In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
2014-07-25WenjingLinYouqiangYangRuihaoChenXiufangWenYuQianChengzhiCaiLijuanZhang
Wenjing Lin,Youqiang Yang,Ruihao Chen,Xiufang Wen,Yu Qian,Chengzhi Cai,Lijuan Zhang,*
Materials and Product Engineering
In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
Wenjing Lin1,Youqiang Yang1,Ruihao Chen1,Xiufang Wen1,Yu Qian1,Chengzhi Cai2,Lijuan Zhang1,*
1School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,China2Department of Chemistry&Center for Materials Chemistry,University of Houston,Houston,TX 77204-5003,USA
A R T I C L EI N F O
Article history:
In-situ IR monitoring
Amphiphilic copolymer
ARGET ATRP
Conversion rate
The amphiphilic copolymer poly(hydroxyethyl methacrylate-co-tert-butyl methacrylate)[P(HEMA-co-tBMA)] was synthesized by activators regenerated by electron transfer atom transfer radical polymerization(ARGET ATRP),with the synthesis process monitored by in-situ infrared spectroscopy(IR).The molecular weight,chemical structure and characteristics of the copolymer were determined by1H NMR,gas chromatography and gel permeation chromatography.The inf l uences of various parameters on the living polymerization were explored. Themolecularweightofthecopolymerwithnarrowmolecularweightdistribution(Mw/Mn<1.50)increasesapproximately linearly with the monomer conversion,indicating a good control of polymerization.In the reaction temperature range from 50°C to 90°C,the monomer conversion is higher at 60°C.The tBMA conversion rate decreases gradually with the increase of tBMA content,while the HEMA conversion is hardly affected by HEMA content.Weak polar solvent is more favorable to the polymerization compared to polar solvent.The molar ratio of reducing agent to catalyst has signif i cant effect on the polymerization and increasing the amount of reducing agent will accelerate the reaction rate but causes wider molecular weight distribution.It is indicated that in-situ IR monitoring contributes to a more in-depth understanding of the mechanism of methacrylate monomer copolymerization.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
Amphiphilic copolymers have unique structures with hydrophilic and hydrophobic chains,and can self-assemble to form core/shell polymeric micelles due to solubility difference of chain segments in selective solvent[1,2].The outer hydrophilic shell of micelles provides a stable interface between the hydrophobic core and aqueous medium,and the inner hydrophobic core of micelles enhances the loading eff i ciency of hydrophobic drugs[3].The polymeric micelles are excellent candidate for the administration of hydrophobic drugs and provide a controlled and targeted way to deliver the encapsulated hydrophobic drugs[4].
Activatorsregeneratedbyelectrontransferatomtransferradicalpolymerization(ARGET ATRP),which is a preferential technique from both industrial and environmental viewpoints,can lower the concentration of catalyst by using an excess amount of reducing agent to regenerate CuIfromCuIIand maintainingappropriate CuI/CuIIbalance[5-8].A varietyofmonomers,suchasmethylmethacrylate[9],styrene[10,11],butyl acrylate[12],and poly(hydroxyethyl methacrylate)[P(HEMA)][13], have been polymerized by ARGET ATRP.
P(HEMA)contains pendant hydroxyl functionalities that render it hydrophilic,and has wide applications in areas of hydrogels[14], contact lenses[15],and drug delivery systems[16].Poly(tert-butyl methacrylate)[P(tBMA)]can be converted to poly(methacrylic acid) (PMAA)by selective hydrolysis of the tert-butyl groups[17].PMAA is a pH-responsive biomaterial,which contains carboxyls that are easily ionized above its pKa of 4.5 and affords the polymer pH-tunability for the controlled-release applications.Therefore,P(HEMA-co-MAA) derived from P(HEMA-co-tBMA)may serve as a pH-responsive amphiphilic carrier for drug delivery.Traditional copolymerization methods for creating hydrophilic HEMA and hydrophobic tBMA micelle networks offer little control over co-monomer distribution,molecular weight,or molecularweightdistribution,whichinturnrestrictsthedesignandsynthesisofhydrophilic/hydrophobicmicellesystems.Inthepresentwork,a linear P(HEMA-co-tBMA)random copolymer is synthesized by ARGET ATRP.The proposed mechanism is shown in Fig.1,using CuBr2/PMDETA as the catalyst and ethyl 2-bromoisobutyrate(EBriB)as the initiator with Sn(Oct)2as reducing agent.Temperature,monomer ratio,as well as the catalyst system will affect the structure of P(HEMA-co-tBMA),thusaffecting its further applications.In our current work,we will explore how those factors affect the polymerization monitored by in-situ IR.
