Oxygen Gasif i cation of Municipal Solid Waste in a Fixed-bed Gasif i er☆
2014-07-25MiaomiaoNiuYajiHuangBaoshengJinXinyeWang
Miaomiao Niu,Yaji Huang*,Baosheng Jin,Xinye Wang
Energy,Resources and Environmental Technology
Oxygen Gasif i cation of Municipal Solid Waste in a Fixed-bed Gasif i er☆
Miaomiao Niu,Yaji Huang*,Baosheng Jin,Xinye Wang
Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education,School of Energy and Environment,Southeast University,Nanjing 210096,China
A R T I C L EI N F O
Article history:
Municipal solid waste
Oxygen gasif i cation
Temperature
Equivalence ratio
Gas f l ow rate
Fourwastematerials,paper,wood,textile andkitchengarbage,inmunicipalsolidwasteweregasif i edseparately with oxygen in a f i xed bed reactor.The yields of products char,tar and gas,the composition of gas components H2,CO,CO2andCH4,andthelowerheatingvalue(LHV)wereexaminedattemperaturesbetween700and900°C andequivalenceratio(ER)between0.14and0.32.Characteristicsofgasevolutionduringgasif i cationwereinvestigated.Results show that a higher temperature improves the formation of H2and CO while lowers the yield of CO2and CH4.The LHV of syngas increases with temperature and varies in the range of 6-10 MJ·m−3,reaching the maximum at 800°C or above.As ER increases,both combustible gas component and LHV of syngas decrease whiletheyield of CO2riseslinearly.TheappropriateER for obtaininghighquality gasisintherangeof 0.18-0.23. TemperatureandERhavesignif i canteffectsontheproductdistribution.HighertemperatureandERarefavorable forhighergasyieldandloweryieldofcharandtarinthegasif i cationoftextileandkitchengarbage.At800°C,the gas evolution may be divided into two regions.In the f i rst region,the f l ow rate of gas increases and then decreases rapidly,while in the second region the f l ow rate decreases monotonically to lower level.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Because of land wastage and seepage of toxic decomposition products out of landf i ll areas,landf i ll disposal of municipal solid waste (MSW)is becoming environmentally less desirable[1-3].Conventional incineration has problems of dioxinsandf i naldisposalof burnt ash,and its energy utilization eff i ciency is low[4,5].Thus the gasif i cation that convertsMSWthroughpartialoxidationintoafuelgaswithappropriate heating value is becoming one of the best alternatives for the reuse of MSW.Especially,the process can reduce the emission of dioxins and heavy metals effectively,which is strongly concerned by the public when MSW is combusted[6].Some researches on MSW gasif i cation have been performed during the past decade.Xiao et al.reported air gasif i cationcharacteristicsofwastewood,paperandplasticsusingaf l uidized bed gasif i er[7].Ahmed and Gupta investigated the characteristics of syngas from pyrolysis and gasif i cation of waste food and rubber [8].However,thegasproducedfromtheairgasif i cationishighlydiluted by nitrogen,loweringthe lower heatingvalue(LHV).The use of oxygen has been proven effective for reducing the dilution effect and achieving a medium heating value.Yoon et al.compared the gasif i cation results with air and oxygen separately for biodiesel by-product and found that the LHV of syngas and carbon conversion with oxygen was higher [9].Therefore,MSW gasif i cation with oxygen may be a promising technique to produce fuel gas of high quality.
So far,little work has been found using oxygen for gasif i cation.In this study,four representative and important materials in MSW, paper,wood,textile and kitchen garbage,are chosen as experimental samples to investigate the characteristics of syngas produced from oxygen gasif i cation in a f i xed bed.The effects of temperature and equivalence ratio(ER)on the product distribution and syngas evolution in the gasif i cation process are examined.
2.Experimental
2.1.Material
Four materials in MSW were involved in the experiments:paper, wood,textile and kitchen garbage,from waste typing paper,waste wood,waste cloth and waste family food,respectively.The materials were fi rstdried under thesunfor 2 days to reduce themoisture content and then shredded into particles in sizes of approximately 2 mm by a shredder.The analysis results are listed in Table 1.
