.Cheong,C.P.Tan,L.Kamariah,S.A.Y.Mohd,Q.M. Lai,Response surface modeling of 1-stearoyl-3(2)-oleoyl glycerol production in a pilot packed-bed immobilized Rhizomucor miehei lipase reactor,J.Mol.Catal.B Enzym.57(2009)136-144.
[16]C.R.Rafael,F.L.Roberto,Lipasefrom Rhizomucormiehei asanindustrial biocatalyst in chemical process,J.Mol.Catal.B.Enzym.64(2010)1-22.
[17]C.R.Rafael,F.L.Roberto,Lipase from Rhizomucor miehei as a biocatalyst in fats and oils modif i cation,J.Mol.Catal.B.Enzym.66(2010)15-32.
[18]X.Xu,T.Porsgaard,H.Zhang,J.Adler-Nissen,C.E.Høy,Production of structured lipids in a packed-bed reactor with Thermomyces lanuginosa Lipase,J.Am.Oil Chem.Soc.79(2002)561-565.
[19]A.F.Vikbjerg,L.Peng,H.Mu,X.Xu,Continuous production of structured phospholipids in a packed bed reactor with lipase from Thermomyces lanuginose,J.Am.Oil Chem.Soc.82(2005)237-242.
[20]F.Shahidi,Edible oil and fat products,Edible Oils,Wiley Press,New York,2005.
[21]C.Paquot,A.Hautfenne,IUPAC Standard Methods for the Analysis of Oils,Fats,and Derivatives,7nd edn Blackwell Scientif i c Publications,London,1987.
[22]T.Yang,M.B.Fruekilde,X.Xu,Applications of immobilized Thermomyces lanuginosa lipase in interesterif i cation,J.Am.Oil Chem.Soc.80(2003)881-887.
[23]AOCS,Off i cial methods and recommended practices of the American oil chemists' society,Methods Cd 3d-63,AOCS,Champaign,1999.
[24]H.Zhang,X.Xu,J.Nilsson,H.Mu,J.Adler-Nissen,C.E.Høy,Production of margarine fats by enzymatic interesterif i cation with silica-granulated Thermomyces lanuginose lipase in a large-scale study,J.Am.Oil Chem.Soc.78(2001)57-64.
[25]T.Yang,X.Xu,C.He,L.Li,Lipase-catalyzed modif i cation of lard to produce human milk fat substitutes,Food Chem.80(2003)473-481.
[26]X.Xu,Enzymaticproductionof structuredlipids:processreactionsandacylmigration, INFORM(InternationalNewsonFats,OilsandRelatedMaterials),112000.1121-1129.
[27]M.Fernandes,N.Krieger,A.Baron,A.Zamora,L.Ramos,D.Mitchell,Hydrolysis and synthesis reactions catalysed by Thermomyces lanuginose lipase in the AOT/Isooctane reversed micellar system,J.Mol.Catal.B Enzym.30(2004)43-49.
[28]W.Du,Y.Y.Xu,D.H.Liu,Z.B.Li,Study on acyl migration in immobilized Lipozyme TL-catalyzed transesterif i cation of soybean oil for biodiesel production,J.Mol. Catal.B Enzym 37(2005)68-71.
[29]S.Halim,A.Kamaruddin,Catalytic studies of lipase on FAME production from waste cooking palm oil in a tert-butanol system,Process Biochem.43(2008)1436-1439.
28 December 2012
☆Supported by the National High Technology Research and Development Program of China(2010AA101505),andtheNationalNaturalScienceFoundationofChina(21106013). *These authors equally contributed to this work.
**Corresponding authors.
E-mailaddresses:yangtiankui@wilmar-intl.com(T.Yang),zhlxiu@dlut.edu.cn(Z.Xiu).
http://dx.doi.org/10.1016/j.cjche.2014.06.027
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 25 April 2013
Accepted 19 June 2013
Available online 1 July 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- Oxygen Gasif i cation of Municipal Solid Waste in a Fixed-bed Gasif i er☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