In recent years,the application of in-situ IR in monitoring reaction process has attracted extensive attention.For the polymerization, in-situ IR can monitor the change of monomer concentration,analyze the monomer conversion and product composition,determine the reaction end point timely and avoid blind operation,and explore the reaction mechanism[18,19].It is used for studying the formation of carbon-carbon and carbon-heteroatom bonds catalyzed by transition metals and exploring new synthesis technology[20].By detecting the starting and end points of reaction precisely and tracking the material accumulation,the in-situ IR can improve the safety of the operating process[21].It is also used to monitor the emulsion homopolymerization and copolymerization of butyl acetate,methyl methacrylate and vinyl acetate[22],and the emulsion crosslinking copolymerization kinetics of methyl acrylate and isobutyric acid [23].In the synthesis of phenol formaldehyde prepolymer,the online infrared can track the change of polymer structure and prevent the formation of byproducts[24].
Herein,for the synthesis of P(HEMA-co-tBMA)with low molecular weight distribution using ARGET ATRP,the effects of temperature, monomer ratio,solvent,and molar ratio of reducing agent to the catalyst on the monomer conversion are monitored by in-situ IR.The reaction kinetics and mechanism of the copolymerization of hydrophilic HEMA/hydrophobic tBMA monomers are systematically investigated, which will provide the guidance for copolymerization of P(HEMA-cotBMA)and this kind of hydrophilic/hydrophobic monomers.
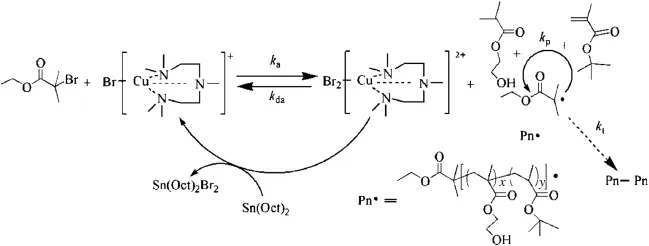
Fig.1.The ARGET ATRP mechanism for P(HEMA-co-tBMA).
2.Experimental
2.1.Materials
Tert-butyl methacrylate(tBMA,TCI-EP)was washed with sodium hydroxide solution(10%),distilled from calcium hydride,and stored under argon at−20°C.2-Hydroxyethyl methacrylate(HEMA,99%, Aldrich)was purif i ed by passing through a neutral alumina column followed by distillation and stored under argon at−20°C.Toluene was distilled from calcium hydride.Ethyl 2-bromoisobutyrate(99%, Aldrich),N,N,N′,N″,N″-pentamethyldiethylenetriamine(PMDETA,99%, Aldrich),anisole,CuBr2,n-hexane,dichloromethane,stannous octoate (Sn(Oct)2),methanol,tetrahydrofuran(THF),acetone,and all other reagents were used as received.
2.2.Measurements
The number average molecular weight(Mn)and polydispersity index (Mw/Mn)were determined by gel permeation chromatography(GPC) adopting an Agilent 1200 series GPC system equipped with a LC quant pump,three columns including PL gel 5 mm 50 nm,1000 nm and 10000 nm columns in series and a RI detector.The column system was calibrated with a set of monodisperse polystyrene standards using HPLC gradeTHFas themobilephasewitha fl owrateof1.0 ml·min−1at30°C.
1HNMRspectralmeasurementswereexecutedona BrukerAVANCE III 400 NMR spectrometer(Switzerland)operated at 400 MHz,using deuterated chloroform(CDCl3)as solvent and tetramethylsilane as the internal standard.The temperature was 25°C.
Gas chromatography(GC)was carried out on an Agilent Technologies 6820 series II Network GC system equipped with a poly(dimethylsiloxane)capillary column(12 m×200 μm×0.25 μm)using H2as eluent at a fl ow rate of 1.5 ml·min−1and a temperature ramp rate of 10°C·min−1. The temperature of the injector and detector was kept constant at 250°C with a H2fl ow rate of 40 ml·min−1.Anisole(1/1 by volume to HEMA) was added to the polymerization medium as an internal GC standard. GC samples were diluted with acetone prior to characterization.
2.3.In-situ IR
A ReactIR iC10 reaction analysis system(Mettler-Toledo AutoChem, Switzerland)equipped with a light conduit and DiComp(diamond composite)insertion probe was used to collect mid-FTIR spectra of the condensation components.FTIR spectra were collected every minute in the wave number range between 4000 cm−1and 650 cm−1at a resolution of 8 cm−1.The reaction information was provided by in-situ IR detecting the disappearance of the C=C vibration peak(1638 cm−1) of the comonomers[25].The total conversion(α)of monomers HEMA and tBMA during the polymerization was calculated according to Eq.(1).The conversion of monomer HEMA or tBMA was obtained by ConcIRT™software(spectral region:800-1200 cm−1,time region: 0-60 min)[17].ConcIRT™,using a type of mathematical algorithm known as curve-resolution,is a powerful tool for analyzing a broad range of reactions because the characteristic peaks of HEMA and tBMA around 800-1200 cm−1are completely different,which can be used tocalculatetheconversionofHEMAandtBMAusingtheConcIRT™software.When adding the f i rst monomer tBMA,ConcIRT™will calculate the associated component spectrum and relative concentration prof i le. And ConcIRT™will re-analyze the former reaction spectra of tBMA and update theindividualcomponentspectra and prof i les when adding thesecondmonomerHEMA.Therefore,wecancalculatetheconversion of HEMA and tBMA through their concentration prof i les:

where A0and Atare the peak heights at reaction times t=0 and 1638 cm−1,respectively.