2.2.Procedure
Gasi fi cation tests of MSWwere carried out atatmospheric pressurein a lab-scale fi xed bed reactor shown in Fig.1.The gasi fi cation system consistedofanoxygensupply,a fi xedbedreactor,acondensatecollecting fl ask,a gas cleaning section,and a gas analyzer system.The reactor with 60mmIDwasmadeofInconelandcouldbeexternallyheatedbyanelectrical ring furnace with total heating length of 1000 mm.A 60 mm OD stainless steel feeding tube containing testing sample was placed in the inlet of reactor before test.In each test the electrical heater was turnedon and the furnace was heated at a rate 10°C·min-1.After reaching the desired f i nal temperature,the feeding tube was pushed into the heating zone and an oxygen f l ow at an appropriate equivalence ratio was introduced as a gasifying agent.The operating conditions for all experiments were as shown in Table 2.The reaction proceeded for the next 25 min. Subsequently,theheatingandoxygensupplywerestoppedandthereactor was allowed to cool.Syngas exited from the reactor and passed throughthecondenserandscrubberinsequenceforcoolingandpurif i cation.Twowayswereusedforgassamplingandanalysis.Fortheinf l uence of temperature and ER,all the gas produced were f i rst collected by a gas sampling bag and then sent to an Emerson Gas Component Analyzer for component analysis and mass measurement.For the gasif i cation evolution,the clean gas was tested online to detect the component change with time.The amount of condensed tar in the glass condenser and the char left inside the reactor were measured by taking the difference in mass before and after the test.
3.Results and Discussion
The main reactions involved in the gasif i cation process are given below[10],inwhichthevalueofreactionheatreferstothetemperature 298.15 K.


Table 1Properties of materials in MSW(dry basis)
TheLHVofsyngasiscalculatedbyLHV(MJ·m−3)=(CO×126.36+ H2×107.98+CH4×358.18)/1000,where CO,H2,and CH4are their volume percentages.

Fig.1.Schematic illustration of the gasif i cation apparatus.

Table 2Summary of gasif i cation operating conditions
3.1.Effect of temperature
Temperature is crucial for the MSW gasif i cation process[11].In the present work,gasif i cation temperature is varied from 700 to 900°C in 50°C increments.The effect of temperature on gas composition is shown in Fig.2.The yield of H2of kitchen garbage increases monotonically from 10 to 20%with temperature,while that of other three materials show the same increase of about 5%from 700 to 800°C and the same decrease of about 5%from 800 to 900°C.For the four samples, the yields of CO2and CH4decline as temperature increases,while the yield of CO increases greatly.In the experimental range,the yield of CO2decreases sharply from 40%to 20%while that of CO increases from 20%to 40%approximately.On one hand,higher temperature accelerates the rate of devolatilization and cracking reactions of tar, producingmore H2and CO[12].Onthe other hand,higher temperature isconducivetomovetheequilibriumofBoudouardandwater-gasreactions to the right hand side,leading to a marked drop in CO2content [13].Besides,CO shift reaction and methanation reaction are exothermic and thus less dominate at higher temperature,increasing the production of CO to a certain extent.The yield of CH4is practically the same(5%-9%)for differentsamples and thevariation with temperature is not signif i cant.
In Fig.2,the main gas composition shows evident differences for different waste materials.The differences are large at high gasif i cation temperatures but reduce dramatically as temperature decreases.The yield of H2in gasif i cation of kitchen garbage is the highest,which could be attributed to its high moisture content. More steam is produced by evaporation and steam reforming reactions of carbon,carbon monoxide and methane are favored.Because of high content of volatiles and low f i xed carbon content, the heat and its transfer in gasif i cation of paper and textile are better and more light gases(mainly CO and CO2)are produced by cracking reactions[14].Due to high content of f i xed carbon in wood material,wood gasif i cation results in a relatively high yield of CO2[10].
The effect of temperature and material on LHV of the syngas is presented in Fig.3.The content of H2and CO with high LHV is larger,so higher gasif i cation temperature leads to higher LHV and improves the syngas quality effectively.In particular,with the continuous decrease of CH4yield,there is a slight drop in LHV for paper,wood and textilegasif i cation above 800°C,while the LHV of syngas produced by kitchen garbage gasif i cation keeps increasing because of the high H2content at high temperatures.The LHV of syngas from oxygengasif i cation of MSW varies in the range of 6-10 MJ·m-3,reaching the maximum at 800°C for paper,wood and textile and 900°C for kitchen garbage under the experimental condition.