2.4.Synthesis of P(HEMA-co-tBMA)
TheARGETATRPprocedureoftBMAandHEMAwas asfollows:CuBr2(11.1 mg,0.0500 mmol)was added to a dry 100 ml three-necked fl ask with a magnetic stirring bar,and the fl ask was evacuated and fl ushed with argon thrice,then the in-situ IR probe was placed into the reaction fl ask.Anhydrous toluene(18 ml),tBMA(5715 μl,35.2 mmol)and HEMA(1215 μl,10.00 mmol)were added to the fl ask using a degassed syringe and the mixture was homogenated by ultrasonic dispersion for 10 min,and PMDETA(105 μl,0.500 mmol)was then introduced into the fl askvia a syringe.After being stirred for10 min,Cu/PMDETAcatalyst complex formed.Then Sn(Oct)2(160 μl,0.500 mmol)in toluene(2 ml) was added to reduce the deactivator.Finally,EBriB(147 μl,1.00 mmol) was added and the fl ask was placed in a preheated oil bath maintained at 70°C.The in-situ IR probe was submerged into the solution and begantocollectthedataatthesametime.Samplesweretakenoutatcertain time intervals and analyzed by GC and GPC to follow the reaction process.After 2 h,the fl ask was removed from the oil bath and cooled to room temperature.THF(50 ml)was added into the fl ask and the mixture was then passed through a neutral alumina column to remove the catalyst.Finally,P(HEMA-co-tBMA)was recovered by precipitating the mixtureinto10-foldexcessofwater/methanol(1:1,byvolume)mixture, fi ltered,and dried under vacuum for 24 h.

Fig.3.Total conversion versus time by in-situ IR and GC(temperature=60°C;[HEMA]: [tBMA]=40:40;[PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).
3.Results and Discussion
3.1.Polymerization kinetics of P(HEMA-co-tBMA)
To investigate the polymerization kinetics of P(HEMA-co-tBMA) with in-situ IR by monitoring the change of C=C bond vibrations at the characteristic peak(1638 cm−1)of HEMA and tBMA,Fig.2 shows a typical three-dimensional IR spectrum(1661-1620 cm−1)during the copolymerization of HEMA and tBMA.The conversion of monomers asafunctionoftimecan bedeterminedbyConcIRT™software.Itcan be seen that the peak at 1638 cm−1decreases gradually as the reaction proceeds.Fig.3 shows the total conversions of HEMA and tBMA calculated by Eq.(1)and determined by GC analysis,both of which are consistent.The data from Fig.A1(see Appendix A)show a linear relation with time within 30 min of polymerization time,indicating f i rst-order kinetics with respect to monomer and constant radical concentrations throughout the polymerization.
Fig.4 shows the relationship between Mnand Mw/Mnof the copolymer and the total conversion.The correlation between Mnand the conversion is approximately linear and Mw/Mnvalues are in the range of 1.2-1.4,indicating a living and well-controlled radical polymerization in the current work.

Fig.2.Three-dimensionalspectrumofthepeakat1638cm−1duringcopolymerizationvia in-situ IR(temperature=60°C;[HEMA]:[tBMA]=40:40;solvent:toluene;[PMDETA]: [Sn(Oct)2]:[CuBr2]=10:10:1).

Fig.4.Dependence of Mnand Mw/Mnon the total conversion(temperature=60°C; [HEMA]:[tBMA]=40:40;[PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).
3.2.Effect of temperature
The total monomer conversions at different reaction temperatures (50-90°C)investigated with in-situ IR are shown in Fig.5(a)and Table 1.The polymerization rate increases with temperature,resulting in shorter equilibrium time[26].Too high or too low reaction temperature would reduce the total monomer conversion and increase Mw/Mn, which is also shown in Table 1.The highest conversion(90%-95%)is achieved at 60°C and 70°C.The Mnvalue of the copolymer is close to the theoretical value and Mw/Mnis the lowest.At 50°C,80°C and 90°C,the total conversions are much lower and Mw/Mnvalues are higher.The data in Fig.5(b)show linear time dependency within 30 min of polymerization time,indicating f i rst-order kinetics([M]0is the initial concentration and[M]is the concentration at time t).