The product distribution at different temperatures for gasif i cation of textile and kitchen garbage is shown in Fig.4.Higher temperature leads to higher gas yield and lower yields of char and tar,since more gas is produced in the initial devolatilization at higher temperatures,and the endothermic reactions of gasif i cation of char and the steam cracking andreformingoftarsincreasewithtemperature[15].Intheexperimentalrange,thegasyieldcouldbeenhancedby20%-30%withtheincrease of temperature.Comparing the two samples,the gas yield of textile is higher,which may be ascribed to easier broken molecular chain structure and higher volatile content.As for the kitchen garbage,relatively stable molecular chain structure and lower volatile content result in lower syngas yield[16].
3.2.Effect of ER
In accordance with previous gasif i cation study[10-15],the equivalenceratioisdef i nedasthefuel/oxygenratiodividedbythefuel/oxygen ratio under stoichiometric conditions with complete fuel combustion. Its calculation is as follows:

Fig.2.Effect of temperature on gas composition.
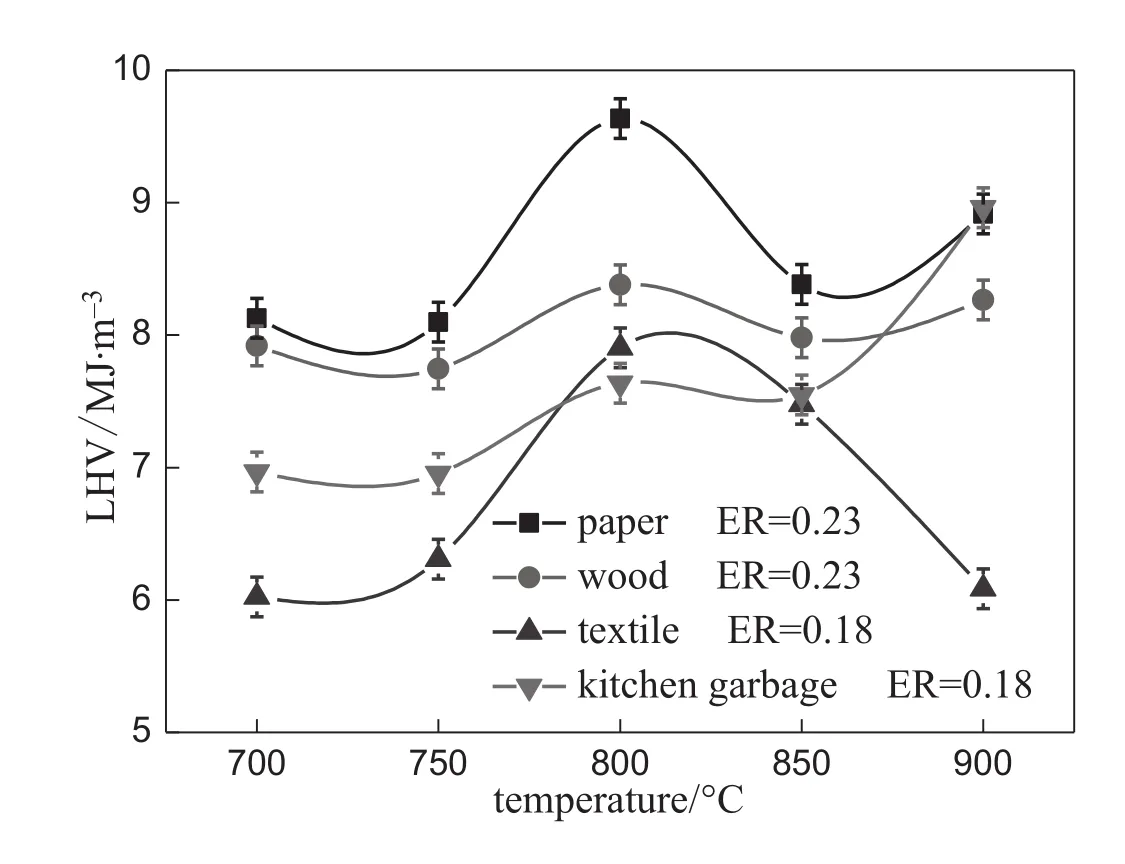
Fig.3.Effect of temperature on LHV.
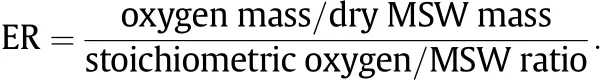
In the present work,ER is varied from 0.14 to 0.32 by changing the oxygen f l ow rate and maintaining other conditions constant.The inf l uence of ER on gas composition is reported in Fig.5.In general,the four waste materials behave similarly.Higher value of ER favors oxidation reactions and deteriorates the syngas quality.The yields of H2and CO decline dramatically as ER increases except that CO yield of wood presents a maximum.The increase of CO2yield from 30%to 50%with ER can be explained by the strengthened oxidation reactions.The yield of CH4keeps at low values and decreases slightly in the experimentalrange.The decrease of syngas LHV is expected due to the reduction of combustible gas components(H2,CO,CH4).A signif i cant decrease, about 4 MJ·m−3,in LHV is observed in Fig.6.The maximum value of LHV could be up to 8-10 MJ·m−3when ER is within 0.18-0.23.Fig.7 showstheeffectofERonproductdistributionoftextileandkitchengarbage gasif i cation.An increase of ER from 0.14 to 0.28 causes a decrease of10%-15%incharyield,reducesthetaryieldto20%orsoandincreases thegasyieldgreatly.ERaffectsthetextilegasif i cationmoresignif i cantlythan the kitchen garbage gasi fi cation due to the high volatile content of textile[16].
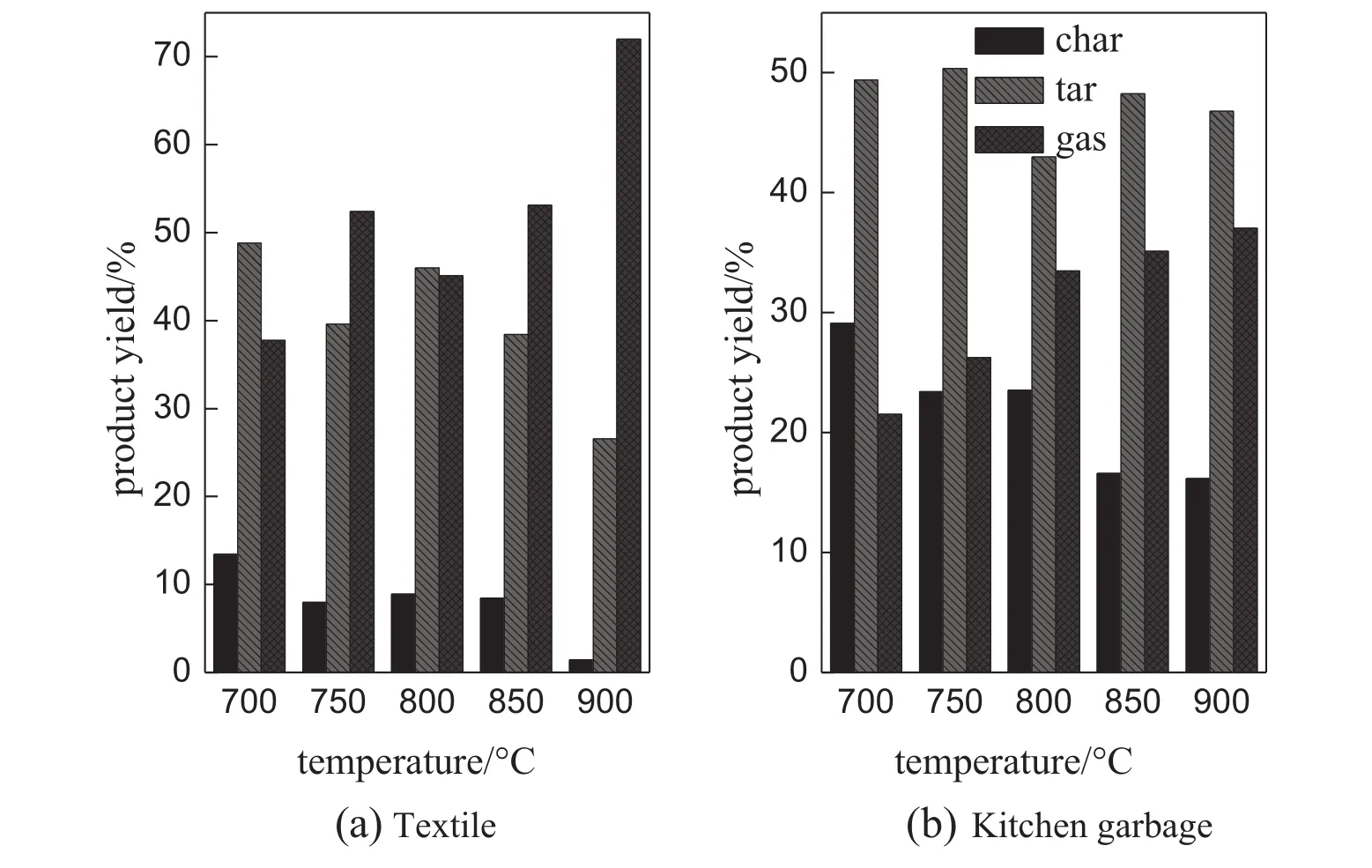
Fig.4.Effect of temperature on product yield.