Tofurtherunderstandtheinf l uenceofreactiontemperatureonmonomer conversion,the total conversion is converted to the individual conversion rate of monomers HEMA and tBMA by ConcIRT™[20],as shown inFig.6.The conversions of HEMA are more than95%and hardly affected by temperature,while the reaction temperature exhibits strong inf l uence on the conversion of tBMA.The conversion of tBMA is 60%at 50°C,then increases as the temperatureincreases to70°C and decreases as the temperatureishigherthan80°C,droppingto50%at90°C.Thef i rst-orderkinetic plots ln([M]0/[M])versus time are linear during the f i rst 30 min of polymerization.The kinetic plots show a short induction period initially due to the solubility of catalyst at a relatively low temperature of 50°C. Increasing the temperature accelerates the polymerization slightly.
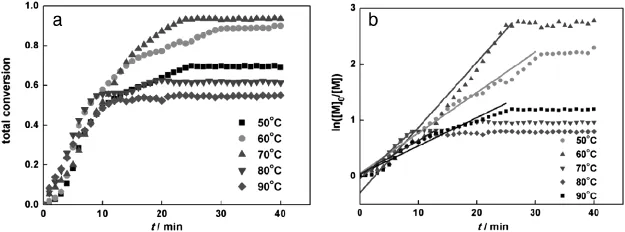
Fig.5.Total conversion and ln([M]0/[M])with time at varied reaction temperatures([HEMA]:[tBMA]=40:40;[PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).

Table 1Characterizations for P(HEMA-co-tBMA)at varied reaction temperatures
The theoretical molecular weight(Mn,th)and molar ratio(ψIR)of HEMA to tBMA of the copolymer product are calculated according to Eqs.(2)and(3),respectively,also summarized in Table 1,showing that Mn,GPCvalues are in close agreement with Mn,th:
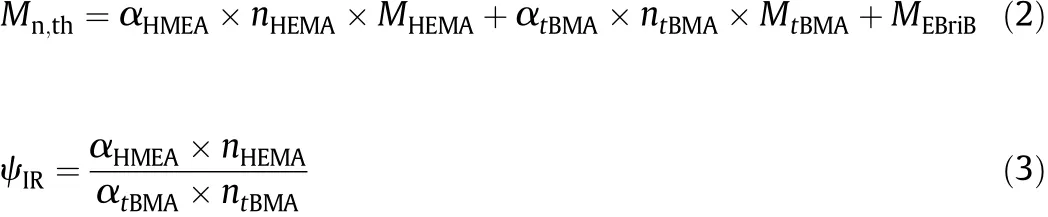
whereαHMEAandαtBMAaretheconversionsof HEMAandtBMA,respectively,nHEMAand ntBMAare the mole numbers of HEMA and tBMA, respectively,and MHEMA,MtBMAand MEBriBare respectively the molar mass values of HEMA,tBMA and EBriB.
The chemical structures and composition of the products at various reaction temperatures characterized by1H NMR spectroscopy are depictedin Fig.7.Thesignals at0.8-1.2 and1.7-2.0areascribedrespectivelyto-CCH3and-CH2-ofmethacrylatebackbone,whilethesignal at1.42is thecharacteristic resonanceof-C(CH3)3in thetBMA unit and the signals at 4.12 and 3.86 attribute to-CH2CH2O-and-CH2CH2O-in the HEMA unit,respectively.The1H NMR results suggest that the P(HEMA-co-tBMA)copolymer is successfully synthesized.From the integration ratio value of the signals originated from the HEMA unit at 3.86(Ib,2H,-CH2CH2O-)and tBMA unit at 1.42(Ic,9H,-C(CH3)3), the composition ratio of the copolymer(ψNMR)can be calculated according to Eq.(4)and the results are also listed in Table 1.

It can be seen that ψNMRis consistent with ψIRcalculated by in-situ monitoring.The molar ratio of HEMA to tBMA is close to 1 at 60°C and 70°C,attributing to thecomplete transformation of the two monomers.Conversely,temperatures lower than 60°C or higher than 70°C cause incomplete conversion of tBMA and higher HEMA/tBMA ratios (>1).Therefore,60°C is an optimum reaction temperature and chosen for the follow-up studies.
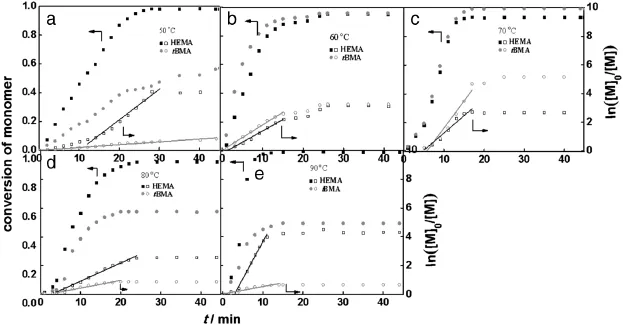
Fig.6.Conversion of HEMA and tBMA and ln([M]0/[M])with time at varied temperatures([HEMA]:[tBMA]=40:40;[PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).