Fig.5.Effect of ER on gas composition.
As shown in Fig.6,the appropriate ER for the oxygen gasi fi cation of single materials is in the range of 0.18-0.23,less than the optimum range for air gasi fi cation reported in literature.Fang et al.studied the fi xed bed air gasi fi cation characteristics of agricultural forestry wastes in Chinaandfoundthat thefavorableER scope for peanutshells andeucalyptus wood chips is 0.28-0.34[17].The effect of oxygen concentration on gas composition can be used for explanation.Compared with air gasi fi cation,oxygen gasi fi cation increases the oxygen concentration in gasifying agent so that the density of activated molecules in the reactor is enhanced.The effective collisions between reactant molecules increase.The heat and mass transfer in gasi fi cation reaction is improved and a higher heating rate is obtained,producing more light gases with less char and tar.
Fig.6.Effect of ER on LHV.
3.3.Evolution of product gas
Fig.8 shows the characteristic of syngas evaluated in terms of gas f l ow rate.The gas f l ow rate at 800°C can be divided into two regions.The initial region is characterized by a high f l ow rate of gas components(H2,CO,CO2and CH4)and a subsequent steep decrease,while the second region is a period of a monotonically decreasing gas f l ow rate.The f l ow rate change in the f i rst region could be attributed to the evolution of volatile matter from the MSW.In the second region,the char gasif i cation and reforming reactions may play a primary role in the gas evolution.Possible reasons for the slow reaction rate in the second region are as follows.First,the produced gas covers the interface between oxygen and char and hinders the char gasif i cation.Second,the ash in solid residue increased with time which makes the steam reforming reactions slower.The two regions are not completely independent since the cracking and char gasif i cation reactions also occur in the initial devolatilization[18].For example,Blasi et al.described the gasif i cation as two stages:solid pyrolysis and char conversion[19].Besides, it should be noticed that the f i rst region of kitchen waste gasif i cation lasts longer than that of other three waste materials,possibly due to the high water content of kitchen garbage.Evaporation and water-gas reaction prolong the duration of the f i rst region and increase the yield of H2[20].
4.Conclusions
Syngasfromoxygengasif i cationofwastesolidmaterialscan beused as an alternate gaseous fuel with a relatively high LHV.In this work,for thefour wastesolid materials,paper,wood,textile andkitchen garbage, ahighertemperatureismoreeffectivetoincreasetheyieldofH2andCO and lower the yield of CO2.The LHV of syngas from oxygen gasif i cation varies in the range of 6-10 MJ·m−3at 800°C-900°C.As the equivalence ratio increases,combustible gas components and LHV of syngas decrease,while the yield of CO2increases linearly.ER values in the range of 0.18 to 0.23 are appropriate to produce syngas with high LHV(8-10 MJ·m−3)at 800°C.Higher temperature and ER are favorable for higher gas yield and lower yields of char and tar in textile and kitchen garbage gasif i cation.The gas evolution of four samples at 800°C could be divided into two regions,with the f i rst region characterized by a steep increase and a steep decrease in the f l ow rate of gas and the second region characterized by a monotonically decreasing f l ow rate at a lower level.
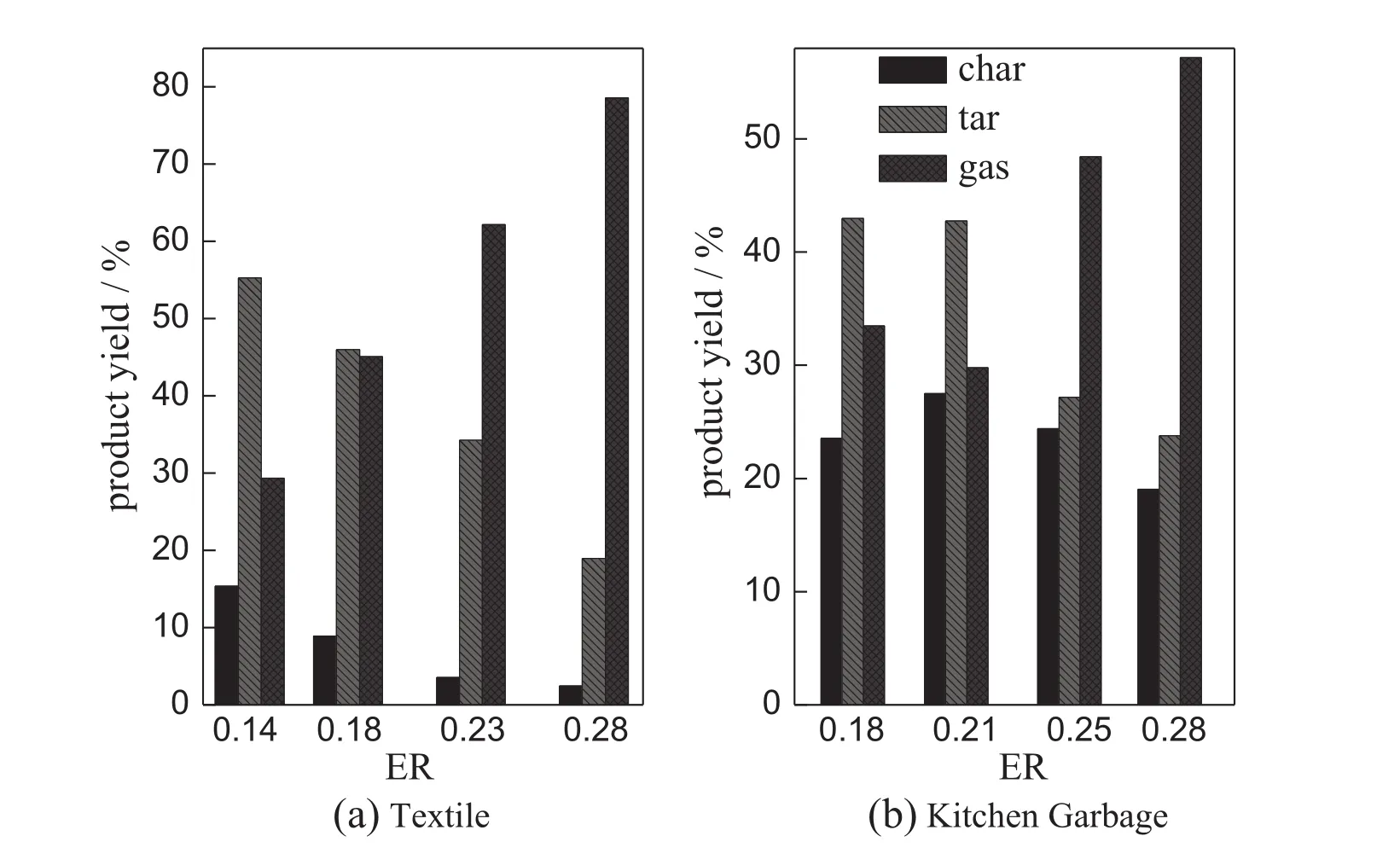
Fig.7.Effect of ER on yield of product.

Fig.8.Evolution of gas f l ow rate at 800°C.
[1]A.Umberto,Process and technological aspects of municipal solid waste gasif i cation. A review,Waste Manag.32(4)(2012)625-639.
[2]M.Thomas,Novel and innovative pyrolysis and gasif i cation technologies for energy eff i cient and environmentally sound MSW disposal,Waste Manag.24(1)(2004) 53-79.
[3]K.L.Lam,A.O.Oyedun,C.W.Hui,Experimental and modeling studies of biomass pyrolysis,Chin.J.Chem.Eng.20(3)(2012)543-550.
[4]I.Naruse,H.Yao,Behavior of lead compounds during municipal solid waste incineration,Proc.Combust.Inst.32(2009)2685-2691.
[5]X.X.Jiang,N.Ellis,Z.P.Zhong,Characterization of pyrolytic ligninextractedfrom biooil,Chin.J.Chem.Eng.18(6)(2010)1018-1022.