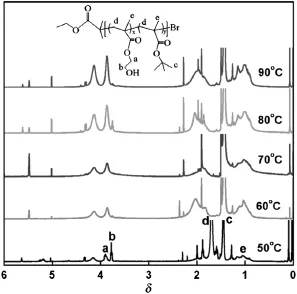
Fig.7.1H NMRspectraof P(HEMA-co-tBMA)polymerizedinCDCl3atvariedtemperatures ([HEMA]:[tBMA]=40:40;[PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).
3.3.Effect of monomer ratio
ThecopolymerizationatdifferentmonomerratiosofHEMAandtBMA conducted in toluene at 60°C at the molar ratio of[PMDETA]:[Sn(Oct)2]: [CuBr2]10:10:1isgiveninTable2.Theinf l uenceofmonomerratioonthe totalmonomerconversionisshowninFig.8.ForHEMAortBMApolymerization,the conversion is high,reaching 96%for tBMA and 91%for HEMA. For higher[HEMA]:[tBMA]molar ratio over 40:40,the copolymerization rate is faster compared to polymerization of tBMA with higher conversion.As the content of tBMA further increases at lower[HEMA]:[tBMA] molarratio(below40:40),thetotal conversion andthemolecularweight of the polymer products decrease gradually with the decrease of molar ratio[HEMA]:[tBMA].The f i rst-order kinetic plots ln([M]0/[M])versus time are linear,which could be seen in Fig.A2(see Appendix A).
The total conversions converted to the conversions of HEMA and tBMA by the ConcIRT™software are shown in Fig.9.It can be seen that the HEMA conversion is hardly affected by the change of molar ratio [HEMA]:[tBMA],while the tBMA conversion shows obvious dependence on the molar ratio.When molar ratio[HEMA]:[tBMA]is below 40:40, tBMA conversiondecreases as theratio decreases,reducingthetotalconversion.It is well known that the polymerization rate of ARGET ATRP depends primarily on the free radical activity,and the radical activity of tBMA is relatively lower than that of HEMA because of larger steric hindrance of the tert-butyl group.As a result,the growth rate constant of tert-butylmethacrylateissmallerthanthatofHEMAunderthesameconditions.Since HEMA has the hydroxyl group-OH,we guess that the polarity and reactivity ratio of HEMA may be larger than those of tBMA.In the present work,the highest conversion of HEMA and tBMA is achieved atanequalmolaramountofHEMAand tBMA(40:40),andthemolecular weight of P(HEMA-co-tBMA)with narrow distribution determined by GPC(10382,Entry 3 in Table 2)is quite close to the theoretical value (10894,calculated from 100%conversion).The ln([M]0/[M])-time plots of all the reactions are linear(see Fig.A3 in Appendix A).
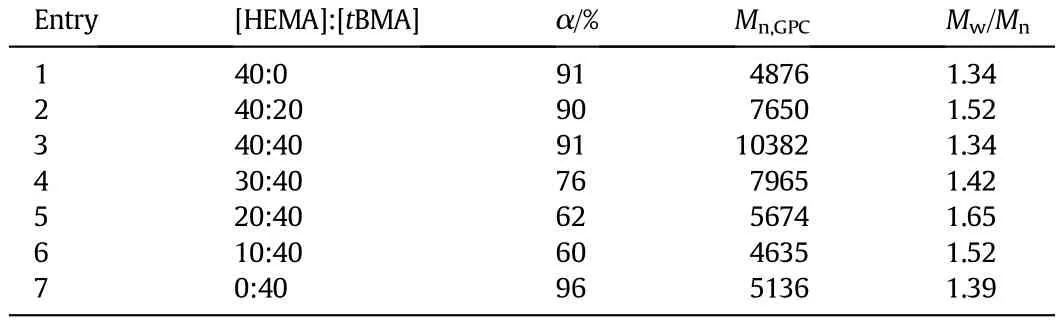
Table 2 Characterizations for P(HEMA-co-tBMA)at varied monomer ratios
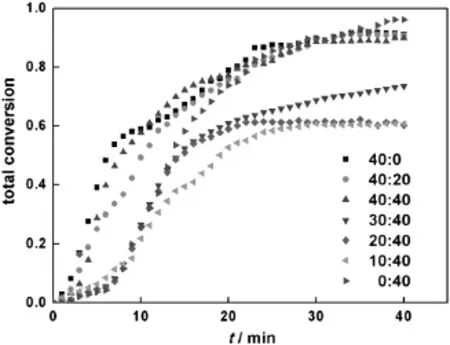
Fig.8.Total conversion with time in varied[HEMA]:[tBMA]ratios(temperature=60°C; [PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).
3.4.Effect of solvent
Fig.10 shows the effects of solvent polarity on the reaction process with four solvents(toluene,anisole,THF,and DMF)with different polarity as the polymerization media.The polymerization rate and total conversion decrease gradually with the increase of polarity of solvents. The totalconversionwasnearlyupto91%after30 min in non-polarsolvent toluene,while only about 18%after 60 min in strong-polar solvent DMF,indicating that the reaction is much faster and more effective in non-polar solvent than in polar solvent.The non-polar solvent is more suitable for the copolymerization of HEMA and tBMA.The molecular weight of P(HEMA-co-tBMA)decreases gradually and exhibits broader molecular weight distribution with the increase of polarity of solvents, as seen in Table 3.
The conversions of HEMA and tBMA decrease gradually with the increase of polarity of solvents(Fig.10),resulting in a large decrease of the total monomer conversion.This result may be due to the reaction/ complexation of the reducing agent in the polar solvent.Toluene is a favorable solvent for the polymerization of HEMA and tBMA.The kinetics is f i rst-order in these four solvents(see Fig.A4 in Appendix A).
3.5.Effect of reducing agent/catalyst ratio
Compared to other controlled living polymerization techniques, ARGET ATRP has a unique advantage that the polymerization can be well controlled by a suff i ciently large excess of reducing agent and very low levels of catalyst[6].The concentration of reducing agent has a great inf l uence on the reduction rate and hence the polymerization rate.In the current work,the molar ratio of reducing agent Sn(Oct)2to catalyst CuBr2varies from 5:1 to 20:1.At[Sn(Oct)2]:[CuBr2]of 5:1,the total conversion was 80%in 60 min and the conversions of HEMA and tBMA were all relatively low[Fig.11(a)]with lower molecular weight (Table 4).When a 10-fold excess of reducing agent(10:1)was used, the polymerization rate speeded up and the total conversion was over 90%in40min[Fig.11(b)].Thepolymerizationratewasfurtherelevated byincreasingratio[Sn(Oct)2]:[CuBr2]to15:1and20:1,resultingineven broadermolecularweightdistributions.Thevalueof[CuI]/[CuII]wastoo small at the ratio[CuBr2]:[Sn(Oct)2]of 5:1,and was too large and unstable as the ratio maintained at 15:1 and 20:1,leading to broader molecular weight distribution[27].Increasing the concentration of reducing agent increases the polymerization rate as it will produce more Cu(I)Br and more radicals,which increases PDI since the number of monomers added per activation deactivation cycle will increase.The optimum[Sn(Oct)2]:[CuBr2]ratio is 10:1,which could maintain a higher conversion and narrow molecular weightdistribution.First-order kinetics were observed in varied[Sn(Oct)2]: [CuBr2]ratios(see Fig.A5 in Appendix A).

Fig.9.HEMA and tBMA conversion with time in varied monomer ratios(temperature=60°C;[PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).

Fig.10.Conversionoftotal,HEMA andtBMA with time invariedsolvents(temperature= 60°C;[HEMA]:[tBMA]=40:40;[PMDETA]:[Sn(Oct)2]:[CuBr2]=10:10:1).
4.Conclusions

Table 3Characterizations for P(HEMA-co-tBMA)in varied solvents
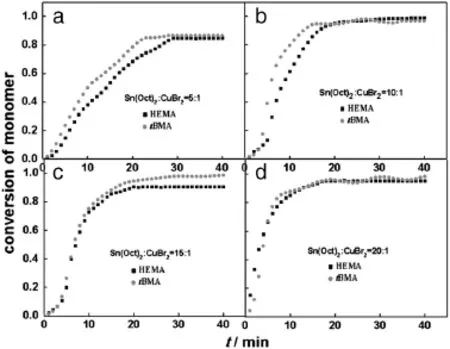
Fig.11.HEMA and tBMA conversion with time in varied[Sn(Oct)2]:[CuBr2]ratios (temperature=60°C,[HEMA]:[tBMA]=40:40).
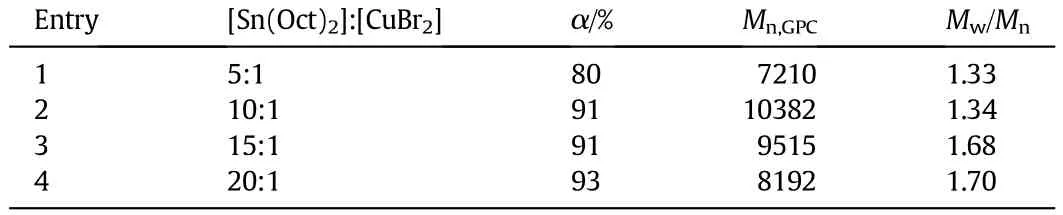
Table 4Characterizations for P(HEMA-co-tBMA)at varied[Sn(Oct)2]:[CuBr2]ratios
The amphiphilic copolymer P(HEMA-co-tBMA)was synthesized via ARGET ATRP.The copolymerization process showed a living and wellcontrolled f i rst-order kinetic characteristic.The effects of reaction parameters,such as temperature,monomer ratio,solvent,and reducing agent/ catalyst ratio on the ARGET ATRP of HEMA and tBMA were investigated by in-situ IR.The reaction temperature has a major impact on the copolymerization:too low or too high temperature could lower the conversion oftBMAandthusreducethetotalconversion.TheconversionoftBMAdecreases due to a tert-butyl steric hindrance effect when the concentrationof tBMA is higher than that of HEMA.The polarity of solvent also affects the polymerization rate and the conversion.Polar solvent is not suitable for the copolymerization of HEMA and tBMA.For the catalytic system, lower concentration of reducing agent will decrease the total monomer conversion,butlargerconcentrationcausesbroadermolecularweightdistribution of the copolymer.In-situ IR monitoring the polymerization process plays an important role for optimizing the reaction conditions and understanding the mechanism of polymerization reaction.