[6]G.Xiao,M.J.Ni,Y.Chi,B.S.Jin,R.Xiao,Z.P.Zhong,Y.J.Huang,Gasif i cation characteristics of MSW and an ANN prediction model,Waste Manag.29(1)(2009) 240-244.
[7]G.Xiao,B.S.Jin,Z.P.Zhong,Y.Chi,M.J.Ni,K.F.Cen,R.Xiao,Y.J.Huang,H.Huang, Experimental study on MSW gasif i cation and melting technology,J.Environ.Sci. (China)19(11)(2007)1398-1403.
[8]I.Ahmed,A.K.Gupta,Characteristic of hydrogen and syngas evolution from gasif i cation and pyrolysis of rubber,Int.J.Hydrogen Energy 36(7)(2011)4340-4347.
[9]S.J.Yoon,Y.C.Choi,Y.I.Son,S.H.Lee,J.G.Lee,Gasif i cation of biodiesel by-product with air or oxygen to make syngas,Bioresour.Technol.(4)(2010)1227-1232.
[10]F.Duan,B.S.Jin,Y.J.Huang,B.Li,Y.M.Wu,M.Y.Zhang,Results of bituminous coal gasif i cation upon exposure to a pressurized pilot-plant circulating f l uidized-bed (CFB)reactor,Energy Fuel 24(2010)3150-3158.
[11]Y.H.Zhao,H.Wen,Z.C.Guo,Z.H.Xu,Development of a fuel-f l exible co-gasif i cation technology,Chin.J.Chem.Eng.13(1)(2005)96-101.
[12]M.Y.He,Z.Q.Hu,B.Xiao,J.F.Li,X.J.Guo,S.Y.Luo,F.Yang,Y.Feng,G.J.Yang,S.M.Liu, Hydrogen-rich gas from catalytic steam gasif i cation of municipal solid waste (MSW):inf l uence of catalyst and temperature on yield and product composition, Int.J.Hydrogen Energy 34(1)(2009)195-203.
[13]L.H.Shen,Y.Gao,J.Xiao,Simulation of hydrogen production from biomass gasif i cation in interconnected f l uidized beds,Biomass Bioenergy 32(2)(2008)120-127.
[14]G.e.R.Jaffri,J.Y.Zhang,Investigation on steam gasif i cation of high-metamorphous anthracite using mixed black liquor and calcium catalyst,Chin.J.Chem.Eng.16(4) (2008)575-583.
[15]I.Narvaez,A.Orio,M.P.Aznar,J.Corella,Biomass gasif i cation with air in an atmospheric bubbling f l uidized bed.Effect of six operational variables on the quality of the produced raw gas,Ind.Eng.Chem.Res.35(7)(1996)2110-2120.
[16]S.Y.Luo,B.Xiao,Z.Q.Hu,S.M.Liu,Effect of particle size on pyrolysis of singlecomponent municipal solid waste in f i xed bed reactor,Int.J.Hydrogen Energy 35 (1)(2010)93-97.
[17]Y.Fang,L.Chen,H.Wang,Fixed-bed gasif i cation investigation of agricultural and forest residues in Southern China,Acta Energ.Sol.Sin.30(8)(2009)1118-1123 (in Chinese).
[18]R.Xiao,B.S.Jin,H.C.Zhou,Z.P.Zhong,M.Y.Zhang,Air gasif i cation of polypropylene plastic waste in f l uidized bed gasif i er,Energ.Convers.Manage.48(3)(2007)778-786.
[19]Di.C.Blasi,G.Signorelli,C.D.Russo,G.Rea,Product distribution from pyrolysis of wood and agricultural residues,Ind.Eng.Chem.Res.38(6)(1999)2216-2224.
[20]P.M.Lv,Z.H.Xiong,J.Chang,C.Z.Wu,Y.Chen,J.X.Zhu,An experimental study on biomass air-steam gasif i cation in a f l uidized bed,Bioresour.Technol.95(1)(2004) 95-101.
17 December 2012
☆Supported by the National Basic Research Program of China(2011CB201505)and the National Natural Science Foundation of China(51006023).
*Corresponding author.
E-mail address:heyyj@seu.edu.cn(Y.Huang).
http://dx.doi.org/10.1016/j.cjche.2014.06.026
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 3 February 2013
Accepted 19 March 2013
Available online 1 July 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