Appendix A

Fig.A1.First order kinetic plots versus time by in-situ IR and GC.

Fig.A2.ln([M]0/[M])with time in varied monomer ratios.
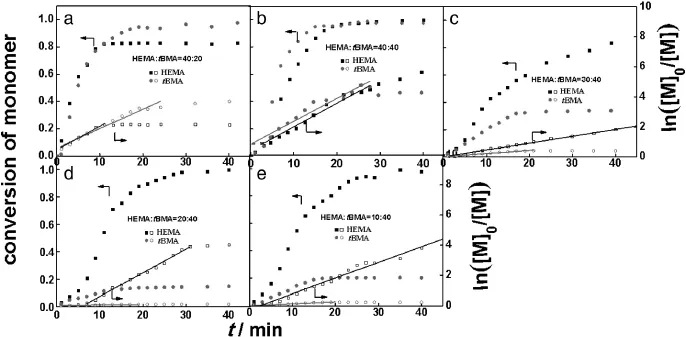
Fig.A3.HEMA and tBMA conversion and ln([M]0/[M])with time in varied monomer ratios.
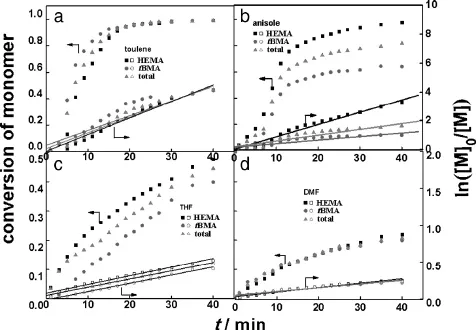
Fig.A4.Conversion of total,HEMA and tBMA and ln([M]0/[M])with time in varied solvents.

Fig.A5.HEMA and tBMA conversion and ln([M]0/[M])with time in varied[Sn(Oct)2]:[CuBr2]ratios.
[1]X.D.Guo,L.J.Zhang,Y.Chen,Y.Qian,Core/shellpH-sensitive micelles self-assembled from cholesterol conjugated oligopeptides for anticancer drug delivery,AICHE J.56 (2010)1922-1931.
[2]X.J.Li,M.H.Yin,G.L.Zhang,F.B.Zhang,Study and characterization of novel temperature and pH responsive hydroxylpropyl cellulose-based graft copolymers,Chin.J. Chem.Eng.17(2009)145-149.
[3]Y.N.Xue,Z.Z.Huang,J.T.Zhang,M.Liu,M.Zhang,S.W.Huang,R.X.Zhuo,Synthesis and self-assembly of amphiphilic poly(acrylic acid-b-DL-lactide)to form micelles for pH-responsive drug delivery,Polymer 50(2009)3706-3713.
[4]K.S.Soppimath,T.M.Aminabhavi,A.R.Kulkarni,W.E.Rudzinski,Biodegradable polymeric nanoparticles as drug delivery devices,J.Control.Release 70(2001)1-20.
[5]P.V.Mendonca,A.C.Serra,J.F.J.Coelho,A.V.Popov,T.Guliashvili,Ambient temperature rapid ATRP of methyl acrylate,methyl methacrylate and styrene in polar solvents with mixed transition metal catalyst system,Eur.Polym.J.47(2011)1460-1466.
[6]W.Jakubowski,K.Matyjaszewski,Activator generated by electron transfer for atom transfer radical polymerization,Macromolecules 38(2005)4139-4146.
[7]J.Magnus,N.Daniel,N.Ove,M.Eva,Surface modif i cation of thermally expandable microspheres by grafting poly(glycidyl methacrylate)using ARGET ATRP,Eur. Polym.J.45(2009)2374-2382.
[8]D.J.Siegwart,J.K.Oh,K.Matyjaszewski,ATRP in the design of functional materials for biomedical applications,Prog.Polym.Sci.37(2012)18-37.
[9]K.Matyjaszewski,W.Jakubowski,K.Min,W.Tang,J.Huang,W.A.Braunecker,N.V. Tsarevsky,Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents,Proc.Natl.Acad.Sci.U.S.A.103(2006)15309-15314.
[10]W.Jakubowski,B.Kirci-Denizli,R.R.Gil,K.Matyjaszewski,Polystyrene with improved chain-end functionality and higher molecular weight by ARGET ATRP, Macromol.Chem.Phys.209(2008)32-39.
[11]W.Jakubowski,K.Min,K.Matyjaszewski,Activatorsregeneratedbyelectrontransfer for atom transfer radical polymerization of styrene,Macromolecules 39(2006) 39-45.
[12]T.Pintauer,K.Matyjaszewski,Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes,Chem.Soc.Rev.37 (2008)1087-1097.
[13]S.M.Paterson,D.H.Brown,T.V.Chirila,I.Keen,A.K.Whittaker,M.V.Baker,The synthesis of water-soluble PHEMA via ARGET ATRP in protic media,J.Polym.Sci.A Polym.Chem.48(2010)4084-4092.
[14]Y.Q.Xiang,D.J.Chen,Preparation of a novel pH-responsive silver nanoparticle/ poly(HEMA-PEGMA-MAA)composite hydrogel,Eur.Polym.J.43(2007)4178-4187.
[15]X.M.Li,Y.D.Cui,Study on synthesis and chloramphenicol release of poly(2-hydroxyethylmethacrylate-co-acrylamide)hydrogels,Chin.J.Chem.Eng.16(2008) 640-645.
[16]M.D.P.Wilcox,N.Harmis,B.A.Cowell,T.Williams,B.A.Holden,Bacterial interactions with contact lenses:effects of lens material,lens wear and microbial physiology, Biomaterials 22(2001)3235-3247.
[17]P.V.D.Wetering,E.E.Moret,N.M.E.Schuurmans-Nieuwenbroek,M.J.V.Steenbergen, W.E.Hennink,Structure-activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery,Bioconjug.Chem.10 (1999)589-597.
[18]P.MacLaurin,N.C.Crabb,I.Wells,P.J.Worsfold,Quantitative in situ monitoring of an elevated temperature reaction using a water-cooled mid-infrared f i ber-optic probe, Anal.Chem.68(1996)1116-1123.
[19]L.K.Breland,F.S.Robson,Polyisobutylene-based miktoarm star polymers via a combination of carbocationic and atom transfer radical polymerizations,Polymer 49 (2008)1154-1163.
[20]Y.S.Zhao,H.B.Wang,X.H.Hou,Y.H.Hu,A.W.Lei,H.Zhang,L.Z.Zhu,Oxidative cross-coupling through double transmetallation:surprisingly high selectivity for palladium-catalyzed cross-coupling of alkylzinc and alkynylstannanes,J.Am. Chem.Soc.128(2006)15048-15049.
[21]A.E.Enriquez,J.L.Templeton,Monomeric and dinuclear tungsten carbyne complexes containing benzyl,allyl,and alkenyl carbyne substituents,Organometallics 21(2002)852-863.
[22]H.Hong,A.D.Marc,In-line monitoring of emulsion homo-and copolymerizations using ATR-FTIR spectrometry,Polym.React.Eng.10(2002)21-39.
[23]A.Matsumoto,T.Otaka,H.Aota,In-situ kinetic pursuit of emulsion crosslinking copolymerizations of monomethacrylate and dimethacrylate by means of reactIR, Macromol.Rapid Commun.22(2001)607-610.
[24]M.Krajnc,I.Poljansek,Characterization of phenol-formaldehyde prepolymer resins by in line FT-IR spectroscopy,Acta Chim.Slov.52(2005)238-244.
[25]J.Scherble,B.Ivan,R.Mulhaupt,Online monitoring of silicone network formation by means of in-situ mid-infrared spectroscopy,Macromol.Chem.Phys.203(2002) 1866-1871.
[26]K.Tanaka,K.Matyjaszewski,Copolymerization of(meth)acrylates with olef i ns using activators regenerated by electron transfer for atom transfer radical polymerization(ARGET ATRP),Macromol.Symp.261(2008)1-9.
[27]W.Jakubowski,K.Matyjaszewski,Activators regenerated by electron transfer for atom-transfer radical polymerization of(meth)acrylates and related block copolymers,Angew.Chem.Int.Ed.45(2006)4482-4486.
3 July 2013
☆Supported by the National Natural Science Foundation of China(21176090,21136003), Team Project of Natural Science Foundation of Guangdong Province(S2011030001366), Science and Technology Foundation of Guangdong Province(2012B050600010),and Fundamental Research Funds for the Central Universities(2013ZP0010).
*Corresponding author.
E-mail address:celjzh@scut.edu.cn(L.Zhang).
http://dx.doi.org/10.1016/j.cjche.2014.06.030
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 21 October 2013
Accepted 1 November 2013
Available online 1 July 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Oxygen Gasif i cation of Municipal Solid Waste in a Fixed-bed Gasif i er☆
- Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
